Quantitative Assessment of Oysters’ Multiple Nitrogen Removal Pathways in a Subtropical Bay
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Oyster Rearing and Lab-to-Bay Extrapolation
2.2. Shenzhen Bay Field Sampling and Analyses
2.3. Flux Inventory Model
2.4. Organic and Total N Removal Calculation in SZB
3. Results
3.1. Laboratory- and Bay-Scale Oyster-Induced Nitrogen Removal
3.2. Quantitative Nitrogen Removal Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Ayvazian, S.; Mulvaney, K.; Zarnoch, C.; Palta, M.; Reichert-Nguyen, J.; McNally, S.; Pilaro, M.; Jones, A.; Terry, C.; Fulweiler, R.W. Beyond bioextraction: The role of oyster-mediated denitrification in nutrient management. Environ. Sci. Technol. 2021, 55, 14457–14465. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N. Chapter 1—The Marine Nitrogen Cycle: Overview and Challenges. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1–50. [Google Scholar]
- Wan, X.S.; Sheng, H.X.; Liu, L.; Shen, H.; Tang, W.; Zou, W.; Xu, M.N.; Zheng, Z.; Tan, E.; Chen, M.; et al. Particle-associated denitrification is the primary source of N2O in oxic coastal waters. Nat. Commun. 2023, 14, 8280. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Krause-Jensen, D. Intervention options to accelerate ecosystem recovery from coastal eutrophication. Front. Mar. Sci. 2018, 5, 470. [Google Scholar] [CrossRef]
- Boesch, D.F. Barriers and bridges in abating coastal eutrophication. Front. Mar. Sci. 2019, 6, 123. [Google Scholar] [CrossRef]
- Botta, R.; Asche, F.; Borsum, J.S.; Camp, E.V. A review of global oyster aquaculture production and consumption. Mar. Policy 2020, 117, 103952. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Ray, N.E.; Hancock, B.; Brush, M.J.; Colden, A.; Cornwell, J.; Labrie, M.S.; Maguire, T.J.; Maxwell, T.; Rogers, D.; Stevick, R.J.; et al. A review of how we assess denitrification in oyster habitats and proposed guidelines for future studies. Limnol. Oceanogr. Methods 2021, 19, 714–731. [Google Scholar] [CrossRef]
- Cubillo, A.M.; Lopes, A.S.; Ferreira, J.G.; Moore, H.; Service, M.; Bricker, S.B. Quantification and valuation of the potential of shellfish ecosystem services in mitigating coastal eutrophication. Estuar. Coast. Shelf Sci. 2023, 293, 108469. [Google Scholar] [CrossRef]
- Bricker, S.B.; Grizzle, R.E.; Trowbridge, P.; Rose, J.M.; Ferreira, J.G.; Wellman, K.; Zhu, C.; Galimany, E.; Wikfors, G.H.; Saurel, C.; et al. Bioextractive removal of nitrogen by oysters in Great Bay Piscataqua River estuary, New Hampshire, USA. Estuaries Coasts 2020, 43, 23–38. [Google Scholar] [CrossRef]
- Lai, Q.T.; Irwin, E.R.; Zhang, Y. Estimating nitrogen removal services of eastern oyster (Crassostrea virginica) in Mobile bay, Alabama. Ecol. Indic. 2020, 117, 106541. [Google Scholar] [CrossRef]
- Clements, J.C.; Comeau, L.A. Nitrogen removal potential of shellfish aquaculture harvests in eastern Canada: A comparison of culture methods. Aquacult. Rep. 2019, 13, 100183. [Google Scholar] [CrossRef]
- Ray, N.E.; Henning, M.C.; Fulweiler, R.W. Nitrogen and phosphorus cycling in the digestive system and shell biofilm of the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 2019, 621, 95–105. [Google Scholar] [CrossRef]
- Rose, J.M.; Gosnell, J.S.; Bricker, S.; Brush, M.J.; Colden, A.; Harris, L.; Karplus, E.; Laferriere, A.; Merrill, N.H.; Murphy, T.B.; et al. Opportunities and challenges for including oyster-mediated denitrification in nitrogen management plans. Estuaries Coasts 2021, 44, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Arfken, A.; Song, B.; Bowman, J.S.; Piehler, M. Denitrification potential of the eastern oyster microbiome using a 16S rRNA gene based metabolic inference approach. PLoS ONE 2017, 12, e0185071. [Google Scholar] [CrossRef]
