Spatial Distribution and Abundance of a Pelagic Squid during the Evolution of Eddies in the Southeast Pacific Ocean
Abstract
1. Introduction
2. Materials and Methods
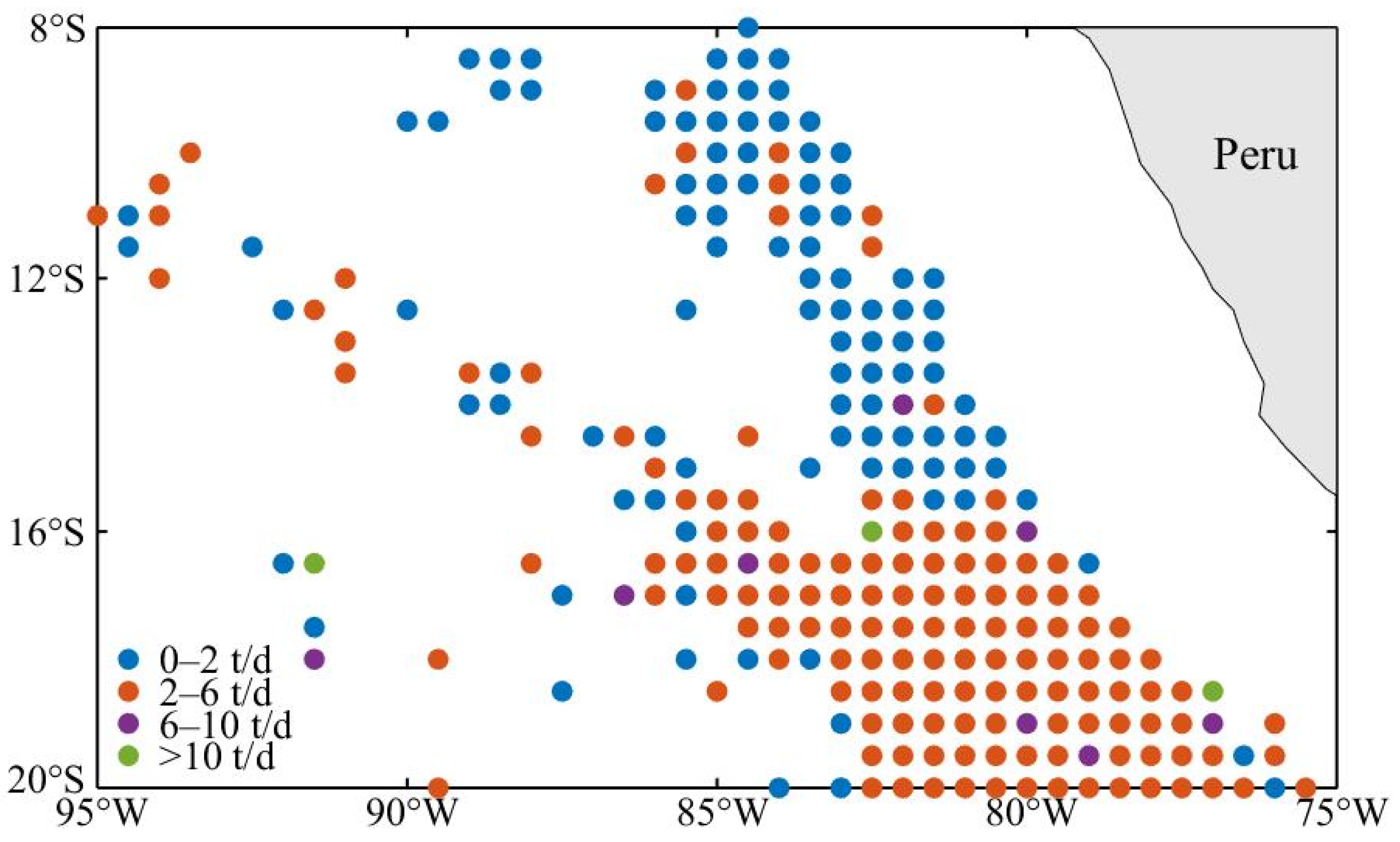
2.1. Fisheries Data
2.2. Environmental Data
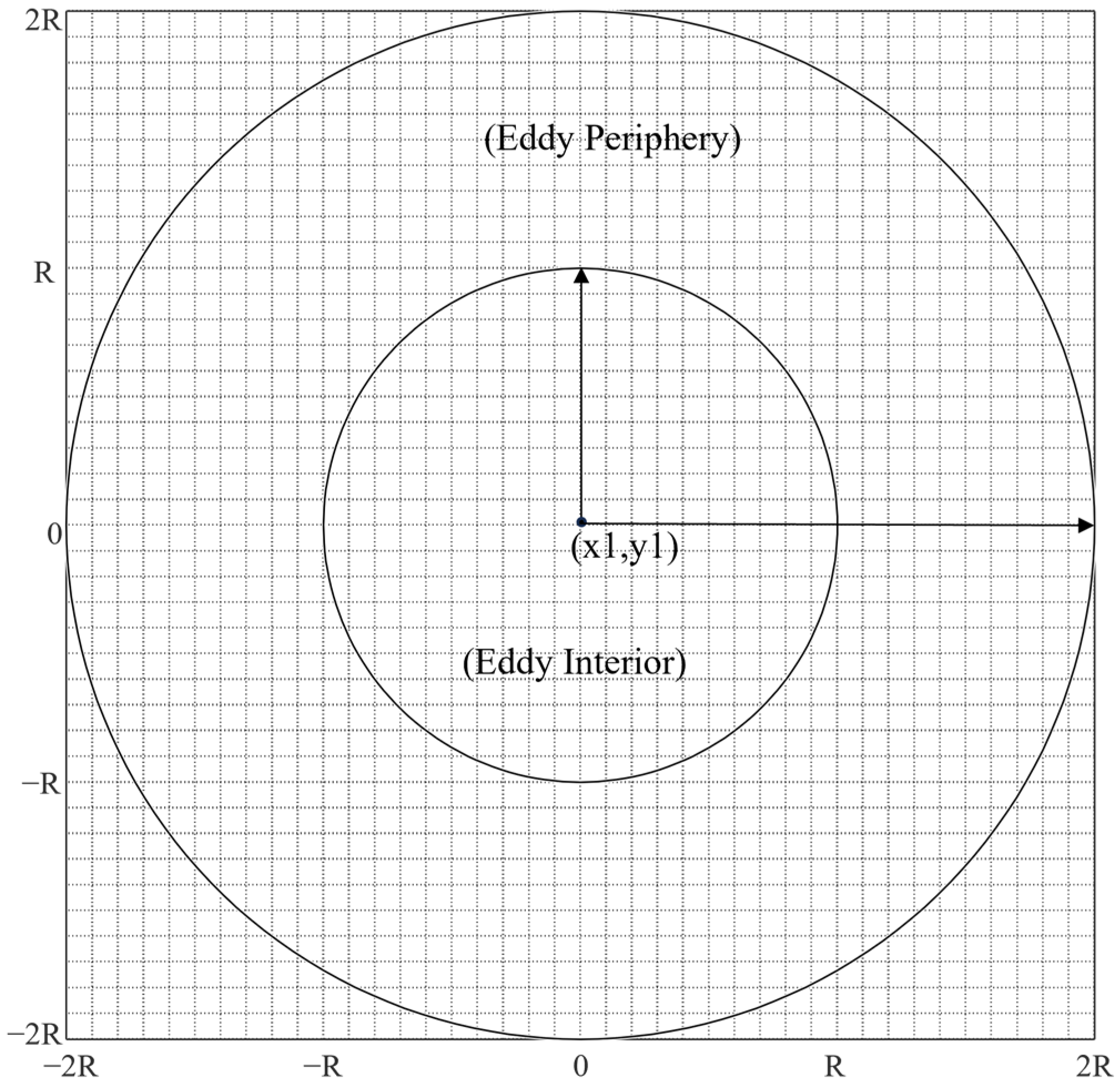
2.3. Mesoscale Eddy Analysis
2.4. The Relationship between the Abundance and Distribution of D. gigas and Different Stages of Eddies
2.5. Environmental Shifts and D. gigas Habitat Changes during Different Stages of Eddies
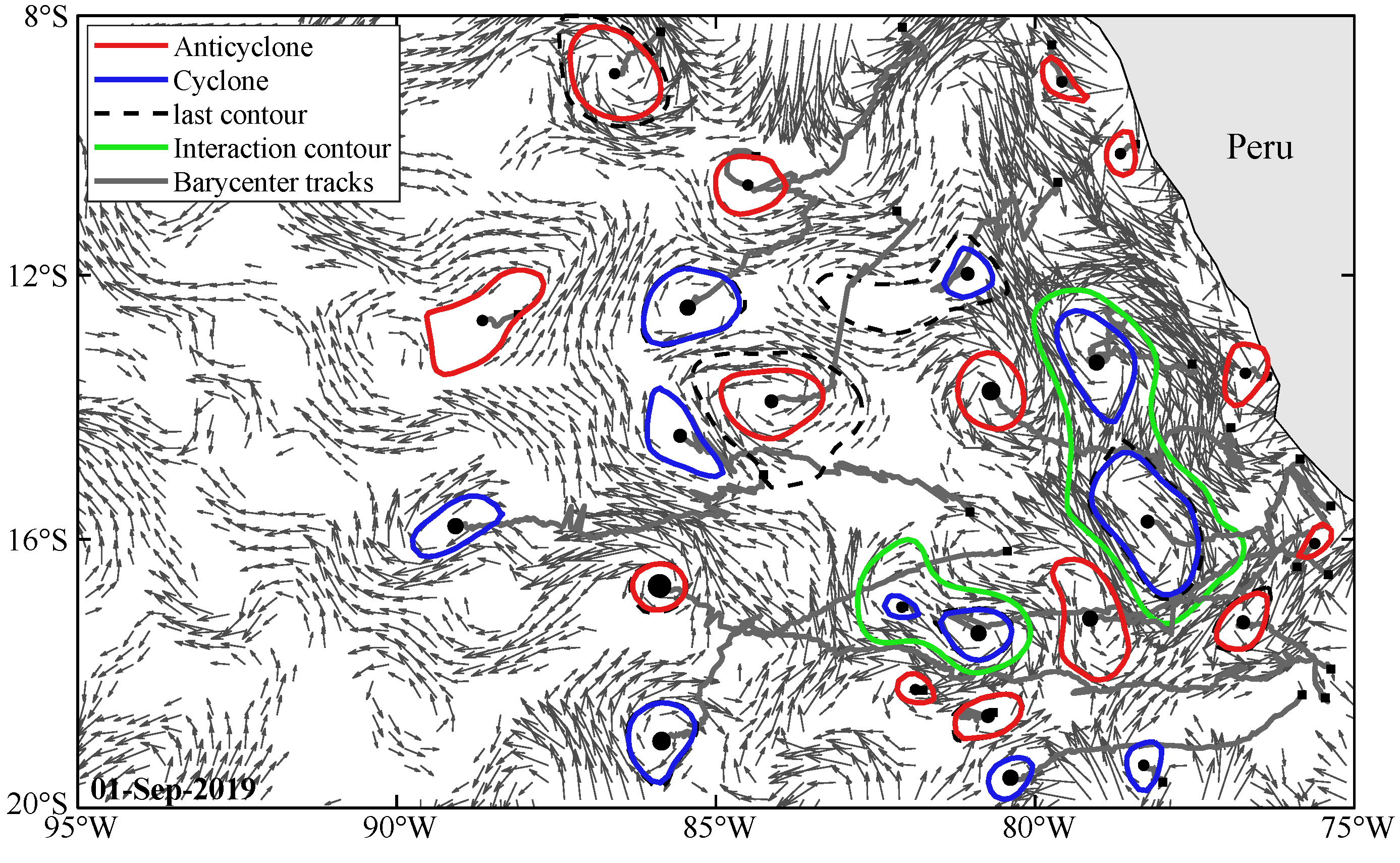
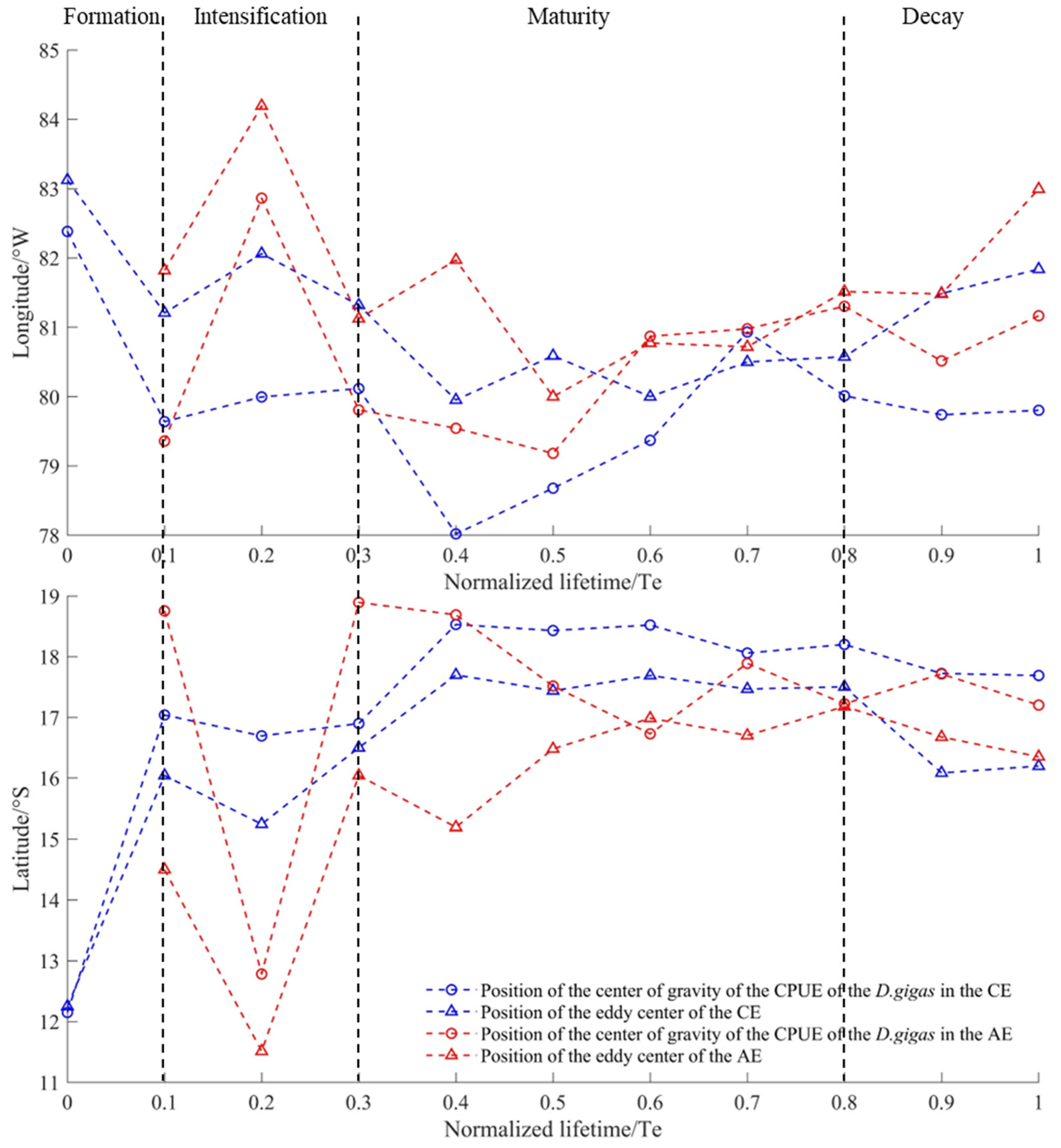
2.6. Evolutionary Analysis of Eddy 284
3. Results
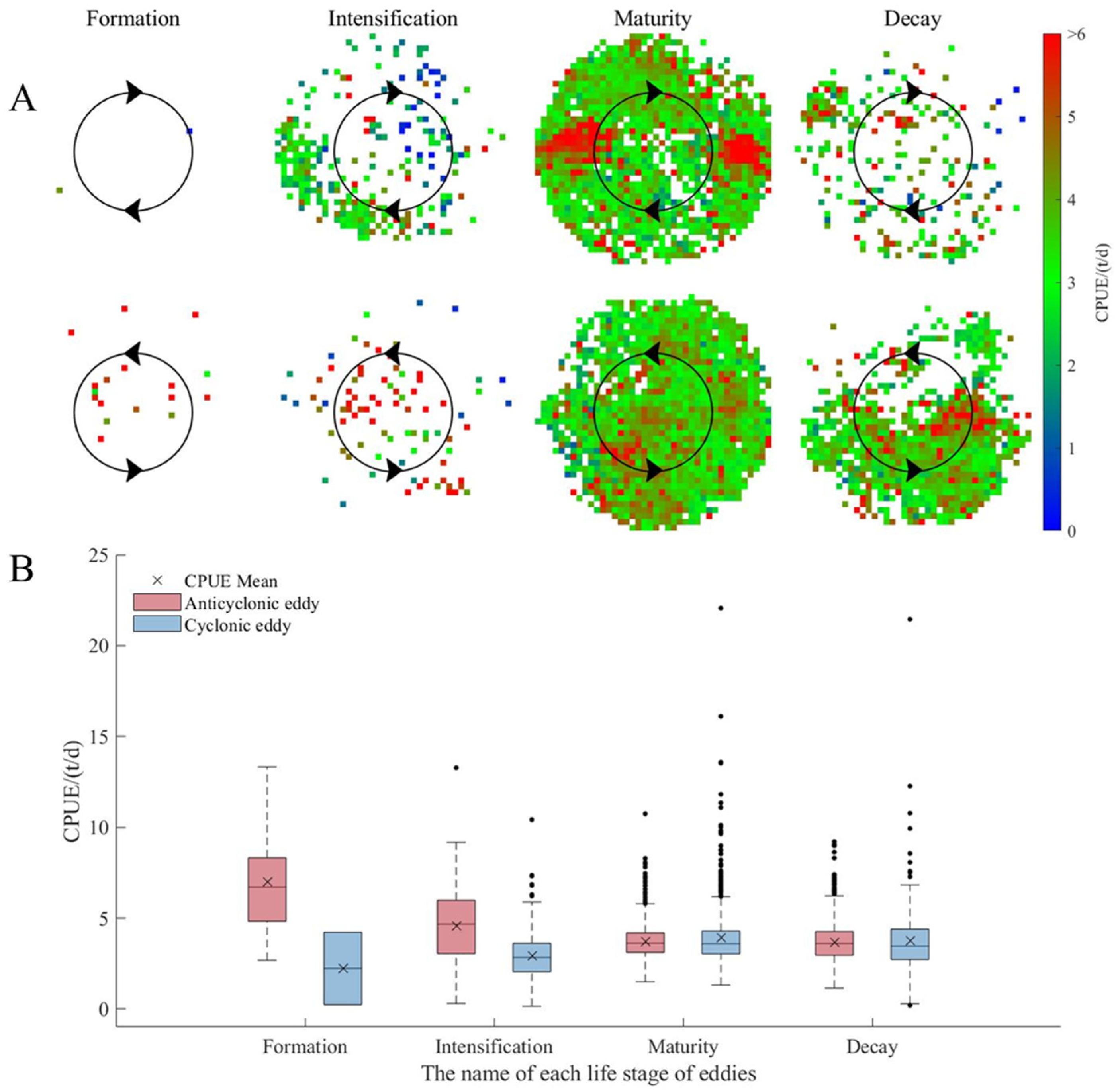
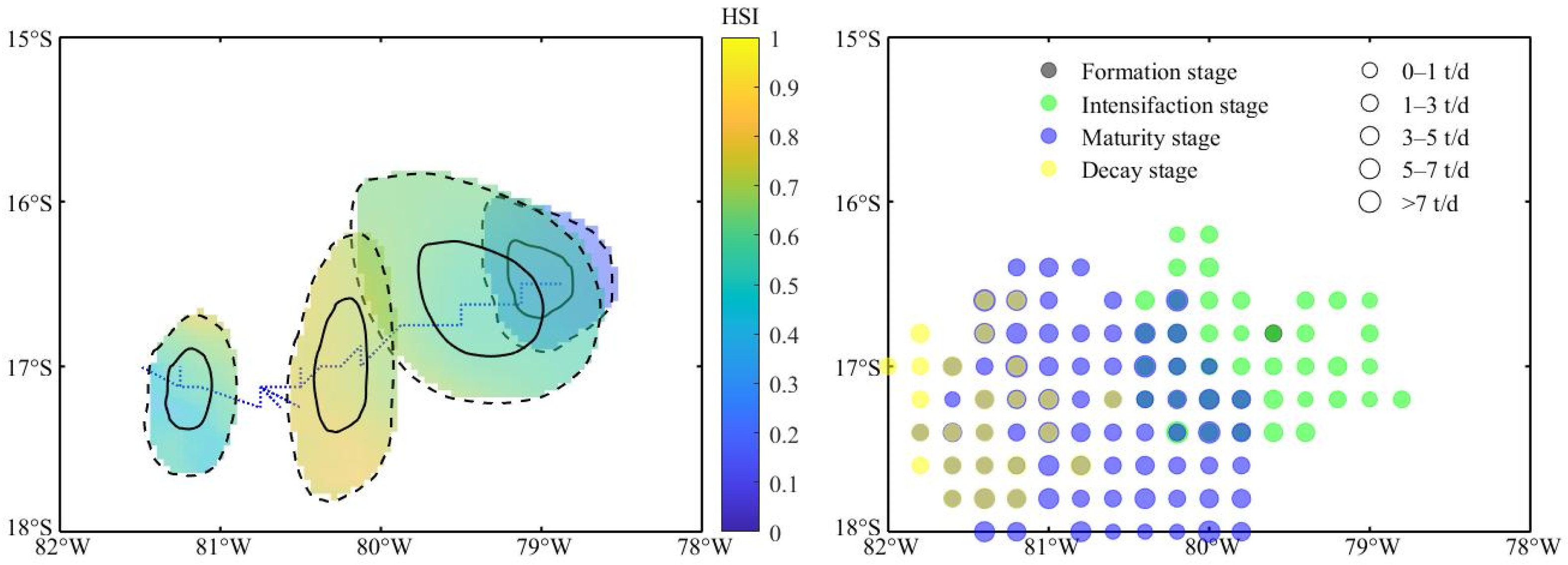
3.1. D. gigas Abundance and Distribution in Different Eddy Stages
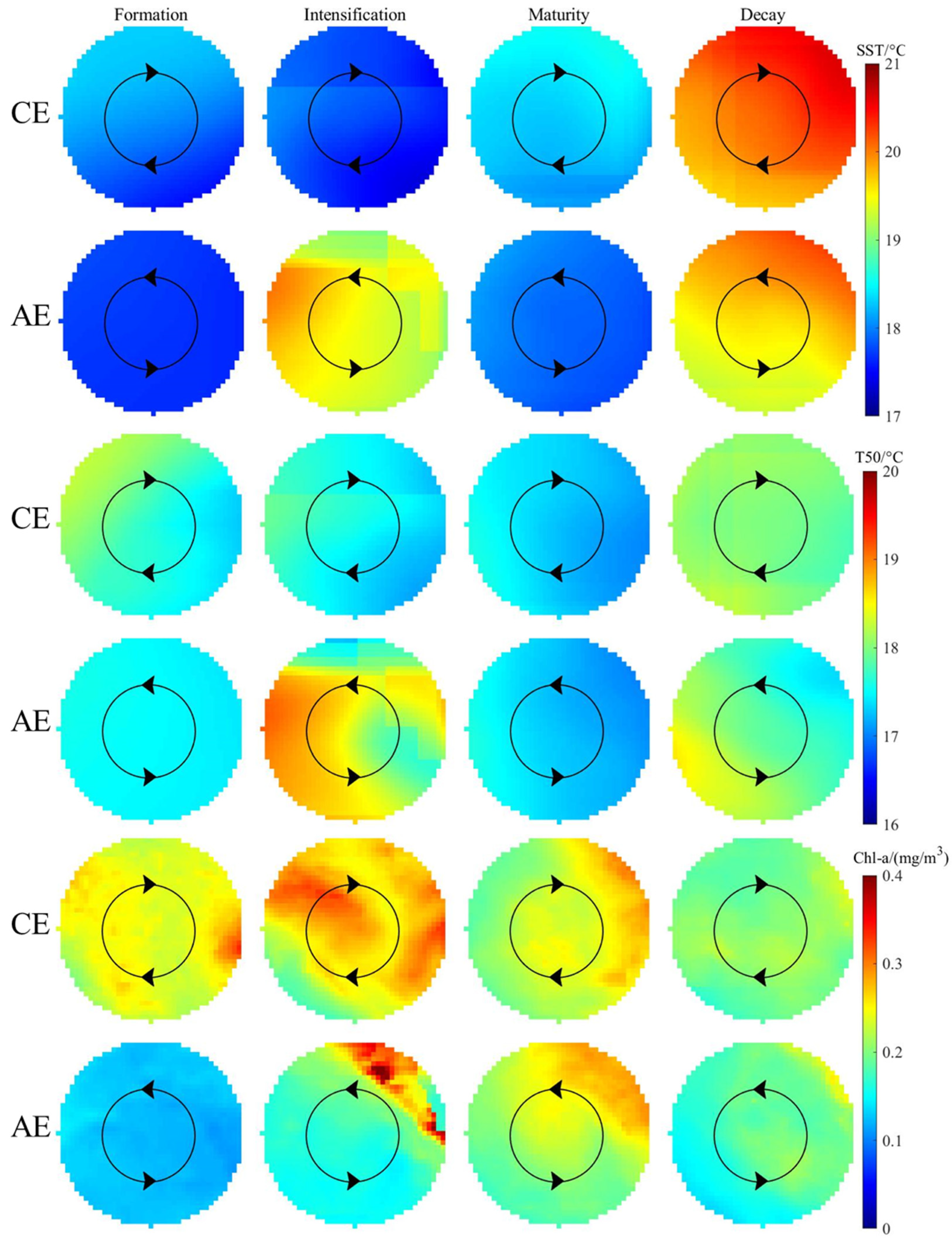
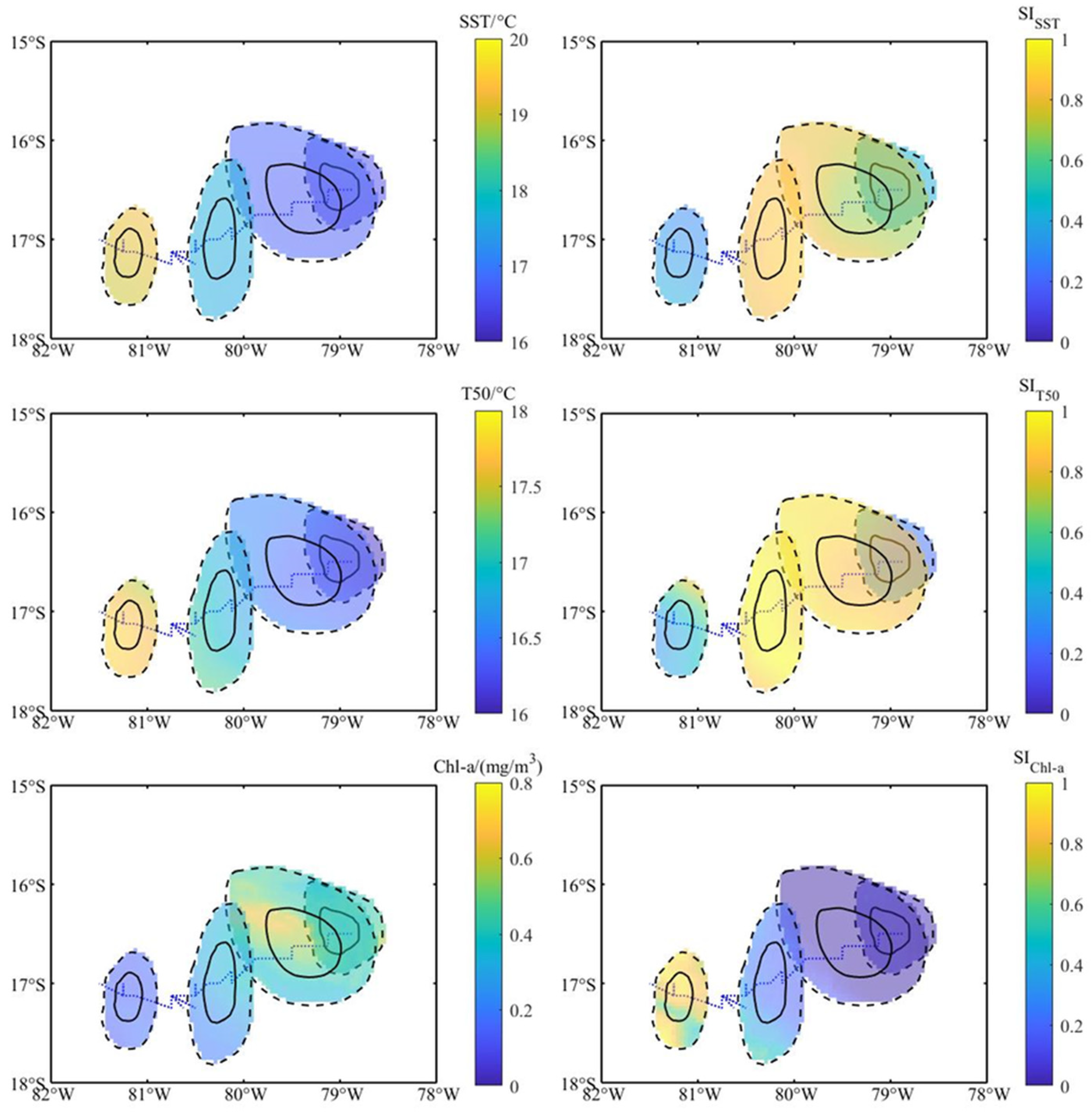
3.2. Environmental Characteristics of Different Stages of Eddies
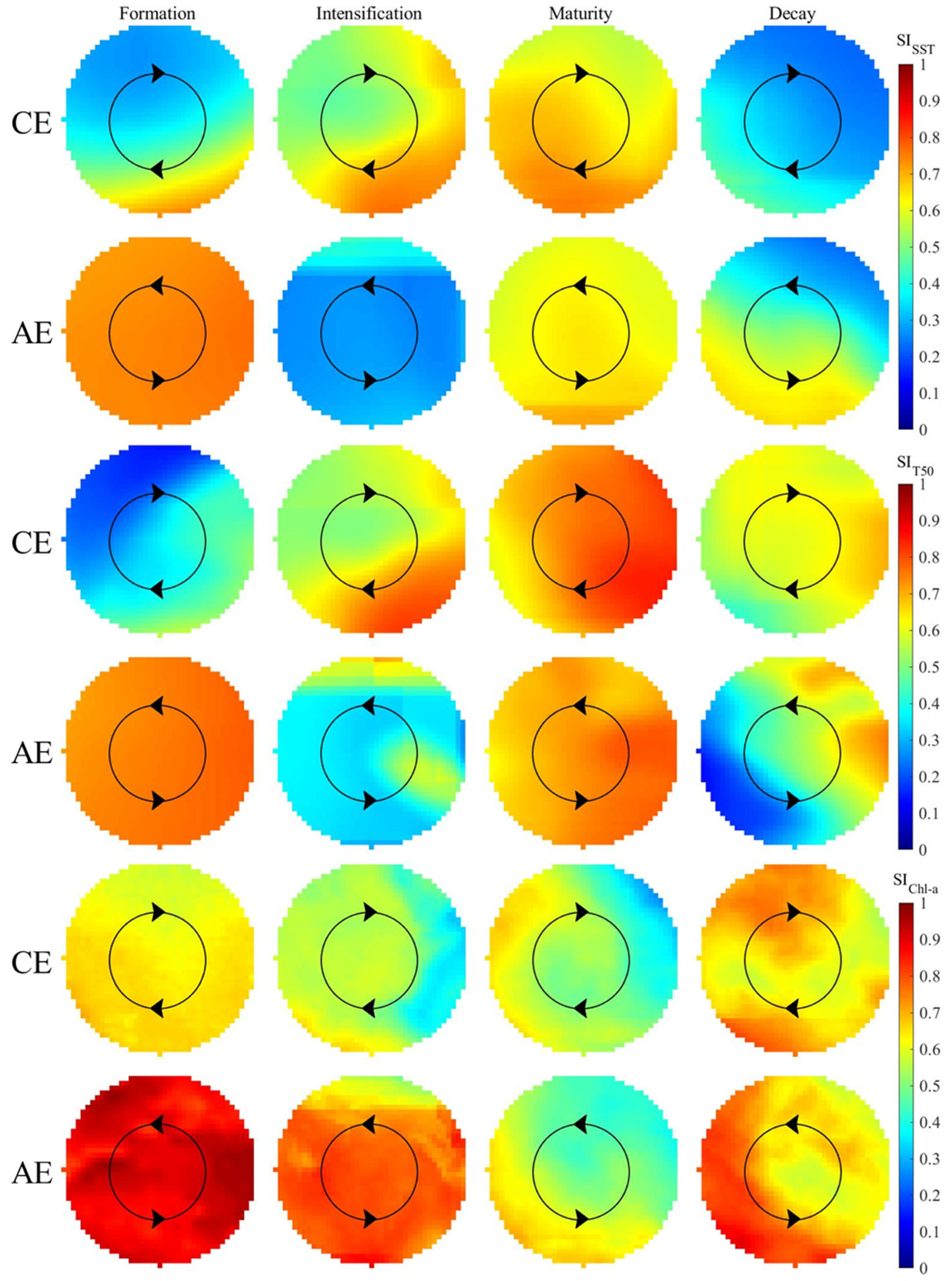
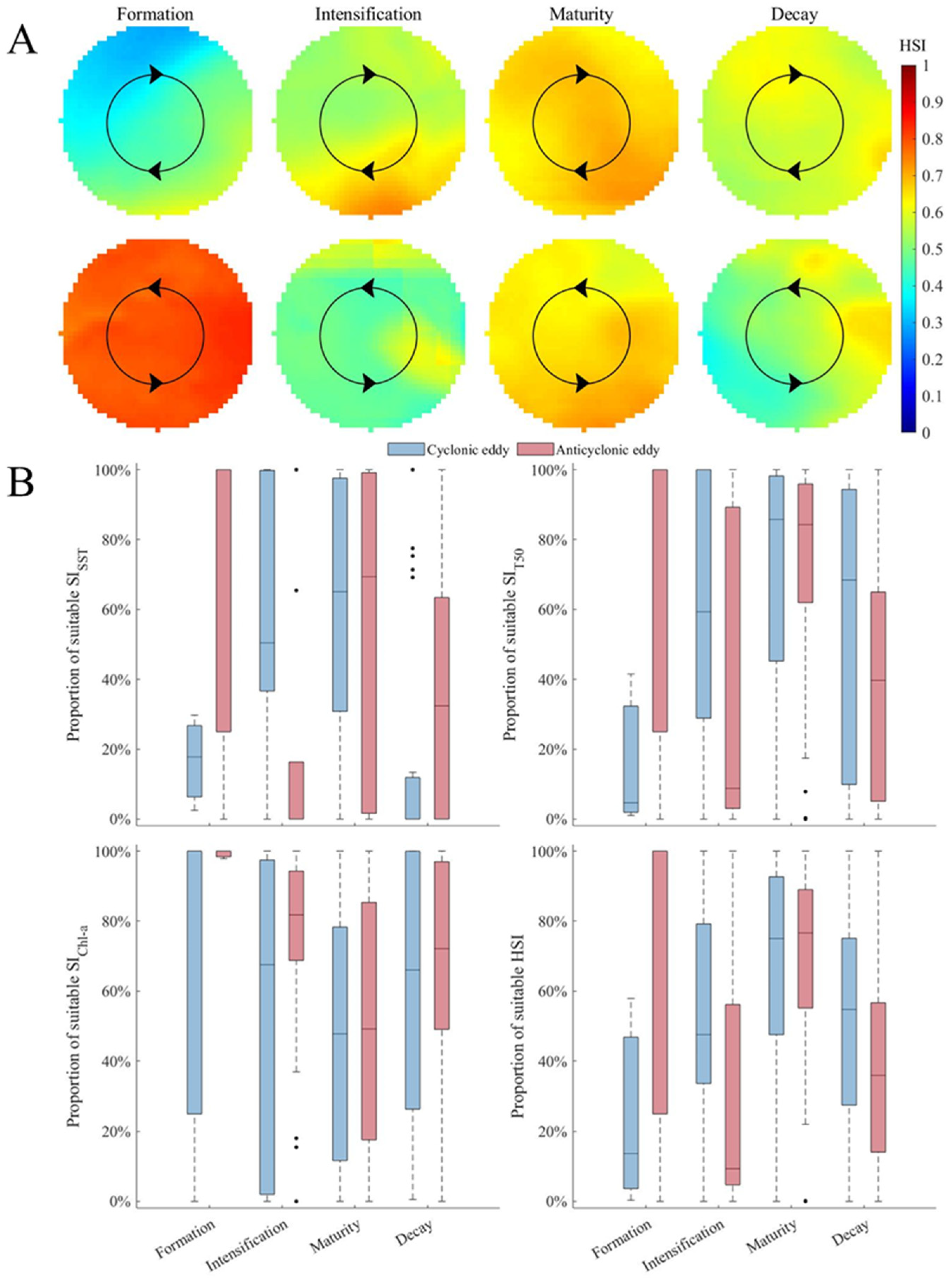
3.3. Changes in Habitat Suitability of D. gigas in Different Stages of Eddies
3.4. Evolutionary Analysis of Eddy 284
4. Discussion
4.1. Eddy-Induced Environmental Change
4.2. Associations between D. gigas and Environmental Factors
4.3. Relationships between Abundance and Distribution of D. gigas and Different Eddy Stages
4.4. Mechanisms Affecting Habitat Suitability of D. gigas during Different Stages of Eddies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chelton, D.B.; Schlax, M.G.; Samelson, R.M.; De Szoeke, R.A. Global Observations of Large Oceanic Eddies. Geophys. Res. Lett. 2007, 34, 2007GL030812. [Google Scholar] [CrossRef]
- Gaube, P.; McGillicuddy, D.J.; Chelton, D.B.; Behrenfeld, M.J.; Strutton, P.G. Regional Variations in the Influence of Mesoscale Eddies on Near-surface Chlorophyll. JGR Ocean. 2014, 119, 8195–8220. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, C.; Guan, Y.; Chen, D.; McWilliams, J.; Nencioli, F. Eddy Analysis in the Subtropical Zonal Band of the North Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 68, 54–67. [Google Scholar] [CrossRef]
- Bakun, A. Fronts and Eddies as Key Structures in the Habitat of Marine Fish Larvae: Opportunity, Adaptive Response and Competitive Advantage. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
