Too Hot to Handle: Effects of Water Temperature on the Early Life Stages of Gongolaria barbata (Fucales)
Abstract
1. Introduction
2. Methods and Materials
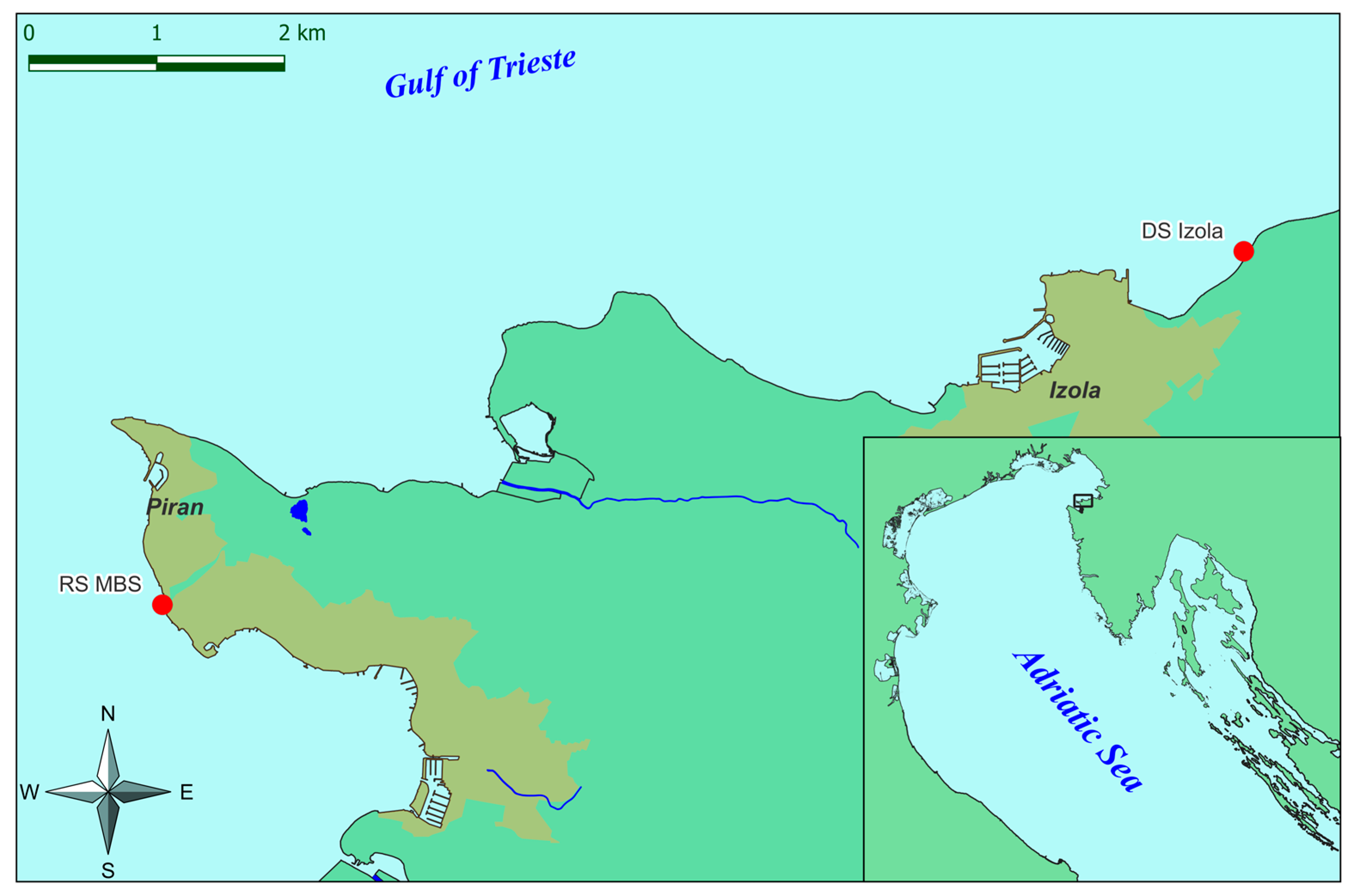
2.1. Study Area
2.2. Reproductive Material Collection
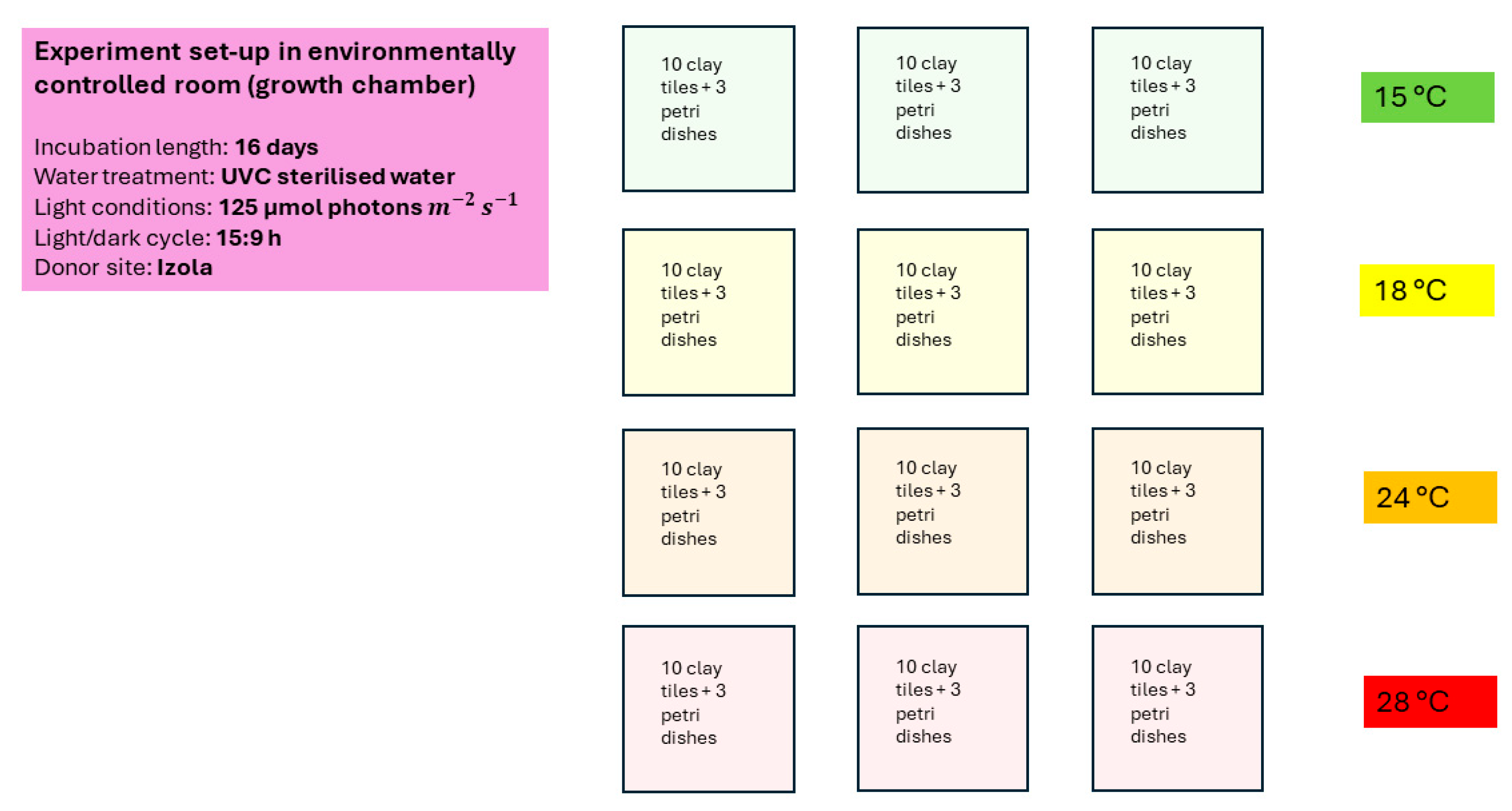
2.3. Laboratory Work
2.4. Photo-Processing and Statistical Analyses
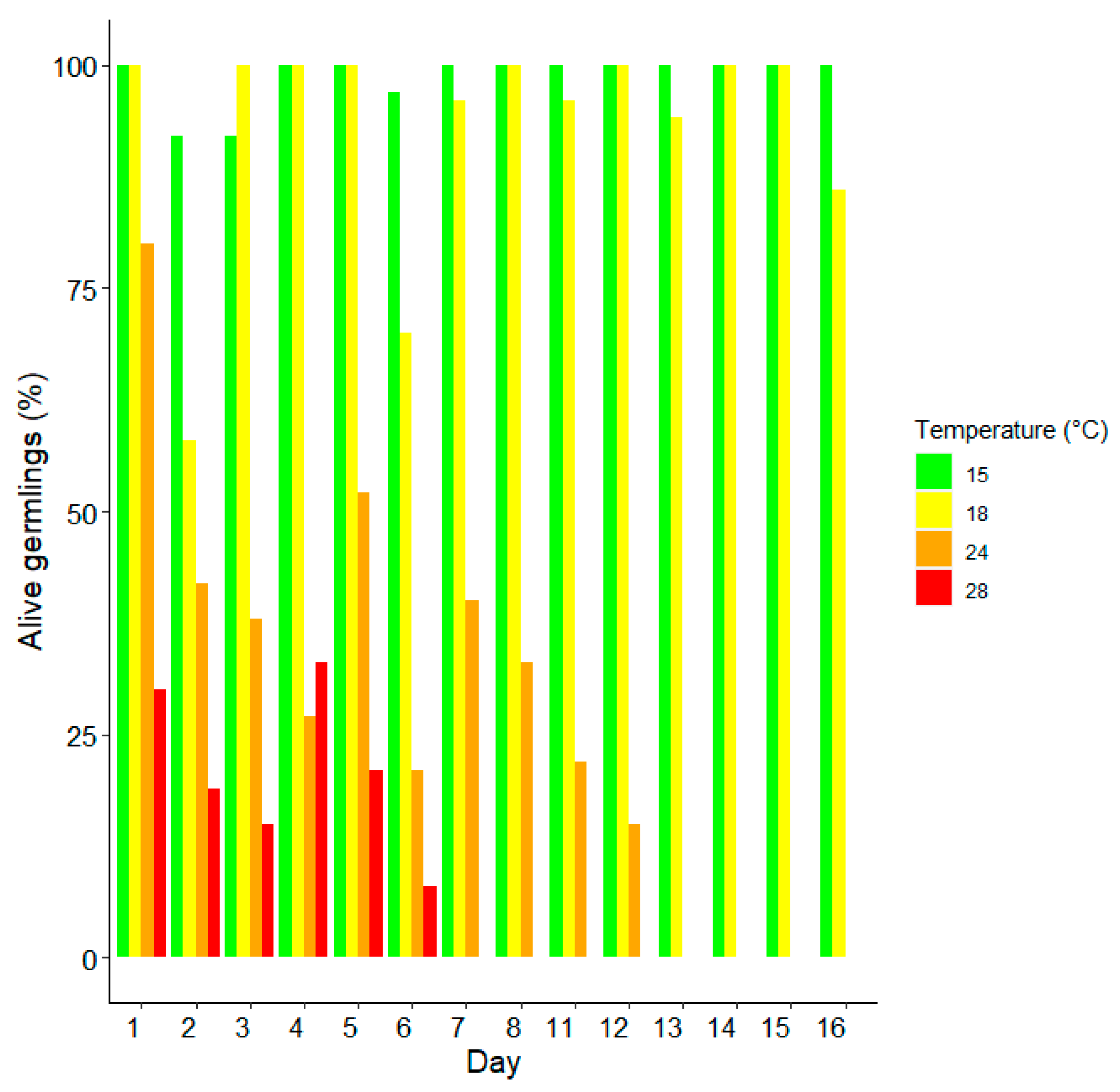
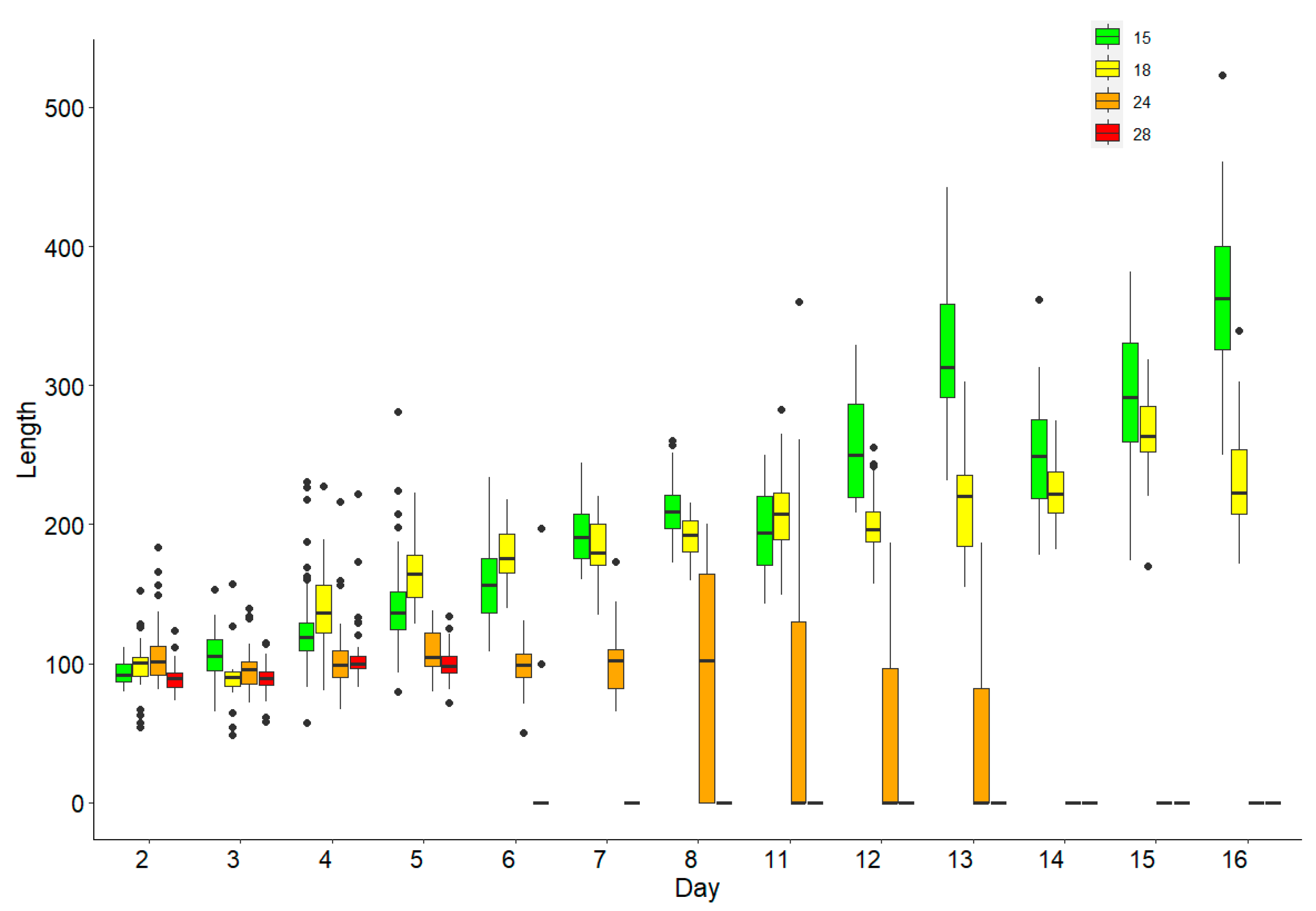