- Voet, H.; Soetaert, K.; Moens, T.; Bodé, S.; Boeckx, P.; Van Colen, C.; Vanaverbeke, J. N2O production by mussels: Quantifying rates and pathways in current and future climate settings. Front. Mar. Sci. 2023, 10, 1101469. [Google Scholar] [CrossRef]
- Newell, R.I.; Jordan, S.J. Preferential ingestion of organic material by the American oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 1983, 13, 47–53. [Google Scholar] [CrossRef]
- Ray, N.E.; Fulweiler, R.W. Meta-analysis of oyster impacts on coastal biogeochemistry. Nat. Sustain. 2021, 4, 261–269. [Google Scholar] [CrossRef]
- Labrie, M.S.; Sundermeyer, M.A.; Howes, B.L. Quantifying the effects of floating oyster aquaculture on nitrogen cycling in a temperate coastal embayment. Estuaries Coasts 2023, 46, 494–511. [Google Scholar] [CrossRef]
- Bronk, D.A.; Steinberg, D.K. Chapter 8—Nitrogen Regeneration. In Nitrogen in the Marine Environment, 2nd ed.; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 385–467. [Google Scholar]
- Hamersley, M.R.; Howes, B.L. Coupled nitrification-denitrification measured in situ in a Spartina alterniflora marsh with a 15NH4+ tracer. Mar. Ecol. Prog. Ser. 2005, 299, 123–135. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Zarnoch, C.B. Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecol. Appl. 2014, 24, 271–286. [Google Scholar] [CrossRef]
- Humphries, A.T.; Ayvazian, S.G.; Carey, J.C.; Hancock, B.T.; Grabbert, S.; Cobb, D.; Strobel, C.J.; Fulweiler, R.W. Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove nitrogen at comparable high rates. Front. Mar. Sci. 2016, 3, 74. [Google Scholar] [CrossRef]
- Filippini, G.; Dafforn, K.A.; Bugnot, A.B. Shellfish as a bioremediation tool: A review and meta-analysis. Environ. Pollut. 2023, 316, 120614. [Google Scholar] [CrossRef] [PubMed]
- Mara, P.; Edgcomb, V.P.; Sehein, T.R.; Beaudoin, D.; Martinsen, C.; Lovely, C.; Belcher, B.; Cox, R.; Curran, M.; Farnan, C.; et al. Comparison of oyster aquaculture methods and their potential to enhance microbial nitrogen removal from coastal ecosystems. Front. Mar. Sci. 2021, 8, 174. [Google Scholar] [CrossRef]
- Filippini, G.; Bugnot, A.B.; Ferguson, A.; Gribben, P.E.; Palmer, J.; Erickson, K.; Dafforn, K.A. The influence of oyster reefs and surrounding sediments on nitrogen removal—An in-situ study along the East coast of Australia. Environ. Res. 2023, 237, 116947. [Google Scholar] [CrossRef]
- Yu, S.; Mu, Y. Evaluation of green development in mariculture: The case of Chinese oyster aquaculture. Aquaculture 2023, 576, 739838. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Cheung, S.C.H. Floating mountain in Pearl River: A study of oyster cultivation and food heritage in Hong Kong. Asian Educ. Dev. Stud. 2019, 8, 433–442. [Google Scholar] [CrossRef]
- Wang, R.; Wu, J.; Yiu, K.-F.; Shen, P.; Lam, P.K.S. Long-term variation in phytoplankton assemblages during urbanization: A comparative case study of Deep Bay and Mirs Bay, Hong Kong, China. Sci. Total Environ. 2020, 745, 140993. [Google Scholar] [CrossRef]
- Wan, Y. Determination of Hydrodynamic Time of Deep Bay and Evaluation of Effect on Leading Nutrient Reduction. Ph.D. Thesis, Tsinghua University, Beijing, China, 2009. [Google Scholar]
- Environmental Protection Department, Hong Kong SAR. Marine Water Quality Data. Available online: https://cd.epic.epd.gov.hk/EPICRIVER/vicmarineannual/result/ (accessed on 29 April 2024).