- Garabato, A.C.N.; Yu, X.; Callies, J.; Barkan, R.; Polzin, K.L.; Frajka-Williams, E.E.; Buckingham, C.E.; Griffies, S.M. Kinetic Energy Transfers between Mesoscale and Submesoscale Motions in the Open Ocean’s Upper Layers. J. Phys. Oceanogr. 2022, 52, 75–97. [Google Scholar] [CrossRef]
- Lv, M.; Wang, F.; Li, Y.; Zhang, Z.; Zhu, Y. Structure of Sea Surface Temperature Anomaly Induced by Mesoscale Eddies in the North Pacific Ocean. JGR Ocean. 2022, 127, e2021JC017581. [Google Scholar] [CrossRef]
- Nukapothula, S.; Chen, C.; Yunus, A.P.; Wu, J. Satellite-Based Observations of Intense Chlorophyll-a Bloom in Response of Cold Core Eddy Formation: A Study in the Arabian Sea, Southwest Coast of India. Reg. Stud. Mar. Sci. 2018, 24, 303–310. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.; Hogg, A.M.; England, M.H.; Constantinou, N.C.; Kiss, A.E.; Morrison, A.K. Global Changes in Oceanic Mesoscale Currents over the Satellite Altimetry Record. Nat. Clim. Change 2021, 11, 397–403. [Google Scholar] [CrossRef]
- Domokos, R.; Seki, M.P.; Polovina, J.J.; Hawn, D.R. Oceanographic Investigation of the American Samoa Albacore (Thunnus alalunga) Habitat and Longline Fishing Grounds. Fish. Oceanogr. 2007, 16, 555–572. [Google Scholar] [CrossRef]
- Xing, Q.; Yu, H.; Wang, H.; Ito, S.; Chai, F. Mesoscale Eddies Modulate the Dynamics of Human Fishing Activities in the Global Midlatitude Ocean. Fish Fish. 2023, 24, 527–543. [Google Scholar] [CrossRef]
- Lindo-Atichati, D.; Bringas, F.; Goni, G.; Muhling, B.; Muller-Karger, F.; Habtes, S. Varying Mesoscale Structures Influence Larval Fish Distribution in the Northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 463, 245–257. [Google Scholar] [CrossRef]
- Durán Gómez, G.S.; Nagai, T.; Yokawa, K. Mesoscale Warm-Core Eddies Drive Interannual Modulations of Swordfish Catch in the Kuroshio Extension System. Front. Mar. Sci. 2020, 7, 680. [Google Scholar] [CrossRef]
- Arur, A.; Krishnan, P.; Kiruba-Sankar, R.; Suryavanshi, A.; Lohith Kumar, K.; Kantharajan, G.; Choudhury, S.B.; Manjulatha, C.; Babu, D.E. Feasibility of Targeted Fishing in Mesoscale Oceanic Eddies: A Study from Commercial Fishing Grounds of Andaman and Nicobar Islands, India. Int. J. Remote Sens. 2020, 41, 5011–5045. [Google Scholar] [CrossRef]