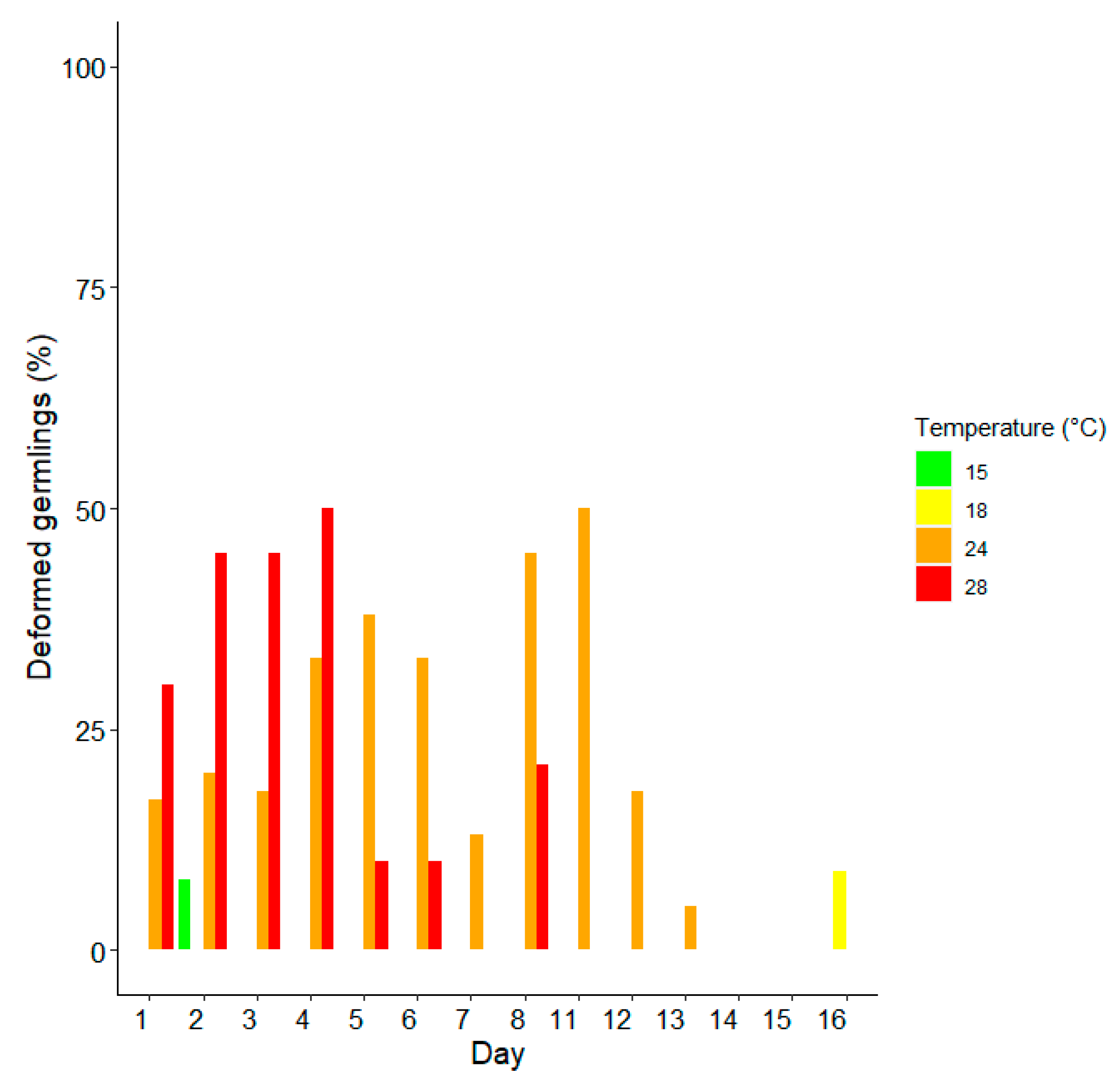
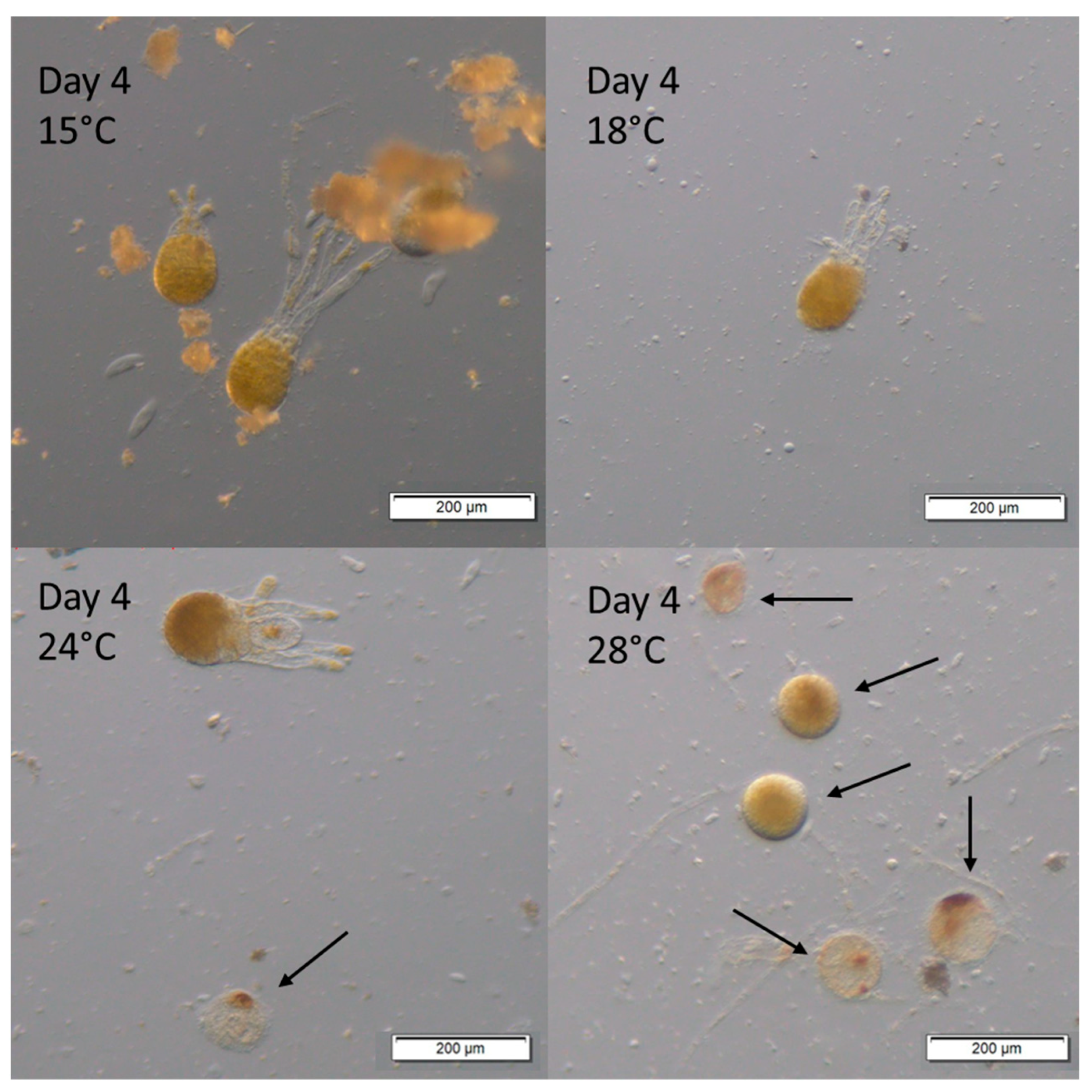
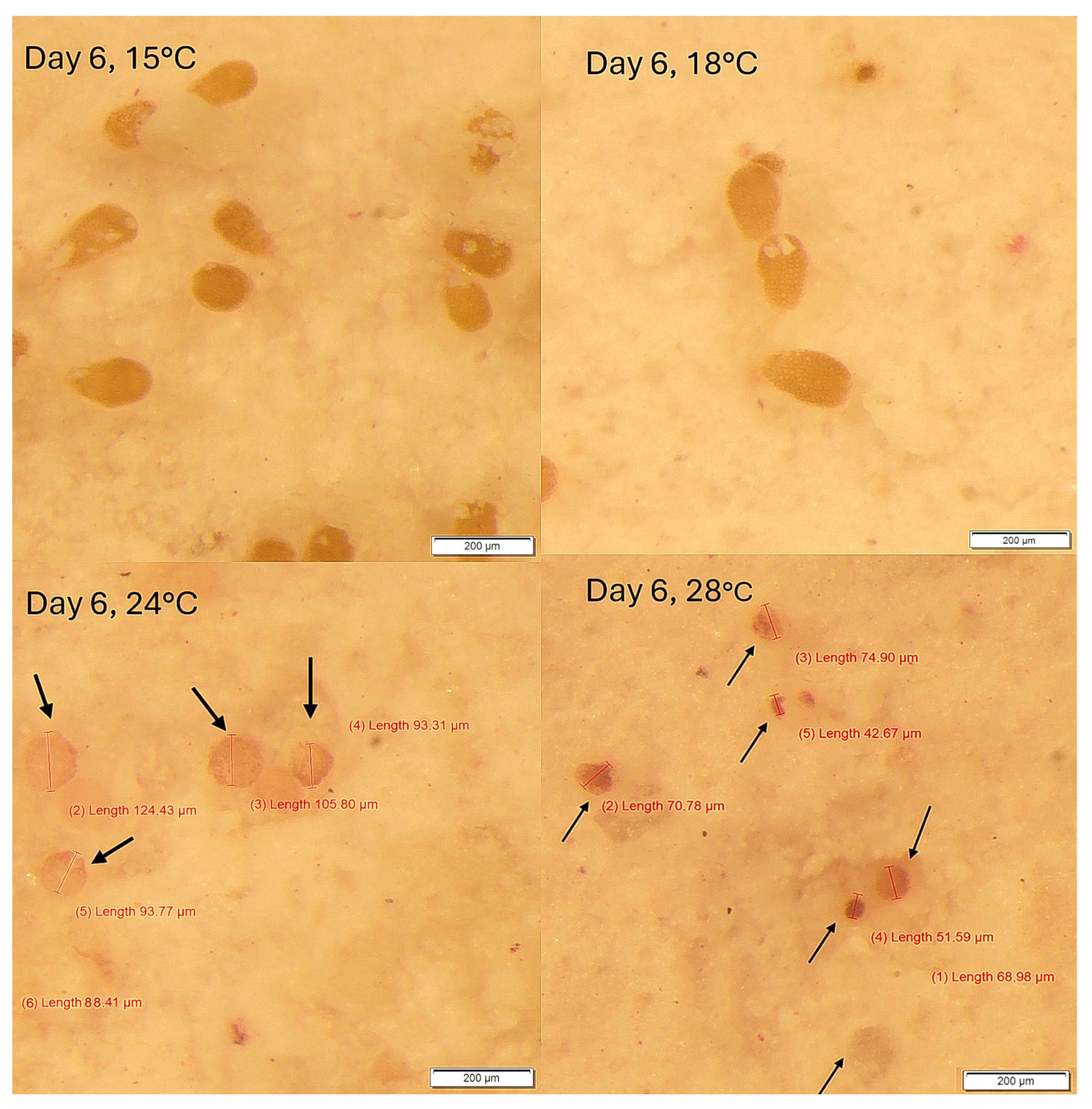
3. Results
Survival and Growth of Germlings in Mesocosm
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Assis, J.; Fragkopoulou, E.; Frade, D.; Neiva, J.; Oliveira, A.; Abecasis, D.; Faugeron, S.; Serrão, E.A. A fine-tuned global distribution dataset of marine forests. Sci. Data 