The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coconut-Oil-Derived Polyol
2.3. Characterization of the CODP
2.4. Preparation of the Coco PU Foam (CCF)
2.5. Characterization of the Physical Properties of the CCF (Cell Size, Density Morphology, and Open Cell Content)
2.6. The Mechanical Properties of the CCF
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. The Thermal Decomposition Analysis
2.9. Water Contact Angle Measurements
2.10. Oil and Water Sorption Measurements
2.11. Foam Regeneration Evaluation
2.12. Determination of the Hydroxyl Values
3. Results and Discussion
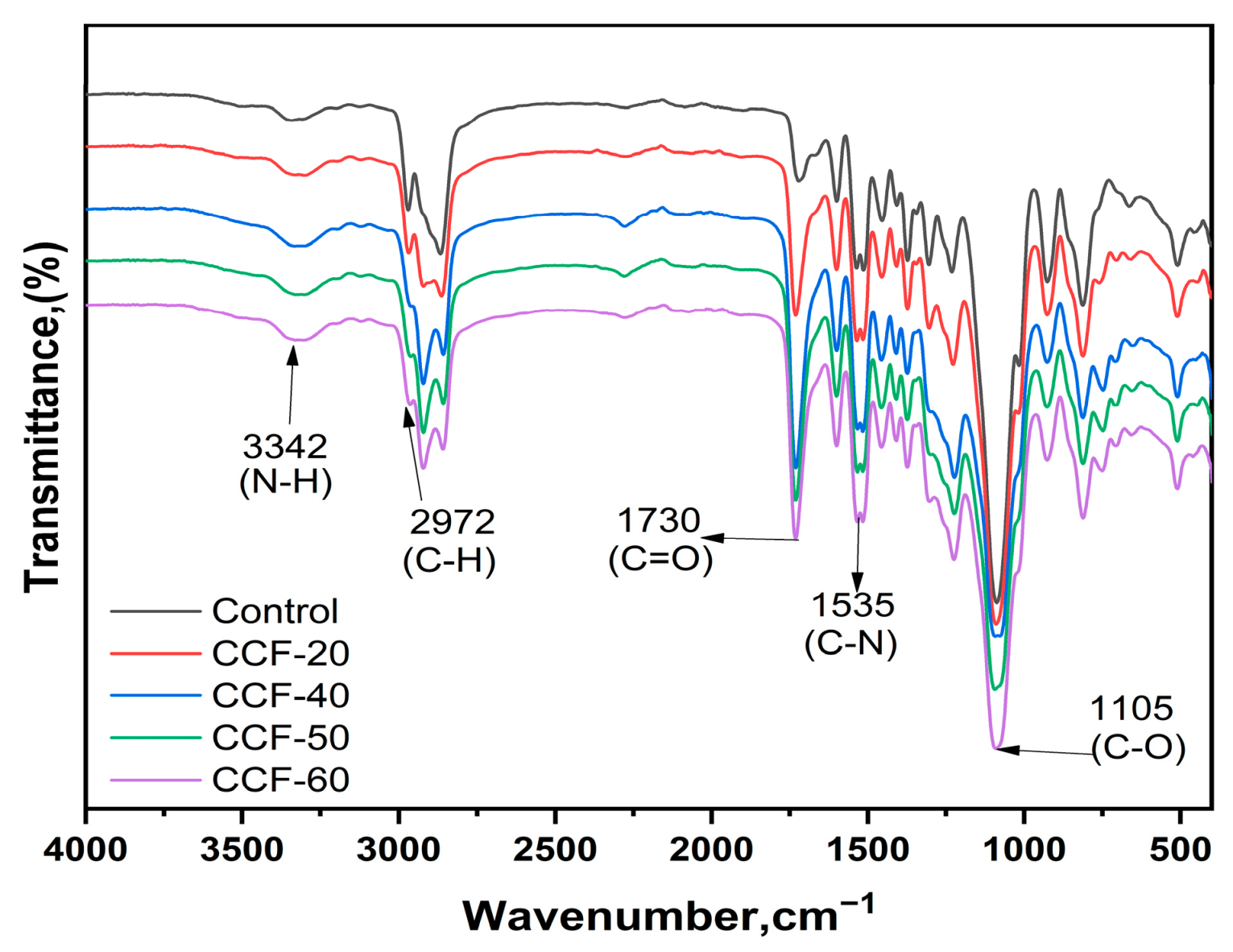
3.1. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
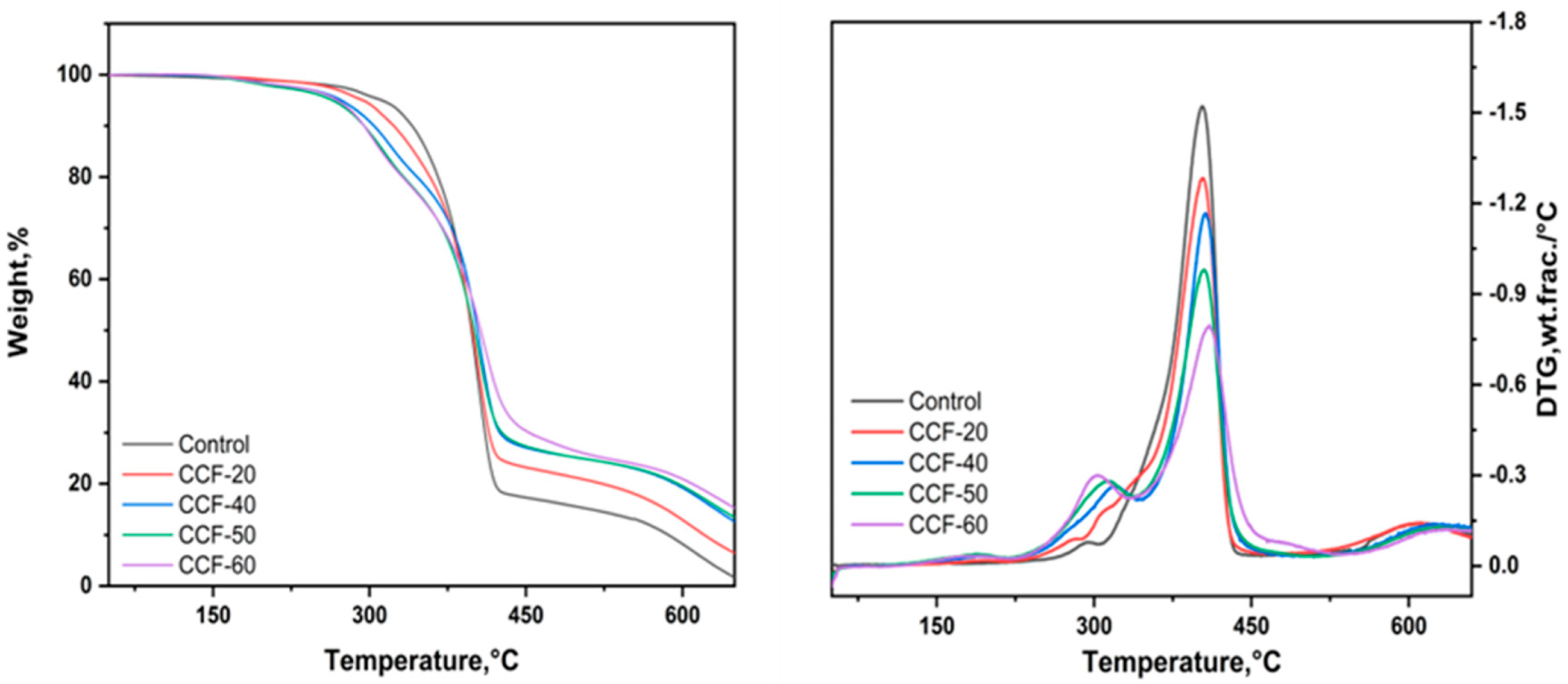
3.2. The Thermal Properties of the Foams
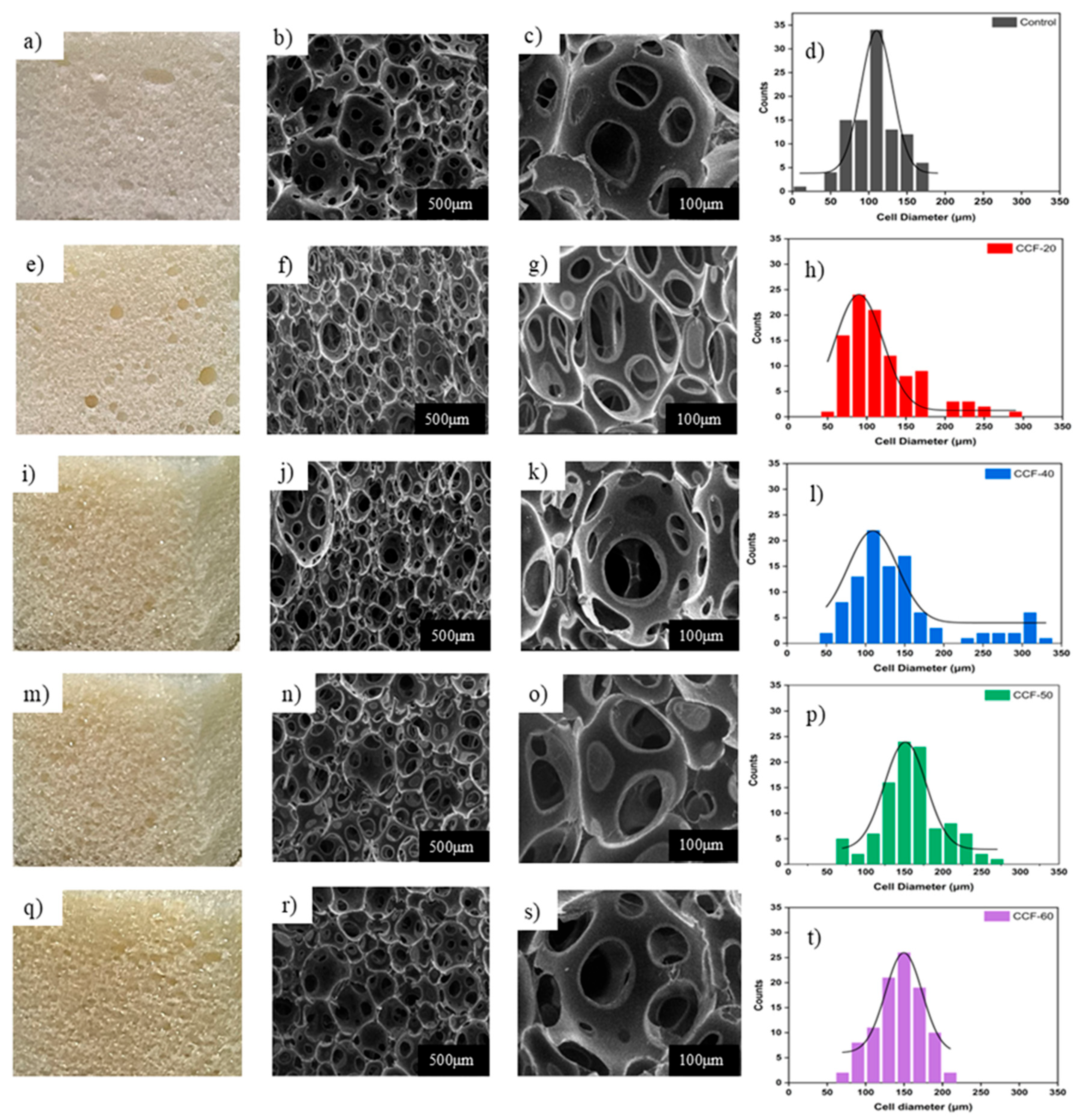
3.3. The Influence of the CODP Replacement on the Morphology and Physico-Mechanical Characteristics of the Foams
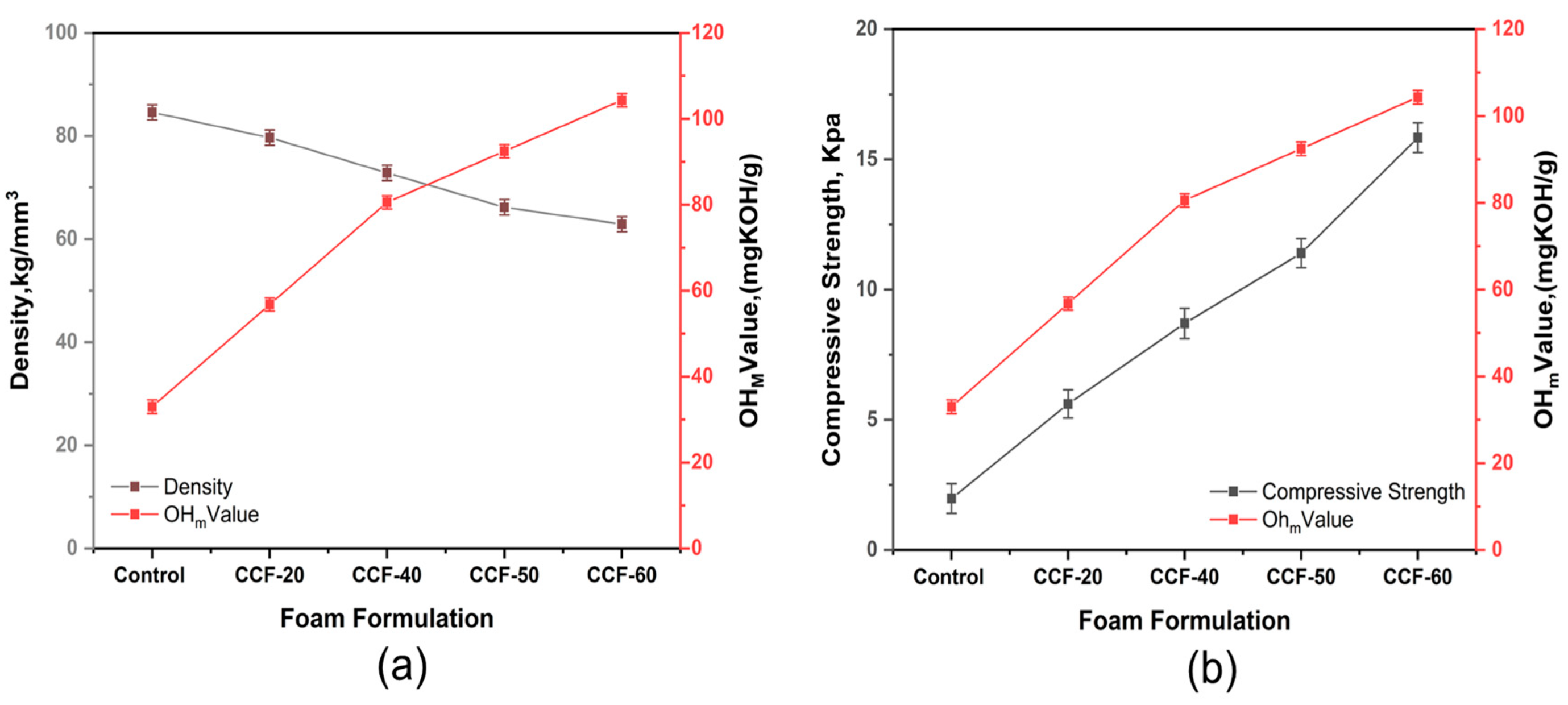
3.4. The Influence of the Hydroxyl Values on the Density and Compressive Strength of the Synthesized Foams
3.5. Wettability and Hydrophobicity of the Synthesized Foams
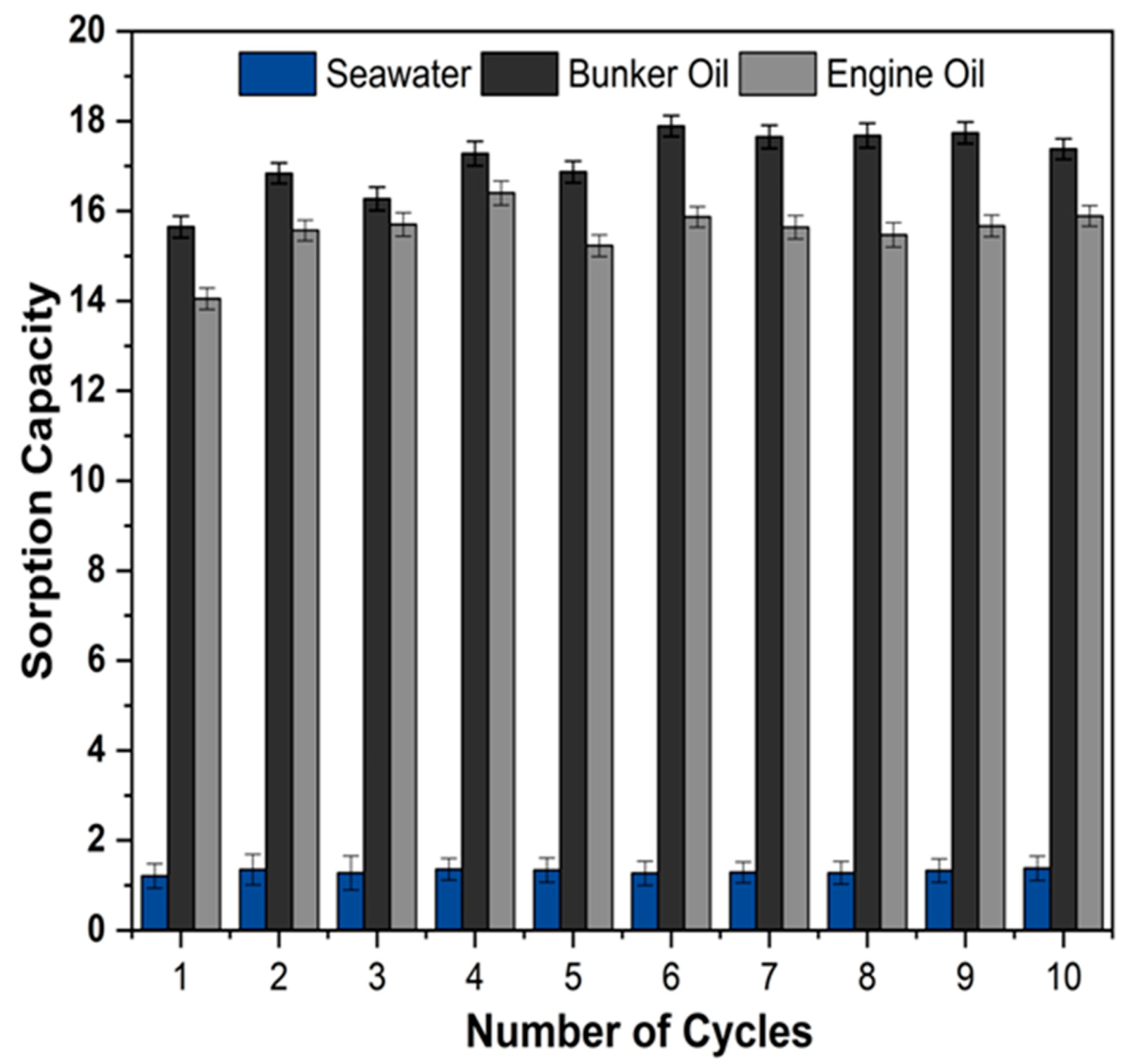
3.6. The Oil Sorption Capacity, Oil/Water Selectivity, and Reusability of the Synthesized Foam
3.7. The Oil Sorption Capacities of Common Sorbents
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing Materials |
References
- Pete, A.J.; Bharti, B.; Benton, M.G. Nano-Enhanced Bioremediation for Oil Spills: A Review. ACS EST Eng. 2021, 1, 928–946. [Google Scholar] [CrossRef]
- Thakur, A.; Koul, B. Impact of Oil Exploration and Spillage on Marine Environments. In Advances in Oil-Water Separation; Elsevier: Amesterdam, The Netherlands, 2022; pp. 115–135. [Google Scholar] [CrossRef]
- O’Callaghan-Gordo, C.; Orta-Martínez, M.; Kogevinas, M. Health Effects of Non-Occupational Exposure to Oil Extraction. Environ. Health 2016, 15, 56. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. The Development of Long-Term Adverse Health Effects in Oil Spill Cleanup Workers of the Deepwater Horizon Offshore Drilling Rig Disaster. Front. Public Health 2018, 6, 117. [Google Scholar] [CrossRef]
- Galieriková, A.; Materna, M. World Seaborne Trade with Oil: One of Main Cause for Oil Spills? Transp. Res. Procedia 2020, 44, 297–304. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Dutta, T. An Overview of Oil Pollution and Oil-Spilling Incidents. In Advances in Oil-Water Separation; Elsevier: Amesterdam, The Netherlands, 2022; pp. 3–15. [Google Scholar] [CrossRef]