- Huo, Z.; Wang, Z.; Liang, J.; Zhang, Y.; Shen, J.; Yao, T.; Su, J.; Yu, R. Effects of salinity on embryonic development, survival, and growth of Crassostrea hongkongensis. J. Ocean Univ. China 2014, 13, 666–670. [Google Scholar] [CrossRef]
- Lee, T.H.; Dang, X.; Mumby, H.S.; Xiao, S.; Chung, S.C.; Thiyagarajan, V. Winter mortality syndrome in Hong Kong oyster (Crassostrea hongkongensis): Causes and impacts. Aquaculture 2024, 591, 741139. [Google Scholar] [CrossRef]
- Wilson, S.T.; Bange, H.W.; Arévalo-Martínez, D.L.; Barnes, J.; Borges, A.V.; Brown, I.; Bullister, J.L.; Burgos, M.; Capelle, D.W.; Casso, M.; et al. An intercomparison of oceanic methane and nitrous oxide measurements. Biogeosciences 2018, 15, 5891–5907. [Google Scholar] [CrossRef]
- Jain, R.K.; Cui, Z.C.; Domen, J.K. Chapter 4—Environmental Impacts of Mining. In Environmental Impact of Mining and Mineral Processing; Jain, R.K., Cui, Z.C., Domen, J.K., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 53–157. [Google Scholar]
- Mareddy, A.R. Chapter 5—Impacts on air environment. In Environmental Impact Assessment; Mareddy, A.R., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 171–216. [Google Scholar]
- Li, H.; Zhang, G.; Zhu, Y.; Kaufmann, H.; Xu, G. Inversion and driving force analysis of nutrient concentrations in the ecosystem of the Shenzhen-Hong Kong Bay area. Remote Sens. 2022, 14, 3694. [Google Scholar] [CrossRef]