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, G.; Asnaghi, V.; Chiantore, M.; Thrush, S.; Povero, P.; Vassallo, P.; Petrillo, M.; Paoli, C. The effect of Cystoseira canopy on the value of midlittoral habitats in NW Mediterranean, an emergy assessment. Ecol. Model. 2019, 404, 1–11. [Google Scholar] [CrossRef]
- Macic, V.; Svirčev, Z. Macroepiphytes on Cystoseira species (Phaeophyceae) on the coast of Montenegro. Fresenius Environ. Bull. 2014, 23, 29–34. [Google Scholar] [CrossRef]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Popović, A.; Lipej, L. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (Northern Adriatic Sea). Mediterr. Mar. Sci. 2014, 15, 225–238. [Google Scholar] [CrossRef]
- Peleg, O.; Guy-Haim, T.; Yeruham, E.; Silverman, J.; Rilov, G. Tropicalization may invert trophic state and carbon budget of shallow temperate rocky reefs. J. Ecol. 2020, 108, 844–854. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Milazzo, M.; Chemello, R. Decreasing in patch-size of Cystoseira forests reduces the diversity of their associated molluscan assemblage in Mediterranean rocky reefs. Estuar. Coast. Shelf Sci. 2021, 250, 107163. [Google Scholar] [CrossRef]
- Guiry, M.D. Algaebase. Available online: https://www.algaebase.org/browse/taxonomy/#120369 (accessed on 9 February 2024).