- Haseeba, K.P.; Vethamony, P.; Veerasingam, S.; Aboobacker, V.M.; Al-Khayat, J.A. A Comprehensive Review of Oil Residues in the World Oceans: Types, Characteristics, Sources and Distribution. Mar. Pollut. Bull. 2025, 217, 118106. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Fate of Total Petroleum Hydrocarbons in the Environment. In Total Petroleum Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2020; pp. 57–77. [Google Scholar] [CrossRef]
- Asif, Z.; Chen, Z.; An, C.; Dong, J. Environmental Impacts and Challenges Associated with Oil Spills on Shorelines. JMSE 2022, 10, 762. [Google Scholar] [CrossRef]
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental Effects of the Deepwater Horizon Oil Spill: A Review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A Review of Bio-Based Materials for Oil Spill Treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Pagnucco, R.; Phillips, M.L. Comparative Effectiveness of Natural By-Products and Synthetic Sorbents in Oil Spill Booms. J. Environ. Manag. 2018, 225, 10–16. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, P.; Xu, Y. Research on Oil Boom Performance Based on Smoothed Particle Hydrodynamics Method. PLoS ONE 2023, 18, e0289276. [Google Scholar] [CrossRef]
- Widiaksana, N.; Yudiana, A.A.; Nugroho, Y.S. Analysis of Effectiveness of Oil Spill Recovery Using Disc-Type Oil Skimmer at Laboratory Scale. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012086. [Google Scholar] [CrossRef]
- Đorđević, M.; Šabalja, Đ.; Mohović, Đ.; Brčić, D. Optimisation Methodology for Skimmer Device Selection for Removal of the Marine Oil Pollution. JMSE 2022, 10, 925. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Farouq, R.; Mohamed, O.A.; Tony, M.A. Oil Spill Clean-up Using Combined Sorbents: A Comparative Investigation and Design Aspects. Int. J. Environ. Anal. Chem. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Etkin, D.S.; Nedwed, T.J. Effectiveness of Mechanical Recovery for Large Offshore Oil Spills. Mar. Pollut. Bull. 2021, 163, 111848. [Google Scholar] [CrossRef]
- Ouyang, D.; Lei, X.; Zheng, H. Recent Advances in Biomass-Based Materials for Oil Spill Cleanup. Nanomaterials 2023, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Kaur, S.; Vaid, S.; Dhaliwal, Y.; Slaich, R.S.; Verma, D. Oil Spill Cleanup: A Review. Int. J. Sci. Res. Arch. 2024, 12, 2737–2754. [Google Scholar] [CrossRef]
- Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review. Molecules 2020, 25, 4522. [Google Scholar] [CrossRef]