- Arur, A.; Krishnan, P.; George, G.; Goutham Bharathi, M.P.; Kaliyamoorthy, M.; Hareef Baba Shaeb, K.; Suryavanshi, A.S.; Kumar, T.S.; Joshi, A.K. The Influence of Mesoscale Eddies on a Commercial Fishery in the Coastal Waters of the Andaman and Nicobar Islands, India. Int. J. Remote Sens. 2014, 35, 6418–6443. [Google Scholar] [CrossRef][Green Version]
- McGillicuddy, D.J. Mechanisms of Physical-Biological-Biogeochemical Interaction at the Oceanic Mesoscale. Annu. Rev. Mar. Sci. 2016, 8, 125–159. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Gaube, P.; Woodworth-Jefcoats, P.A.; Kobayashi, D.R.; Braun, C.D. Anticyclonic Eddies Aggregate Pelagic Predators in a Subtropical Gyre. Nature 2022, 609, 535–540. [Google Scholar] [CrossRef]
- Braun, C.D.; Gaube, P.; Sinclair-Taylor, T.H.; Skomal, G.B.; Thorrold, S.R. Mesoscale Eddies Release Pelagic Sharks from Thermal Constraints to Foraging in the Ocean Twilight Zone. Proc. Natl. Acad. Sci. USA 2019, 116, 17187–17192. [Google Scholar] [CrossRef]
- Argüelles, J.; Rodhouse, P.G.; Villegas, P.; Castillo, G. Age, Growth and Population Structure of the Jumbo Flying Squid Dosidicus gigas in Peruvian Waters. Fish. Res. 2001, 54, 51–61. [Google Scholar] [CrossRef]
- Markaida, U.; Quiñónez-Velázquez, C.; Sosa-Nishizaki, O. Age, Growth and Maturation of Jumbo Squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. Fish. Res. 2004, 66, 31–47. [Google Scholar] [CrossRef]
- Stewart, J.; Hazen, E.; Foley, D.; Bograd, S.; Gilly, W. Marine Predator Migration during Range Expansion: Humboldt Squid Dosidicus gigas in the Northern California Current System. Mar. Ecol. Prog. Ser. 2012, 471, 135–150. [Google Scholar] [CrossRef][Green Version]
- Nigmatullin, C. A Review of the Biology of the Jumbo Squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish. Res. 2001, 54, 9–19. [Google Scholar] [CrossRef]
- Markaida, U. Food and Feeding of Jumbo Squid Dosidicus gigas in the Gulf of California and Adjacent Waters after the 1997–98 El Niño Event. Fish. Res. 2006, 79, 16–27. [Google Scholar] [CrossRef]