- Einav, R. Proximity and distance—Review of seaweed communities and the marine environment along the coasts of the Levant Basin. Isr. J. Plant Sci. 2023, 70, 213–232. [Google Scholar] [CrossRef]
- Ould-Ahmed, N.; Gómez Garreta, A.; Ribera Siguan, M.A.; Bouguedoura, N. Checklist of the benthic marine macroalgae from Algeria. An. Jardín Botánico Madr. 2013, 70, 136–143. [Google Scholar] [CrossRef]
- Ribera, M.A.; Gómez Garreta, A.; Gallardo, T.; Cormaci, M.; Furnari, G.; Giaccone, G. Check-list of Mediterranean Seaweeds. I. Fucophyceae. Bot. Mar. 1992, 35, 109–130. [Google Scholar] [CrossRef]
- Sabri, H.; Cherifi, O.; Maarouf, A.; Bahammou, N.; Boundir, Y. The checklist and the ecological index of the brown seaweeds from Essaouira coastline (Morocco). J. Appl. Sci. Environ. Stud. 2021, 4, 406–417. [Google Scholar]
- Maiz, N.B.; Boudouresque, C.F.; Ouahchi, F. Inventaire des algues et phanérogames marines benthiques de la Tunisie. Gioranale Bot. Ital. 1987, 121, 5–6. [Google Scholar] [CrossRef]
- Iveša, L.; Bilajac, A.; Gljušćić, E.; Najdek, M. Gongolaria barbata forest in the shallow lagoon on the southern Istrian Coast (northern Adriatic Sea). Bot. Mar. 2022, 65, 255–268. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Iveša, L.; Devescovi, M. Distribution and composition of stands along the west Istrian coast (northern Adriatic, Croatia) and comparison with historical data. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014; pp. 102–107. [Google Scholar]
- Boudouresque, C.-F.; Perret-Boudouresque, M.; Blanfuné, A. Diversity of Marine and Brackish Macrophytes in the Port-Cros National Park (Provence, France, Mediterranean Sea): Taxa and Research Effort over Space and Time. Diversity 2022, 14, 329. [Google Scholar] [CrossRef]
- Trikka, F.; Israel, P.; Koukaras, K.; Argiriou, A. Biochemical characterization of eight Greek algae as candidate species for local seaweed cultivation. Bot. Mar. 2021, 64, 313–326. [Google Scholar] [CrossRef]
- Rendina, F.; Falace, A.; Alongi, G.; Buia, M.C.; Neiva, J.; Appolloni, L.; Marletta, G.; Russo, G.F. The Lush Fucales Underwater Forests off the Cilento Coast: An Overlooked Mediterranean Biodiversity Hotspot. Plants 2023, 12, 1497. [Google Scholar] [CrossRef]
- Marletta, G.; Lombardo, A. The Fucales (Ochrophyta, Phaeophyceae) of the Island of Pantelleria (Sicily Channel, Mediterranean Sea): A new contribution. Ital. Bot. 2023, 15, 137–163. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Pitacco, V.; Lipej, L. Loss of canopy-forming algal richness and coverage in the northern Adriatic Sea. Ecol. Indic. 2021, 125, 107501. [Google Scholar] [CrossRef]
- Báez, J.C.; Olivero, J.; Real, R.; Vargas, J.M.; Flores-Moya, A. Analysis of geographical variation in species richness within the genera Audouinella (Rhodophyta), Cystoseira (Phaeophyceae) and Cladophora (Chlorophyta) in the western Mediterranean Sea. Bot. Mar. 2005, 48, 30–37. [Google Scholar] [CrossRef]
- Marin, O.; Spinu, A. Assessing the Ecological Status of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Ochrophyta) Habitat Along the Romanian Black Sea Coast- A Source of Multiple Ecosystem Services. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2023, 12, 42–56. [Google Scholar] [CrossRef]
- Bartolo, A.G.; Zammit, G.; Russell, H.; Peters, A.F.; Küpper, F.C. DNA barcoding of marine algae from Malta: New records from the central Mediterranean. Acta Bot. Croat. 2021, 80, 176–183. [Google Scholar] [CrossRef]
- Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Rozis, E.; Thibaut, T. Les Forêts Marines de France et de Méditerranée. Guide de Détermination des Espèces-Ingénieurs. Sargassaceae, Fucales, Phaeophyceae; Presses Universitaries de Provence: Aix-en-Provence, France, 2022; Volume 207. [Google Scholar]
- Gran, A.; Movilla, J.; Ballesteros, E.; Sales, M.; Bolado, I.; Galobart, C.; CefalÌ, M.E. Assessing the expansion and success of a restored population of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Phaeophyceae) using high-precision positioning tools and size distribution frequencies. Mediterr. Mar. Sci. 2022, 23, 907–916. [Google Scholar] [CrossRef]
- UNEP-MAP; SPA/RAC. SPA-BD Protocol—Annex II: List of Endangered or Threatened Species; SPA/RAC: Tunis, Tunisia, 2018. [Google Scholar]
- Verlaque, M.; Boudouresque, C.-F.; Perret-Boudouresque, M. Mediterranean seaweeds listed as threatened under the Barcelona Convention: A critical analysis. Sci. Rep. Port-Cros Natl. Park 2019, 33, 179–214. [Google Scholar]