- Carmody, O.; Frost, R.; Xi, Y.; Kokot, S. Adsorption of Hydrocarbons on Organo-Clays—Implications for Oil Spill Remediation. J. Colloid Interface Sci. 2007, 305, 17–24. [Google Scholar] [CrossRef]
- Bastani, D.; Safekordi, A.A.; Alihosseini, A.; Taghikhani, V. Study of Oil Sorption by Expanded Perlite at 298.15K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Cozzarini, L.; Marsich, L.; Ferluga, A.; Schmid, C. Life Cycle Analysis of a Novel Thermal Insulator Obtained from Recycled Glass Waste. Dev. Built Environ. 2020, 3, 100014. [Google Scholar] [CrossRef]
- Gómez-Jiménez-Aberasturi, O.; Ochoa-Gómez, J.R. New Approaches to Producing Polyols from Biomass. J. Chem. Technol. Biotechnol. 2017, 92, 705–711. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A Review of Unconventional Sustainable Building Insulation Materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Carvalho, K.; Alves, R.M.B.; Kulay, L. Environmental Performance of Alternative Green Polyol Synthesis Routes: A Proposal for Improvement. Processes 2021, 9, 1122. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid Polyurethane Foams from a Soybean Oil-Based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Casado, U.; Marcovich, N.E.; Aranguren, M.I. Vegetable Oil Based-Polymers Reinforced with Wood Flour. Mol. Cryst. Liq. Cryst. 2008, 484, 143–509. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, G.; Zhou, X.; Jiang, W.; Wang, T.; Zhang, N.; Yu, L. One-Pot Synthesis of Robust Superhydrophobic, Functionalized Graphene/Polyurethane Sponge for Effective Continuous Oil–Water Separation. Chem. Eng. J. 2016, 302, 155–162. [Google Scholar] [CrossRef]
- Aydoğmuş, E.; Kamişli, F. New Commercial Polyurethane Synthesized with Biopolyol Obtained from Canola Oil: Optimization, Characterization, and Thermophysical Properties. J. Mol. Struct. 2022, 1256, 132495. [Google Scholar] [CrossRef]
- Asare, M.A.; Kote, P.; Chaudhary, S.; De Souza, F.M.; Gupta, R.K. Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants. Polymers 2022, 14, 5282. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Ryszkowska, J.; Szczepkowski, L.; Kurańska, M.; Prociak, A.; Leszczyński, M.K.; Gloc, M.; Antos-Bielska, M.; Mizera, K. Cooperative Effect of Rapeseed Oil-Based Polyol and Egg Shells on the Structure and Properties of Rigid Polyurethane Foams. Polym. Test. 2020, 90, 106696. [Google Scholar] [CrossRef]