- Grizzle, R.E.; Ward, K.M.; Peter, C.R.; Cantwell, M.; Katz, D.; Sullivan, J. Growth, morphometrics and nutrient content of farmed eastern oysters, Crassostrea virginica (Gmelin), in New Hampshire, USA. Aquacult. Res. 2017, 48, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, I.; Wong, C.; Thiyagarajan, V. Comparative analysis of nutritional quality of edible oysters cultivated in Hong Kong. J. Food Compos. Anal. 2023, 118, 105159. [Google Scholar] [CrossRef]
- Buzin, F.; Dupuy, B.; Lefebvre, S.; Barillé, L.; Haure, J. Storage of Pacific oysters Crassostrea gigas in recirculating tank: Ammonia excretion and potential nitrification rates. Aquacult. Eng. 2015, 64, 8–14. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q. Effects of temperature, salinity and body size on the physiological responses of the Iwagaki oyster Crassostrea nippona. Aquacult. Res. 2020, 51, 728–737. [Google Scholar] [CrossRef]
- Kellogg, M.L.; Cornwell, J.C.; Owens, M.S.; Paynter, K.T. Denitrification and nutrient assimilation on a restored oyster reef. Mar. Ecol. Prog. Ser. 2013, 480, 1–19. [Google Scholar] [CrossRef]
- Smyth, A.R.; Geraldi, N.R.; Thompson, S.P.; Piehler, M.F. Biological activity exceeds biogenic structure in influencing sediment nitrogen cycling in experimental oyster reefs. Mar. Ecol. Prog. Ser. 2016, 560, 173–183. [Google Scholar] [CrossRef]
- Loren, D.C.; Robert, D.B.; David, B.; Ray, G.; Mark, W.L.; Martin, H.P.; Sean, P.P.; Tolley, S.G. Ecosystem services related to oyster restoration. Mar. Ecol. Prog. Ser. 2007, 341, 303–307. [Google Scholar] [CrossRef]