- Gonzalez-Pestana, A.; Alfaro-Shigueto, J.; Mangel, J.C. A Review of High Trophic Predator-Prey Relationships in the Pelagic Northern Humboldt System, with a Focus on Anchovetas. Fish. Res. 2022, 253, 106386. [Google Scholar] [CrossRef]
- Preti, A.; Soykan, C.U.; Dewar, H.; Wells, R.J.D.; Spear, N.; Kohin, S. Comparative Feeding Ecology of Shortfin Mako, Blue and Thresher Sharks in the California Current. Environ. Biol. Fish 2012, 95, 127–146. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Qian, W.; Jin, Y.; Li, J. δ13C and δ15N in Humboldt Squid Beaks: Understanding Potential Geographic Population Connectivity and Movement. Acta Oceanol. Sin. 2019, 38, 53–59. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Chen, Y.; Tian, S.; Li, J.; Fang, Z.; Yang, M. Age, Maturation, and Population Structure of the Humboldt Squid Dosidicus gigas off the Peruvian Exclusive Economic Zones. Chin. J. Ocean. Limnol. 2013, 31, 81–91. [Google Scholar] [CrossRef]
- Rosas-Luis, R.; Tafur-Jimenez, R.; Alegre-Norza, A.R.; Castillo-Valderrama, P.R.; Cornejo-Urbina, R.M.; Salinas-Zavala, C.A.; Sánchez, P. Trophic Relationships between the Jumbo Squid (Dosidicus gigas) and the Lightfish (Vinciguerria lucetia) in the Humboldt Current System off Peru. Sci. Mar. 2011, 75, 549–557. [Google Scholar] [CrossRef]
- Pizarro, O.; Shaffer, G.; Dewitte, B.; Ramos, M. Dynamics of Seasonal and Interannual Variability of the Peru-Chile Undercurrent. Geophys. Res. Lett. 2002, 29, 22-1–22-4. [Google Scholar] [CrossRef]
- Chaigneau, A.; Le Texier, M.; Eldin, G.; Grados, C.; Pizarro, O. Vertical Structure of Mesoscale Eddies in the Eastern South Pacific Ocean: A Composite Analysis from Altimetry and Argo Profiling Floats. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Chaigneau, A.; Gizolme, A.; Grados, C. Mesoscale Eddies off Peru in Altimeter Records: Identification Algorithms and Eddy Spatio-Temporal Patterns. Prog. Oceanogr. 2008, 79, 106–119. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Yu, W.; Chen, X. Geographical Distribution Variations of Humboldt Squid Habitat in the Eastern Pacific Ocean. Ecosyst. Health Sustain. 2023, 9, 0010. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X. Ocean Warming-Induced Range-Shifting of Potential Habitat for Jumbo Flying Squid Dosidicus gigas in the Southeast Pacific Ocean off Peru. Fish. Res. 2018, 204, 137–146. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Watanabe, T.; Yatsu, A.; Inagake, D.; Okada, Y. Occurrence of Jumbo Flying Squid Dosidicus gigas Aggregations Associated with the Countercurrent Ridge off the Costa Rica Dome during 1997 El Niño and 1999 La Niña. Mar. Ecol. Prog. Ser. 2002, 231, 151–166. [Google Scholar] [CrossRef]
- Yu, W.; Yi, Q.; Chen, X.; Chen, Y. Climate-Driven Latitudinal Shift in Fishing Ground of Jumbo Flying Squid (Dosidicus gigas) in the Southeast Pacific Ocean off Peru. Int. J. Remote Sens. 2017, 38, 3531–3550. [Google Scholar] [CrossRef]
- Stewart, J.S.; Hazen, E.L.; Bograd, S.J.; Byrnes, J.E.K.; Foley, D.G.; Gilly, W.F.; Robison, B.H.; Field, J.C. Combined Climate- and Prey-Mediated Range Expansion of Humboldt Squid (Dosidicus gigas), a Large Marine Predator in the California Current System. Glob. Change Biol. 2014, 20, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Staaf, D.; Zeidberg, L.; Gilly, W. Effects of Temperature on Embryonic Development of the Humboldt Squid Dosidicus gigas. Mar. Ecol. Prog. Ser. 2011, 441, 165–175. [Google Scholar] [CrossRef]
- Fang, X.; Yu, W.; Chen, X.; Zhang, Y. Response of Abundance and Distribution of Humboldt Squid (Dosidicus gigas) to Short-Lived Eddies in the Eastern Equatorial Pacific Ocean from April to June 2017. Front. Mar. Sci. 2021, 8, 721291. [Google Scholar] [CrossRef]
- Le Vu, B.; Stegner, A.; Arsouze, T. Angular Momentum Eddy Detection and Tracking Algorithm (AMEDA) and Its Application to Coastal Eddy Formation. J. Atmos. Ocean. Technol. 2018, 35, 739–762. [Google Scholar] [CrossRef]
- Aroucha, L.C.; Veleda, D.; Lopes, F.S.; Tyaquiçã, P.; Lefèvre, N.; Araujo, M. Intra- and Inter-Annual Variability of North Brazil Current Rings Using Angular Momentum Eddy Detection and Tracking Algorithm: Observations from 1993 to 2016. JGR Ocean. 2020, 125, e2019JC015921. [Google Scholar] [CrossRef]
- Garreau, P.; Dumas, F.; Louazel, S.; Stegner, A.; Le Vu, B. High-Resolution Observations and Tracking of a Dual-Core Anticyclonic Eddy in the Algerian Basin. JGR Ocean. 2018, 123, 9320–9339. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Chen, X.; Zhou, M.; Zhang, C. Evaluating the Impacts of Mesoscale Eddies on Abundance and Distribution of Neon Flying Squid in the Northwest Pacific Ocean. Front. Mar. Sci. 2022, 9, 862273. [Google Scholar] [CrossRef]