- Savonitto, G.; De La Fuente, G.; Tordoni, E.; Ciriaco, S.; Srijemsi, M.; Bacaro, G.; Chiantore, M.; Falace, A. Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1611–1623. [Google Scholar] [CrossRef]
- Falace, A.; Manfrin, C.; Furnari, G.; Burchio, S.D.; Pallavicini, A.; Descourvieres, E.; Kaleb, S.; Lokovšek, A.; Grech, D.; Alongi, G. Contribution to the knowledge of Gongolaria barbata (Sargassaceae, Fucales) from the Mediterranean: Insights into infraspecific diversity. Phytotaxa 2024, 635, 191–205. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, T.; Blanfune, A.; Markovic, L.; Verlaque, M.; Boudouresque, C.; Perret-Boudouresque, M.; Macic, V.; Bottin, L. Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Mar. Pollut. Bull. 2014, 89, 305–323. [Google Scholar] [CrossRef]
- Falace, A.; Alongi, G.; Cormaci, M.; Furnari, G.; Curiel, D.; Cecere, E.; Petrocelli, A. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Perkol-Finkel, S.; Airoldi, L. Loss and recovery potential of marine habitats: An experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLoS ONE 2010, 5, e10791. [Google Scholar] [CrossRef]
- Blanfune, A.; Boudouresque, C.; Thibaut, T.; Verlaque, M. The sea level rise and the collapse of a Mediterranean ecosystem, the Lithophyllum byssoides algal rim. In The Mediterranean Region under Climate Change. A Scientific Update; Thiebault, S., Moatti, J.P., Eds.; IRD Editions Publisher: Marseille, France, 2016; pp. 285–289. [Google Scholar]
- Mariani, S.; Cefalì, M.E.; Chappuis, E.; Terradas, M.; Pinedo, S.; Torras, X.; Jordana, E.; Medrano, A.; Verdura, J.; Ballesteros, E. Past and present of Fucales from shallow and sheltered shores in Catalonia. Reg. Stud. Mar. Sci. 2019, 32, 100824. [Google Scholar] [CrossRef]
- Orfanidis, S. Temperature Responses and Distribution of Macroalgae Belonging to the Warm-temperate Mediterranean-Atlantic Distribution Group. Bot. Mar. 1991, 34, 541–552. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T. Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122829. [Google Scholar] [CrossRef]
- Falace, A.; Zanelli, E.; Bressan, G. Morphological and reproductive phenology of Cystoseira compressa (Esper) Gerloff & Nizamuddin (Fucales, Fucophyceae) in the Gulf of Trieste (North Adriatic Sea). Ann. Hist. Sci. Soc. 2005, 15, 71. [Google Scholar]
- Andrews, S.; Bennett, S.; Wernberg, T. Reproductive seasonality and early life temperature sensitivity reflect vulnerability of a seaweed undergoing range reduction. Mar. Ecol. Prog. Ser. 2014, 495, 119–129. [Google Scholar] [CrossRef]
- Capdevila, P.; Hereu, B.; Salguero-Gómez, R.; Rovira, G.l.; Medrano, A.; Cebrian, E.; Garrabou, J.; Kersting, D.K.; Linares, C. Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat-forming macroalga. J. Ecol. 2019, 107, 1129–1140. [Google Scholar] [CrossRef]
- Falace, A.; Kaleb, S.; De La Fuente, G.; Asnaghi, V.; Chiantore, M. Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS ONE 2018, 13, e0193011. [Google Scholar] [CrossRef] [PubMed]
- Clausing, R.J.; De La Fuente, G.; Falace, A.; Chiantore, M. Accounting for environmental stress in restoration of intertidal foundation species. J. Appl. Ecol. 2022, 60, 305–318. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Savonitto, G.; Lipizer, M.; Mancuso, P.; Ciriaco, S.; Srijemsi, M.; Falace, A. Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 2019, 100, e02838. [Google Scholar] [CrossRef]
- De Bettignies, T.; Wernberg, T.; Gurgel, C.F.D. Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front. Mar. Sci. 2018, 5, 218. [Google Scholar] [CrossRef]
- Steen, H.; Scrosati, R. Intraspecific competition in Fucus serratus and F. evanescens (Phaeophyceae: Fucales) germlings: Effects of settlement density, nutrient concentration, and temperature. Mar. Biol. 2004, 144, 61–70. [Google Scholar] [CrossRef]
- Lotze, H.K.; Worm, B.; Sommer, U. Strong bottom-up and top-down control of early life stages of macroalgae. Limnol. Oceanogr. 2001, 46, 749–757. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Sommer, U. Algal propagule banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia 2001, 128, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Celis-Plá, P.S.M.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Photoprotective responses in a brown macroalgae Cystoseira tamariscifolia to increases in CO2 and temperature. Mar. Environ. Res. 2017, 130, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, P.; Linares, C.; Aspillaga, E.; Riera, J.L.; Hereu, B. Effective dispersal and density-dependence in mesophotic macroalgal forests: Insights from the Mediterranean species Cystoseira zosteroides. PLoS ONE 2018, 13, e0191346. [Google Scholar] [CrossRef] [PubMed]