- Dingcong, R.G.; Malaluan, R.M.; Alguno, A.C.; Estrada, D.J.E.; Lubguban, A.A.; Resurreccion, E.P.; Dumancas, G.G.; Al-Moameri, H.H.; Lubguban, A.A. A Novel Reaction Mechanism for the Synthesis of Coconut Oil-Derived Biopolyol for Rigid Poly(Urethane-Urea) Hybrid Foam Application. RSC Adv. 2023, 13, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Li, C.; Cai, X.; Yang, Z.; Zhang, F.; Liu, Y.; Yang, G.; Wang, Q.; Fang, G.; Zhang, X. A Study on Coconut Fatty Acid Diethanolamide-Based Polyurethane Foams. RSC Adv. 2022, 12, 13548–13556. [Google Scholar] [CrossRef] [PubMed]
- Tomon, T.R.B.; Estrada, R.J.R.; Fernandez, R.M.D.; Capangpangan, R.Y.; Lubguban, A.A.; Dumancas, G.G.; Alguno, A.C.; Malaluan, R.M.; Bacosa, H.P.; Lubguban, A.A. Coconut Power: A Sustainable Approach for the Removal of Cr6+ Ions Using a New Coconut-Based Polyurethane Foam/Activated Carbon Composite in a Fixed-Bed Column. RSC Adv. 2023, 13, 20941–20950. [Google Scholar] [CrossRef]
- Fernandez, R.M.D.; Estrada, R.J.R.; Tomon, T.R.B.; Dingcong, R.G.; Amparado, R.F.; Capangpangan, R.Y.; Malaluan, R.M.; Dumancas, G.G.; Lubguban, A.A.; Alguno, A.C.; et al. Experimental Design and Breakthrough Curve Modeling of Fixed-Bed Columns Utilizing a Novel 3D Coconut-Based Polyurethane-Activated Carbon Composite Adsorbent for Lead Sequestration. Sustainability 2023, 15, 14344. [Google Scholar] [CrossRef]
- Salcedo, M.L.D.; Omisol, C.J.M.; Maputi, A.O.; Estrada, D.J.E.; Aguinid, B.J.M.; Asequia, D.M.A.; Erjeno, D.J.D.; Apostol, G.; Siy, H.; Malaluan, R.M.; et al. Production of Bio-Based Polyol from Coconut Fatty Acid Distillate (CFAD) and Crude Glycerol for Rigid Polyurethane Foam Applications. Materials 2023, 16, 5453. [Google Scholar] [CrossRef] [PubMed]
- Hipulan, L.N.A.; Dingcong, R.G.; Estrada, D.J.E.; Dumancas, G.G.; Bondaug, J.C.S.; Alguno, A.C.; Bacosa, H.P.; Malaluan, R.M.; Lubguban, A.A. Development of High-Performance Coconut Oil-Based Rigid Polyurethane-Urea Foam: A Novel Sequential Amidation and Prepolymerization Process. ACS Omega 2024, 9, 13112–13124. [Google Scholar] [CrossRef]
- Omisol, C.J.M.; Aguinid, B.J.M.; Abilay, G.Y.; Asequia, D.M.; Tomon, T.R.; Sabulbero, K.X.; Erjeno, D.J.; Osorio, C.K.; Usop, S.; Malaluan, R.; et al. Flexible Polyurethane Foams Modified with Novel Coconut Monoglycerides-Based Polyester Polyols. ACS Omega 2024, 9, 4497–4512. [Google Scholar] [CrossRef]