- Bayer, S.R.; Cubillo, A.M.; Rose, J.M.; Ferreira, J.G.; Dixon, M.; Alvarado, A.; Barr, J.; Bernatchez, G.; Meseck, S.; Poach, M.; et al. Correction: Refining the farm aquaculture resource management model for shellfish nitrogen removal at the local scale. Estuaries Coasts 2024, 47, 1331. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Hollibaugh, J.T.; Mortazavi, B. Living oysters and their shells as sites of nitrification and denitrification. Mar. Pollut. Bull. 2016, 112, 86–90. [Google Scholar] [CrossRef]
- Dvarskas, A.; Bricker, S.B.; Wikfors, G.H.; Bohorquez, J.J.; Dixon, M.S.; Rose, J.M. Quantification and valuation of nitrogen removal services provided by commercial shellfish aquaculture at the subwatershed scale. Environ. Sci. Technol. 2020, 54, 16156–16165. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Andersson, H.; Corner, R.; Desmit, X.; Fang, Q.; De Goede, E.; Groom, S.; Gu, H.; Gustafsson, B.; Hawkins, A. Sustainable Options for People Catchment and Aquatic Resources: The SPEAR Project an International Collaboration on Integrated Coastal Zone Management. In Proceedings of the Coimbra: IMAR—Institute of Marine Research/European Commission, Coimbra, Portugal, 31 March 2008. [Google Scholar]
- Ferreira, J.G.; Sequeira, A.; Hawkins, A.J.S.; Newton, A.; Nickell, T.D.; Pastres, R.; Forte, J.; Bodoy, A.; Bricker, S.B. Analysis of coastal and offshore aquaculture: Application of the FARM model to multiple systems and shellfish species. Aquaculture 2009, 289, 32–41. [Google Scholar] [CrossRef]
- Grizzle, R.E.; Burdick, D.M.; Greene, J.; Abeels, H.; Capone, M. Reef Structure Alternatives for Restoration of Oyster (Crassostrea virginica) Populations in New Hampshire; University of New Hampshire; Piscataqua Region Estuaries Partnership: Durham, NH, USA, 2006; p. 272. [Google Scholar]