- Xu, G.; Dong, C.; Liu, Y.; Gaube, P.; Yang, J. Chlorophyll Rings around Ocean Eddies in the North Pacific. Sci. Rep. 2019, 9, 2056. [Google Scholar] [CrossRef] [PubMed]
- Gaube, P.; Barceló, C.; McGillicuddy, D.J.; Domingo, A.; Miller, P.; Giffoni, B.; Marcovaldi, N.; Swimmer, Y. The Use of Mesoscale Eddies by Juvenile Loggerhead Sea Turtles (Caretta caretta) in the Southwestern Atlantic. PLoS ONE 2017, 12, e0172839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Benitez-Nelson, C.R.; Huang, J.; Xiu, P.; Sun, Z.; Dai, M. Cyclonic Eddies Modulate Temporal and Spatial Decoupling of Particulate Carbon, Nitrogen, and Biogenic Silica Export in the North Pacific Subtropical Gyre. Limnol. Oceanogr. 2021, 66, 3508–3522. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.; Zhang, H.; Fang, Z.; Jiang, B.; Su, B.; Sui, J.; Yan, Y.; Xiang, D. Environment Variables Affect CPUE and Spatial Distribution of Fishing Grounds on the Light Falling Gear Fishery in the Northwest Indian Ocean at Different Time Scales. Front. Mar. Sci. 2022, 9, 939334. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Wan, R.; Zhang, C. Model Selection between Traditional and Popular Methods for Standardizing Catch Rates of Target Species: A Case Study of Japanese Spanish Mackerel in the Gillnet Fishery. Fish. Res. 2015, 161, 312–319. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.; Sanchez-Castillo, M.; Chica-Olmo, M.; Chica-Rivas, M. Machine Learning Predictive Models for Mineral Prospectivity: An Evaluation of Neural Networks, Random Forest, Regression Trees and Support Vector Machines. Ore Geol. Rev. 2015, 71, 804–818. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lee, Y.-C.; Lu, P.-E.; Lu, S.-S.; Lai, H.-T.; Huang, C.-C.; Wang, C.; Lin, Y.-J.; Su, W.-T. Social Media Prediction Based on Residual Learning and Random Forest. In Proceedings of the 25th ACM international Conference on Multimedia, Mountain View, CA, USA, 23 October 2017; ACM: New York, NY, USA, 2017; pp. 1865–1870. [Google Scholar]