- Tomon, T.R.B.; Omisol, C.J.M.; Aguinid, B.J.M.; Sabulbero, K.X.L.; Alguno, A.C.; Malaluan, R.M.; Lubguban, A.A. A Novel Naturally Superoleophilic Coconut Oil-Based Foam with Inherent Hydrophobic Properties for Oil and Grease Sorption. Sci. Rep. 2024, 14, 14223. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Chollet, G.; Grau, E.; Cramail, H. Vegetable Oils: A Source of Polyols for Polyurethane Materials. OCL 2016, 23, D508. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, F.; Zhou, Y.; Xu, Z.; Jiang, X.; Luo, X.; Yuan, G.; Hu, Y.; Hu, W. High-Performance Flexible Polyurethane from Renewable Castor Oil: Preparation, Properties and Mechanism. Compos. Part A Appl. Sci. Manuf. 2022, 159, 107034. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zhang, W.; Javni, I. Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis. Biomacromolecules 2005, 6, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, Z. Polyurethanes from Vegetable Oils. Polym. Revs. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Sipaut, C.S.; Murni, S.; Saalah, S.; Hoon, T.C.; Ibrahim, M.N.M.; Rahman, I.A.; Abdullah, A.A. Synthesis and Characterization of Polyols from Refined Cooking Oil for Polyurethane Foam Formation. Cell. Polym. 2012, 31, 19–38. [Google Scholar] [CrossRef]
- ASTM D4274-21; Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. ASTM International: West Conshocken, PA, USA, 2023. [CrossRef]
- Akdogan, E.; Erdem, M. A Comprehensive Research of Low-Density Bio-Based Rigid Polyurethane Foams from Sugar Beet Pulp-Based Biopolyol: From Closed-Cell towards Open-Cell Structure. Ind. Crops Prod. 2023, 200, 116809. [Google Scholar] [CrossRef]
- ASTM 3574; Test Methods for Flexible Cellular MaterialsSlab, Bonded, and Molded Urethane Foams. ASTM International: West Conshocken, PA, USA, 2017. [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym.-Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Liu, X.; Jia, X.; Liu, W.; Nie, B.; Zhang, C.; Song, D. Mechanical Strength and Porosity Changes of Bituminous Coal Induced by Supercritical CO2 Interactions: Influence of Saturation Pressure. Geoenergy Sci. Eng. 2023, 225, 211691. [Google Scholar] [CrossRef]
- ASTM F726-99; Test Method for Sorbent Performance of Adsorbents. ASTM International: West Conshocken, PA, USA, 2017. [CrossRef]
- ASTM D95-13; Test Method for Water in Petroleum Products and Bituminous Materials by Distillation. ASTM International: West Conshocken, PA, USA, 2018. [CrossRef]
- Xu, W.; Wang, W.; Hao, L.; Liu, H.; Hai, F.; Wang, X. Synthesis and Properties of Novel Triazine-Based Fluorinated Chain Extender Modified Waterborne Polyurethane Hydrophobic Films. Prog. Org. Coat. 2021, 157, 106282. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, T.; Liu, L. Effect of Multi-Armed Chain Extender on Microphase Morphology, Stress-Strain Behavior and Electromechanical Properties of Polyurethane Elastomers. Polymer 2021, 235, 124279. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, S.; Su, Z.; Wang, P. A Novel Vegetable Oil–Lactate Hybrid Monomer for Synthesis of High- Tg Polyurethanes. J. Polym. Sci. A Polym. Chem. 2010, 48, 243–250. [Google Scholar] [CrossRef]