- Zhao, C.-C.; Zhang, S.; Mao, X.-Z. Variations of annual load of TN and TP in the Deep Bay Watershed, Shenzhen. Huan Jing Ke Xue (Chin. Engl. Abstr.) 2014, 35, 4111–4117. [Google Scholar] [CrossRef]
- Bayne, B.L. Chapter 4—Ecology II: Distribution at Local Scales. In Developments in Aquaculture and Fisheries Science; Bayne, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 41, pp. 139–208. [Google Scholar]
- Yu, L.; Gan, J. Mitigation of eutrophication and hypoxia through oyster aquaculture: An ecosystem model evaluation off the Pearl River Estuary. Environ. Sci. Technol. 2021, 55, 5506–5514. [Google Scholar] [CrossRef]
- Welsh, D.T.; Nizzoli, D.; Fano, E.A.; Viaroli, P. Direct contribution of clams (Ruditapes philippinarum) to benthic fluxes, nitrification, denitrification and nitrous oxide emission in a farmed sediment. Estuar. Coast. Shelf Sci. 2015, 154, 84–93. [Google Scholar] [CrossRef]
- Galimany, E.; Wikfors, G.H.; Dixon, M.S.; Newell, C.R.; Meseck, S.L.; Henning, D.; Li, Y.; Rose, J.M. Cultivation of the ribbed mussel (Geukensia demissa) for nutrient bioextraction in an urban estuary. Environ. Sci. Technol. 2017, 51, 13311–13318. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Ji, X.; Zhu, X.; Zheng, J.; Guo, S. Impact of typhoons on the ecological environment of the Pearl River Estuary in the summer of 2021—A study of an algal bloom event. Front. Mar. Sci. 2024, 11, 1395804. [Google Scholar] [CrossRef]