- Díaz-Uriarte, R.; Alvarez De Andrés, S. Gene Selection and Classification of Microarray Data Using Random Forest. BMC Bioinform. 2006, 7, 3. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Du, Y.; Chen, X.; Kindong, R.; Xue, H.; Chai, F.; Yu, W. Eddy Impacts on Abundance and Habitat Distribution of a Large Predatory Squid off Peru. Mar. Environ. Res. 2024, 195, 106368. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, Y.; Zhang, X.; Huang, C. Global Chlorophyll Distribution Induced by Mesoscale Eddies. Remote Sens. Environ. 2021, 254, 112245. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Ziemann, D.; Kolber, Z.; Bienfang, P.K. Role of Eddy Pumping in Enhancing Primary Production in the Ocean. Nature 1991, 352, 55–58. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.-R.; Chai, F.; Yuan, Y. Impact of Mesoscale Eddies on Chlorophyll Variability off the Coast of Chile. PLoS ONE 2018, 13, e0203598. [Google Scholar] [CrossRef] [PubMed]
- Cprrea-Ramirez, M.A.; Hormazábal, S.; Yuras, G. Mesoscale Eddies and High Chlorophyll Concentrations off Central Chile (29°–39°S). Geophys. Res. Lett. 2007, 34, 2007GL029541. [Google Scholar] [CrossRef]
- Paulino, C.; Segura, M.; Chacón, G. Spatial Variability of Jumbo Flying Squid (Dosidicus gigas) Fishery Related to Remotely Sensed SST and Chlorophyll-a Concentration (2004–2012). Fish. Res. 2016, 173, 122–127. [Google Scholar] [CrossRef]
- Sakai, M.; Tsuchiya, K.; Mariategui, L.; Wakabayashi, T.; Yamashiro, C. Vertical Migratory Behavior of Jumbo Flying Squid (Dosidicus gigas) off Peru: Records of Acoustic and Pop-up Tags. JARQ 2017, 51, 171–179. [Google Scholar] [CrossRef]
- Yu, W.; Jin, P.; Zhu, G. Decadal variations in habitat suitability of jumbo flying squid Dosidicus gigas off Peru during spring and summer based on vertical water temperature. J. Fish. Sci. China 2023, 30, 1374–1386. [Google Scholar]
- Robinson, C.J.; Gómez-Gutiérrez, J.; De León, D.A.S. Jumbo Squid (Dosidicus gigas) Landings in the Gulf of California Related to Remotely Sensed SST and Concentrations of Chlorophyll a (1998–2012). Fish. Res. 2013, 137, 97–103. [Google Scholar] [CrossRef]
- Chambault, P.; Baudena, A.; Bjorndal, K.A.; Santos, M.A.R.; Bolten, A.B.; Vandeperre, F. Swirling in the Ocean: Immature Loggerhead Turtles Seasonally Target Old Anticyclonic Eddies at the Fringe of the North Atlantic Gyre. Prog. Oceanogr. 2019, 175, 345–358. [Google Scholar] [CrossRef]