- Palle, I.; Lodin, V.; Mohd Yunus, A.A.; Lee, S.H.; Md Tahir, P.; Hori, N.; Antov, P.; Takemura, A. Effects of NCO/OH Ratios on Bio-Based Polyurethane Film Properties Made from Acacia Mangium Liquefied Wood. Polymers 2023, 15, 1154. [Google Scholar] [CrossRef]
- Macalino, A.; Salen, V.; Reyes, L. Castor Oil Based Polyurethanes: Synthesis and Characterization. IOP Conf. Ser. Mater. Sci. Eng. 2017, 229, 012016. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-Cell Polyurethane Foams of Very Low Density Modified with Various Palm Oil-Based Bio-Polyols in Accordance with Cleaner Production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, T.; Wu, W.; Zhao, J.; Xue, X.; Ao, C.; Zhang, J.; Deng, X.; Zhang, X.; Zhang, W.; et al. Flexible, All-Solid-State Supercapacitors Derived from Waste Polyurethane Foams. Chem. Eng. J. 2022, 431, 133228. [Google Scholar] [CrossRef]
- Hebda, E.; Bukowczan, A.; Michałowski, S.; Wroński, S.; Urbaniak, P.; Kaczmarek, M.; Hutnik, E.; Romaniuk, A.; Wolun-Cholewa, M.; Pielichowski, K. Examining the Influence of Functionalized POSS on the Structure and Bioactivity of Flexible Polyurethane Foams. Mater. Sci. Eng. C 2020, 108, 110370. [Google Scholar] [CrossRef] [PubMed]
- Artavia, L.D.; Macosko, C.W. Polyurethane Flexible Foam Formation. In Low Density Cellular Plastics; Hilyard, N.C., Cunningham, A., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 22–55. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Ma, C.; Zhou, F.; Hu, Y.; Schartel, B. Flame Retardant Flexible Polyurethane Foams Based on Phosphorous Soybean-Oil Polyol and Expandable Graphite. Polym. Degrad. Stab. 2021, 191, 109656. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. Towards Sustainable Catalyst-Free Biomass-Based Polyurethane-Wood Composites (PU-WC): From Valorization and Liquefaction to Future Generation of Biocomposites. J. Clean. Prod. 2024, 468, 143046. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Chen, Y.-C. Synthesis of Bio-Based Polyurethane Foam Modified with Rosin Using an Environmentally-Friendly Process. J. Clean. Prod. 2020, 276, 124203. [Google Scholar] [CrossRef]