- Lu, Z.; Gan, J. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep. Sea Res. Part II 2015, 117, 86–96. [Google Scholar] [CrossRef]


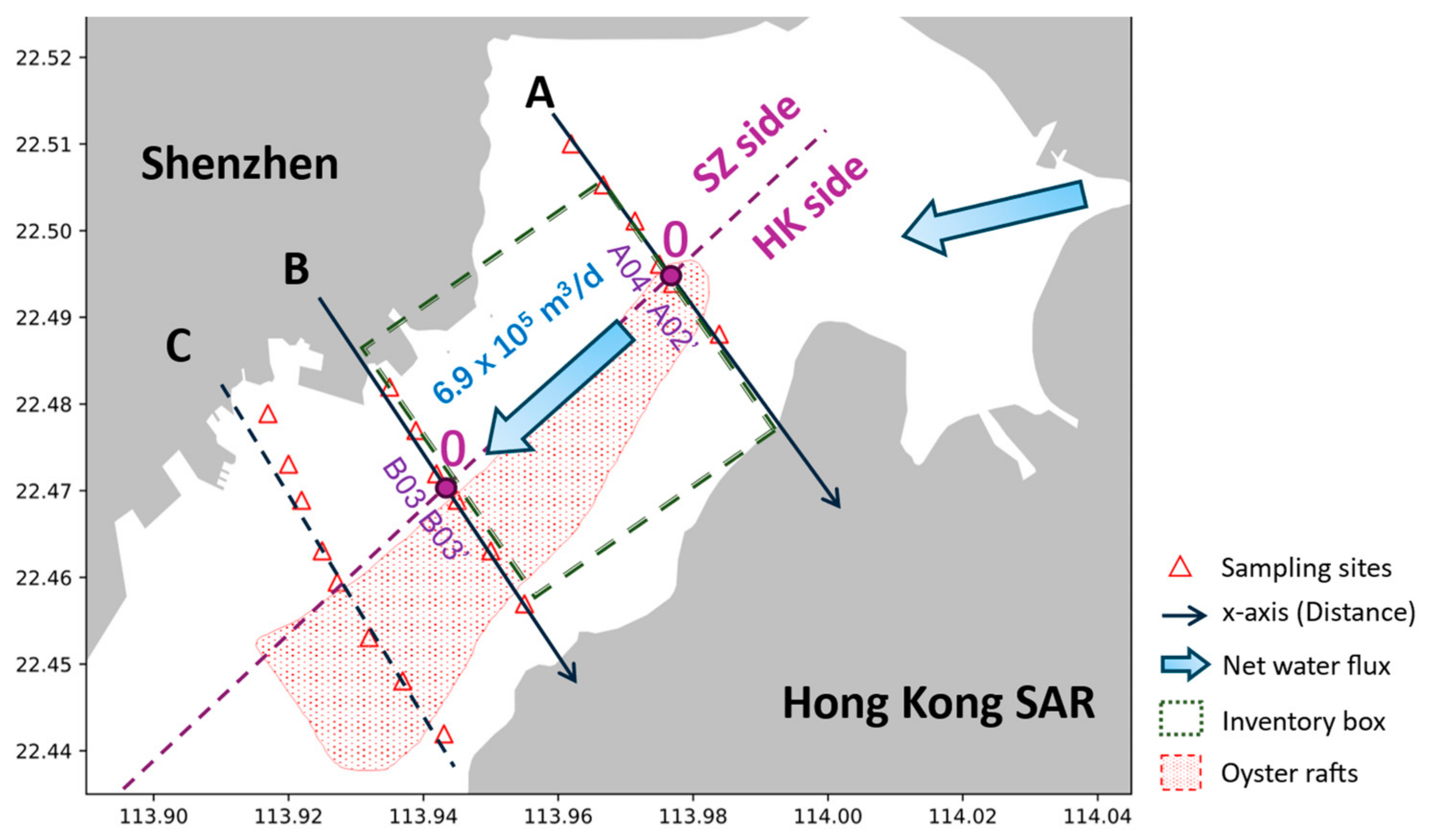
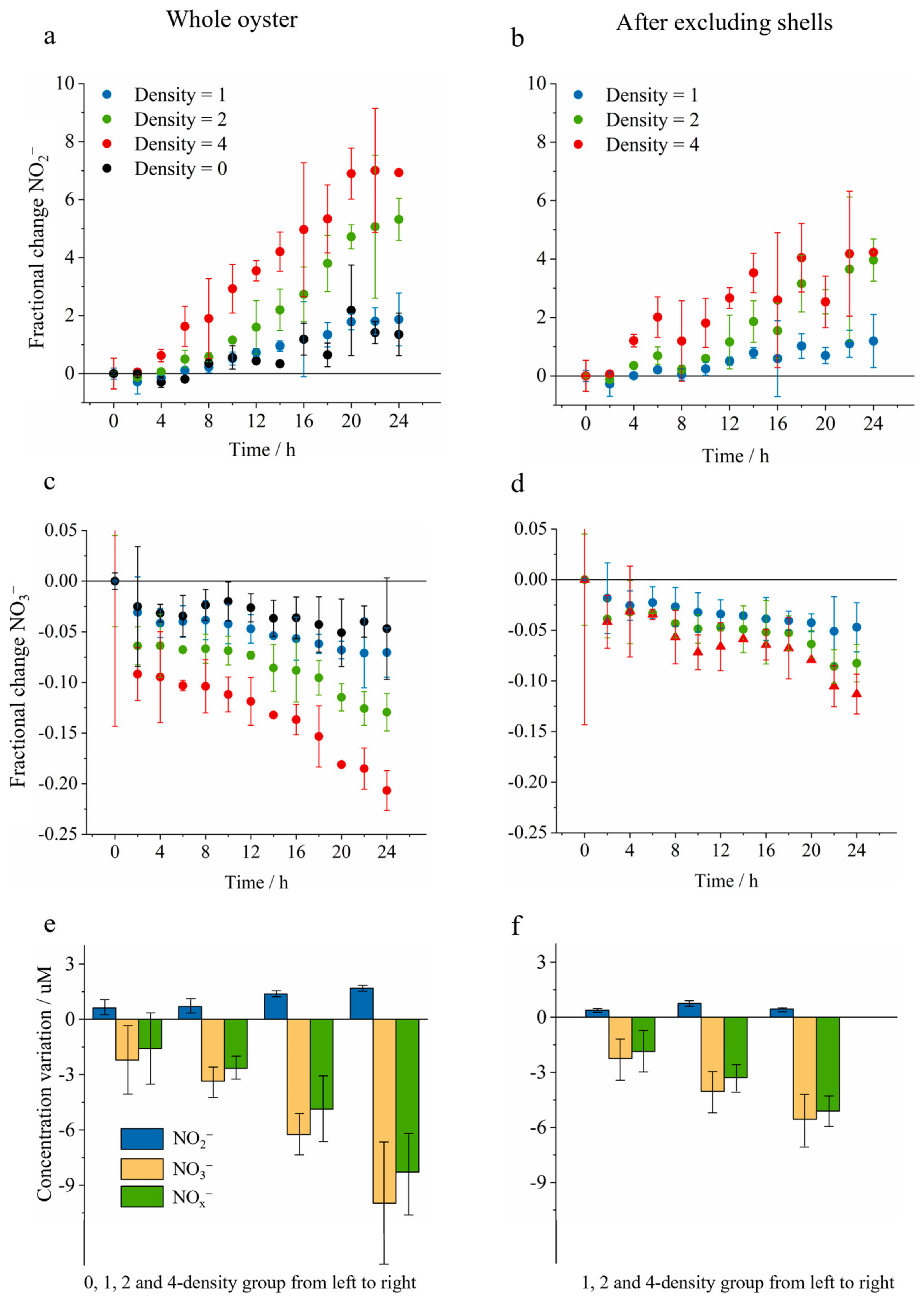
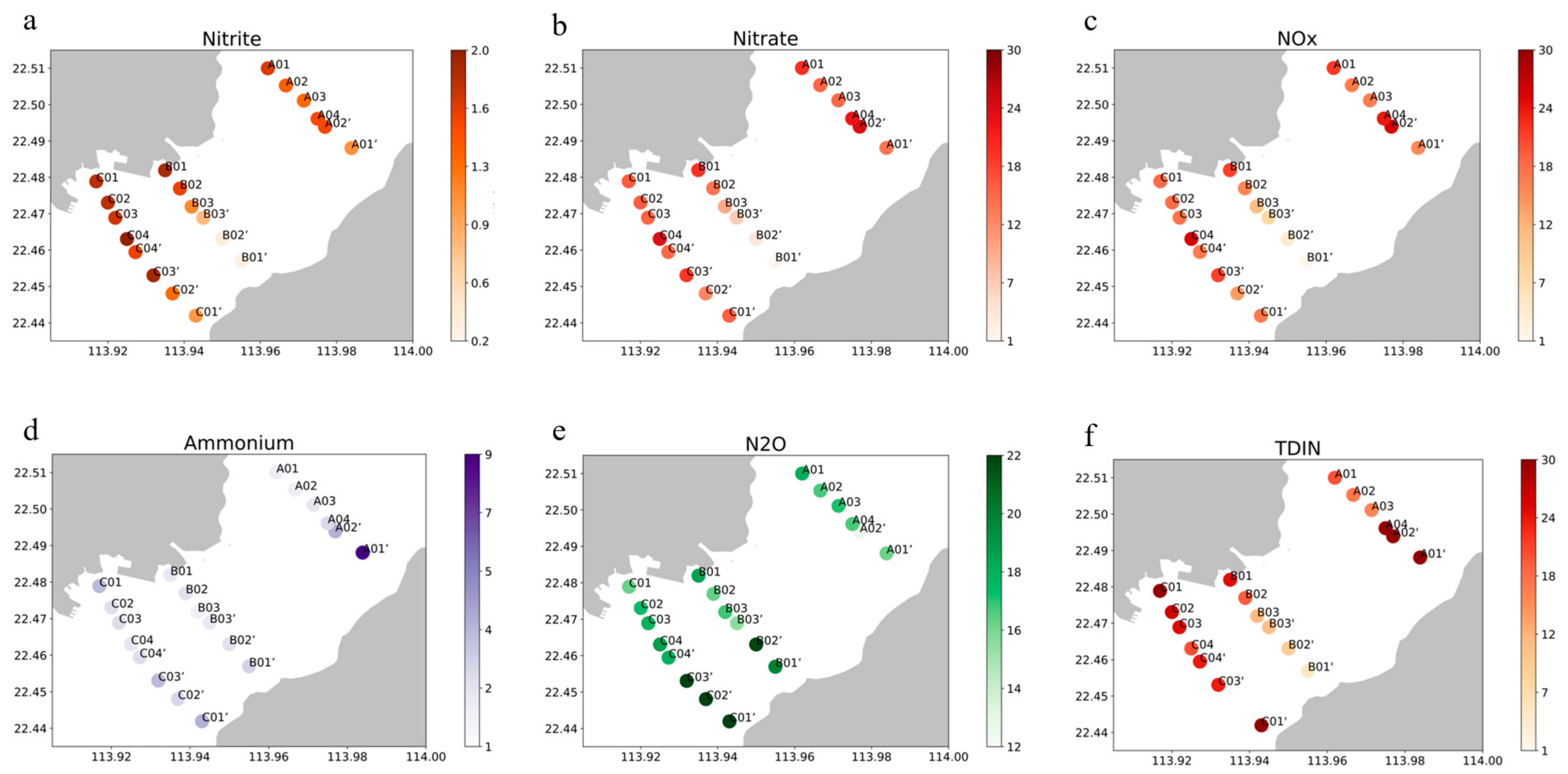
| Transect | Side | Site | Distance (km) | Concentration (μM) | |
|---|---|---|---|---|---|
| NO2− | NO3− | ||||
| A | HK | A01’ | 1.122 | 1.076 ± 0.017 | 14.960 ± 3.962 |
| A02’ | 0.159 | 1.543 ± 0.116 | 24.429 ± 0.964 | ||
| SZ | A01 | −2.294 | 1.672 ± 0.364 | 20.363 ± 3.970 | |
| A02 | −1.490 | 1.397 ± 0.094 | 16.040 ± 3.157 | ||
| A03 | −0.827 | 1.321 ± 0.177 | 15.904 ± 3.626 | ||
| A04 | −0.159 | 1.529 ± 0.148 | 22.545 ± 3.287 | ||
| B | HK | B01’ | 1.910 | 0.273 ± 0.171 | 1.371 ± 0.124 |
| B02’ | 1.058 | 0.340 ± 0.242 | 4.071 ± 2.145 | ||
| B03’ | 0.231 | 0.791 ± 0.011 | 7.288 ± 0.903 | ||
| SZ | B01 | −1.549 | 1.865 ± 0.169 | 19.873 ± 0.543 | |
| B02 | −0.865 | 1.600 ± 0.214 | 14.905 ± 1.006 | ||
| B03 | −0.231 | 1.121 ± 0.145 | 10.528 ± 1.577 | ||
| Transect | Species | Best Regression Equation | R2 | Integral (mol m−2) | |
|---|---|---|---|---|---|
| x < 0 (SZ) | x > 0 (HK) | ||||
| A | NO2− | y = 1.524 + 0.042x − 0.245x2 − 0.128x3 | 0.957 | 2.422 | 1.988 |
| NO3− | y = 18.763 + 4.536sin[π(x+0.610)/1.403] | 0.926 | 30.24 | 31.69 | |
| B | NO2− | y = 1.072 + 0.833sin[π(x−3.299)/3.443] | 1.000 | 3.708 | 1.903 |
| NO3− | y = e2.188−0.693x−0.114x^2 | 0.999 | 25.60 | 8.294 | |
| Study Area | Oyster Species | Pathway | Removal | Reference |
|---|---|---|---|---|
| New Hampshire, US | Crassostrea virginica | DN | 5.0 | Grizzle et al. [53] |