- Wu, X.; Jin, P.; Zhang, Y.; Yu, W. Changing Humboldt Squid Abundance and Distribution at Different Stages of Oceanic Mesoscale Eddies. JMSE 2024, 12, 626. [Google Scholar] [CrossRef]
- Lima, I.D.; Olson, D.B.; Doney, S.C. Biological Response to Frontal Dynamics and Mesoscale Variability in Oligotrophic Environments: Biological Production and Community Structure. J. Geophys. Res. 2002, 107, 25-1–25-21. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Chen, Y.; Xu, L.; Dai, X. Evaluating Habitat Suitability Indices Derived from CPUE and Fishing Effort Data for Ommatrephes bratramii in the Northwestern Pacific Ocean. Fish. Res. 2009, 95, 181–188. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, Z.; Zhang, Y.; Yu, W.; Chen, B.; Chen, X. Climate-Related Habitat Variations of Humboldt Squid in the Eastern Equatorial Pacific Ocean. J. Mar. Syst. 2024, 243, 103960. [Google Scholar] [CrossRef]
- Chen, X.; Han, F.; Zhu, K.; Punt, A.E.; Lin, D. The Breeding Strategy of Female Jumbo Squid Dosidicus gigas: Energy Acquisition and Allocation. Sci. Rep. 2020, 10, 9639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jin, P.; Zhang, Y.; Yu, W. Spatial Distribution and Abundance of a Pelagic Squid during the Evolution of Eddies in the Southeast Pacific Ocean. J. Mar. Sci. Eng. 2024, 12, 1015. https://doi.org/10.3390/jmse12061015
Wu X, Jin P, Zhang Y, Yu W. Spatial Distribution and Abundance of a Pelagic Squid during the Evolution of Eddies in the Southeast Pacific Ocean. Journal of Marine Science and Engineering. 2024; 12(6):1015. https://doi.org/10.3390/jmse12061015
Chicago/Turabian StyleWu, Xiaoci, Pengchao Jin, Yang Zhang, and Wei Yu. 2024. "Spatial Distribution and Abundance of a Pelagic Squid during the Evolution of Eddies in the Southeast Pacific Ocean" Journal of Marine Science and Engineering 12, no. 6: 1015. https://doi.org/10.3390/jmse12061015
APA StyleWu, X., Jin, P., Zhang, Y., & Yu, W. (2024). Spatial Distribution and Abundance of a Pelagic Squid during the Evolution of Eddies in the Southeast Pacific Ocean. Journal of Marine Science and Engineering, 12(6), 1015. https://doi.org/10.3390/jmse12061015