- Muhammad Zain, N.; Ahmad, S.H.; Ahad, N.A.; Ali, E.S. Influence of Isocyanate Structures on Mechanical Performance of Aluminum Bonded with Green Polyurethane Adhesive. AMR 2014, 879, 119–127. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, Z.; Chen, H.; Xiang, Q.; Wang, Q.; Xie, C.; Liu, Y. Superhydrophobic Polyurethane Sponge Based on Sepiolite for Efficient Oil/Water Separation. J. Hazard. Mater. 2022, 434, 128833. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Fragouli, D.; Athanassiou, A. Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams. Materials 2018, 11, 2382. [Google Scholar] [CrossRef]
- Perera, H.J.; Blum, F.D. Alkyl Chain Modified Diatomaceous Earth Superhydrophobic Coatings. In Proceedings of the 2018 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, Sharjah, Abu Dhabi, United Arab Emirates, 6 February–5 April 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Sedai, B.R.; Khatiwada, B.K.; Mortazavian, H.; Blum, F.D. Development of Superhydrophobicity in Fluorosilane-Treated Diatomaceous Earth Polymer Coatings. Appl. Surf. Sci. 2016, 386, 178–186. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Burford, R.P. Effect of Foam Density, Oil Viscosity, and Temperature on Oil Sorption Behavior of Polyurethane. J. Appl. Polym. Sci. 2006, 99, 360–367. [Google Scholar] [CrossRef]
- Piao, J.; Lu, M.; Ren, J.; Wang, Y.; Feng, T.; Wang, Y.; Jiao, C.; Chen, X.; Kuang, S. MOF-Derived LDH Modified Flame-Retardant Polyurethane Sponge for High-Performance Oil-Water Separation: Interface Engineering Design Based on Bioinspiration. J. Hazard. Mater. 2023, 444, 130398. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of Nonwoven Polypropylene Oil Sorbents in Marine Oil-Spill Recovery. Mar. Pollut. Bull. 2003, 46, 780–783. [Google Scholar] [CrossRef]
- Vlaev, L.; Petkov, P.; Dimitrov, A.; Genieva, S. Cleanup of Water Polluted with Crude Oil or Diesel Fuel Using Rice Husks Ash. J. Taiwan Inst. Chem. Eng. 2011, 42, 957–964. [Google Scholar] [CrossRef]
- Hoang, A.T.; Le, V.V.; Al-Tawaha, A.R.M.S.; Nguyen, D.N.; Al-Tawaha, A.R.M.S.; Noor, M.M.; Pham, V.V. An Absorption Capacity Investigation of New Absorbent Based on Polyurethane Foams and Rice Straw for Oil Spill Cleanup. Pet. Sci. Technol. 2018, 36, 361–370. [Google Scholar] [CrossRef]


| Polyols | Voranol ®4701 | CODP |
|---|---|---|
| OH Value | 33–36 | 122 |
| Molecular Weight (Mw) g/mol | 728 | 2519 |
| Average Functionality (fav) | 3.00 | 480 |
| Source | Fossil Fuel | Coconut Oil |
| Foam Formulation | Components | Control | CCF-20 | CCF-40 | CCF-50 | CCF-60 |
|---|---|---|---|---|---|---|
| Polyol | Voranol ®4701 | 100 | 80 | 60 | 50 | 40 |
| CODP | 0 | 20 | 40 | 50 | 60 |