| Pensacola Bay, US | Total | 15.1 | Caffrey et al. [49] | |
| DN | 5.6 | |||
| Mobile Bay, US | Total | 9.2 | Lai et al. [12] | |
| BH | 0.5 | |||
| DN | 5.8 | |||
| Piscataqua River, US | Total | 48.8 | Bricker et al. [11] | |
| Greenwich Bay, US | BH | 25.8 | Dvarskas et al. [50] | |
| 43.6 | Bayer et al. [48] | |||
| Sanggou Bay, China | Crassostrea gigas | Total | 34.5 | Ferreira et al. [51] |
| Loch Creran, UK | 63.6 | Ferreira et al. [52] | ||
| Northern Ireland, UK | Ostrea edulis | BH | 42.2 | Cubillo et al. [10] |
| Shenzhen Bay, China | C. hongkongensis | Total | 33.3 | this study |
| BH | 8.6 | |||
| DN | 24.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Ji, Q.; Chen, Z.; Zhang, H. Quantitative Assessment of Oysters’ Multiple Nitrogen Removal Pathways in a Subtropical Bay. J. Mar. Sci. Eng. 2025, 13, 21. https://doi.org/10.3390/jmse13010021
Liu R, Ji Q, Chen Z, Zhang H. Quantitative Assessment of Oysters’ Multiple Nitrogen Removal Pathways in a Subtropical Bay. Journal of Marine Science and Engineering. 2025; 13(1):21. https://doi.org/10.3390/jmse13010021
Chicago/Turabian StyleLiu, Rongxin, Qixing Ji, Zhengping Chen, and Heng Zhang. 2025. "Quantitative Assessment of Oysters’ Multiple Nitrogen Removal Pathways in a Subtropical Bay" Journal of Marine Science and Engineering 13, no. 1: 21. https://doi.org/10.3390/jmse13010021
APA StyleLiu, R., Ji, Q., Chen, Z., & Zhang, H. (2025). Quantitative Assessment of Oysters’ Multiple Nitrogen Removal Pathways in a Subtropical Bay. Journal of Marine Science and Engineering, 13(1), 21. https://doi.org/10.3390/jmse13010021