| Foam Samples | Tmax, °C | Mass Remaining, % | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | m1 | m2 | m3 | |
| Control | 376.65 | 419.58 | 661.87 | 73.16 | 20.48 | 0.42 |
| CCF-20 | 374.53 | 421.80 | 659.66 | 72.47 | 25.24 | 5.17 |
| CCF-40 | 374.12 | 425.54 | 664.61 | 72.04 | 29.79 | 10.73 |
| CCF-50 | 363.9 | 422.46 | 664.41 | 72.03 | 31.32 | 11.73 |
| CCF-60 | 361.37 | 427.1 | 663.24 | 72.79 | 35.59 | 13.71 |
| Foam Formulation | Cross-Sectional Image of Single Water Droplet | Average Contact Angle (θ) |
|---|---|---|
| Control |  | 104.54 ± 2.58 |
| CCF-20 |  | 107.89 ± 2.47 |
| CCF-40 |  | 112.47 ± 3.76 |
| CCF-50 |  | 115.67 ± 2.07 |
| CCF-60 |  | 118.69 ± 2.14 |
| Sorbent Material | Material Type | Preparation Method | Pollutants | Oil Sorption Capacity (g/g) | References |
|---|---|---|---|---|---|
| Non-Woven Polypropylene Blankets | Polypropylene | Melt-spinning | Crude Oil | 8–12 | [73] |
| Rice Husks | Biomass-based sorbent | Pyrolysis | Crude Oil, Diesel Oil | 2.98–6.22 | [74] |
| Organo-Clay | Modified clay mineral sorbent | Surface modification | Diesel Oil, Engine Oil | 2.1–7.2 | [22] |
| PU Foam with 25% Rice Straw (3 mm in Size) | Bio-based PU | Incorporation of filler into the polyurethane matrix | Diesel Oil | 4.1–6.1 | [75] |
| Synthesized CCFs with 50% CODP | Bio-based PU | One-shot foaming method | Bunker Oil, Engine Oil | 14.0–16.0 | This Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tefora, J.L.L.; Tomon, T.R.B.; Ungang, J.I.D.S.; Malaluan, R.M.; Lubguban, A.A.; Bacosa, H.P. The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications. J. Mar. Sci. Eng. 2025, 13, 1176. https://doi.org/10.3390/jmse13061176
Tefora JLL, Tomon TRB, Ungang JIDS, Malaluan RM, Lubguban AA, Bacosa HP. The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications. Journal of Marine Science and Engineering. 2025; 13(6):1176. https://doi.org/10.3390/jmse13061176
Chicago/Turabian StyleTefora, John Louie L., Tomas Ralph B. Tomon, Joy Ian Dan S. Ungang, Roberto M. Malaluan, Arnold A. Lubguban, and Hernando P. Bacosa. 2025. "The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications" Journal of Marine Science and Engineering 13, no. 6: 1176. https://doi.org/10.3390/jmse13061176
APA StyleTefora, J. L. L., Tomon, T. R. B., Ungang, J. I. D. S., Malaluan, R. M., Lubguban, A. A., & Bacosa, H. P. (2025). The Development of a Coconut-Oil-Based Derived Polyol in a Polyurethane Matrix: A Potential Sorbent Material for Marine Oil Spill Applications. Journal of Marine Science and Engineering, 13(6), 1176. https://doi.org/10.3390/jmse13061176