- Savva, I.; Bennett, S.; Roca, G.; Jordà, G.; Marbà, N. Thermal tolerance of Mediterranean marine macrophytes: Vulnerability to global warming. Ecol. Evol. 2018, 8, 12032–12043. [Google Scholar] [CrossRef] [PubMed]
- Cáliz, A.C.; Fernández, A.N.; de Pedro, R.S.; Flores-Moya, A.; Bañares-España, E. Physiological responses of adults and juveniles of Cystoseira tamariscifolia to projected warming scenarios along Alboran sea populations. In Proceedings of the II International Congress of Young Marine Researchers. Book of Abstracts, Malaga, Spain, 1–4 October 2019; pp. 426–430. [Google Scholar]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of ambient temperature on the photosynthetic activity and phenolic content of the intertidal Cystoseira compressa along the Italian coastline. J. Appl. Phycol. 2019, 31, 3069–3076. [Google Scholar] [CrossRef]
- Verdura, J.; Santamaría, J.; Ballesteros, E.; Smale, D.A.; Cefalì, M.E.; Golo, R.; de Caralt, S.; Vergés, A.; Cebrian, E. Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 2021, 109, 1758–1773. [Google Scholar] [CrossRef]
- Fabbrizzi, E.; Giakoumi, S.; De Leo, F.; Tamburello, L.; Chiarore, A.; Colletti, A.; Coppola, M.; Munari, M.; Musco, L.; Rindi, F.; et al. The challenge of setting restoration targets for macroalgal forests under climate changes. J. Environ. Manag. 2023, 326, 116834. [Google Scholar] [CrossRef]
- Falace, A.; Marletta, G.; Savonitto, G.; Candotto Carniel, F.; Srijemsi, M.; Bevilacqua, S.; Tretiach, M.; Alongi, G. Is the South-Mediterranean canopy-forming Ericaria giacconei (=Cystoseira hyblaea) a loser from ocean warming? Front. Mar. Sci. 2021, 8, 760637. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Pey, A.; Laurent, M.; Martins, G.M.; Airoldi, L.; Mangialajo, L. Threats to large brown algal forests in temperate seas: The overlooked role of native herbivorous fish. Sci. Rep. 2017, 7, 6012. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Ante, Z.; Mangialajo, L.; Antolić, B.; Kušpilić, G.; Ballesteros, E. Cartography of littoral rocky-shore communities (CARLIT) as a tool for ecological quality assessment of coastal waters in the Eastern Adriatic Sea. Ecol. Indic. 2013, 34, 87–93. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Mangialajo, L. Reduction of herbivorous fish pressure can facilitate focal algal species forestation on artificial structures. Mar. Environ. Res. 2018, 138, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gianni, F.; Mačić, V.; Bartolini, F.; Pey, A.; Laurent, M.; Mangialajo, L. Optimizing canopy-forming algae conservation and restoration with a new herbivorous fish deterrent device. Restor. Ecol. 2020, 28, 750–756. [Google Scholar] [CrossRef]
- Mulas, M.; Silverman, J.; Guy-Haim, T.; Noè, S.; Rilov, G. Thermal vulnerability of the Levantine endemic and endangered habitat-forming macroalga, Gongolaria rayssiae: Implications for reef carbon. Front. Mar. Sci. 2022, 9, 862332. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, L11706. [Google Scholar] [CrossRef]
- Pastor, F.; Valiente, J.A.; Khodayar, S. A warming Mediterranean: 38 years of increasing sea surface temperature. Remote Sens. 2020, 12, 2687. [Google Scholar] [CrossRef]
- Pisano, A.; Marullo, S.; Artale, V.; Falcini, F.; Yang, C.; Leonelli, F.E.; Santoleri, R.; Buongiorno Nardelli, B. New evidence of Mediterranean climate change and variability from sea surface temperature observations. Remote Sens. 2020, 12, 132. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Pitacco, V.; Slavinec, P.; Šiško, M.; Makovec, T.; Falace, A. First restoration experiment for Gongolaria barbata in Slovenian coastal waters. What can go wrong? Plants 2021, 10, 239. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Savonitto, G.; Asnaghi, V.; Trkov, D.; Pitacco, V.; Šiško, M.; Makovec, T.; Slavinec, P.; Lokovšek, A.; Ciriaco, S.; et al. Where and how—New insight for brown algal forest restoration in the Adriatic. Front. Mar. Sci. 2022, 9, 988584. [Google Scholar] [CrossRef]
- Lokovšek, A.; Pitacco, V.; Trkov, D.; Zamuda, L.L.; Falace, A.; Orlando-Bonaca, M. Keep It Simple: Improving the Ex Situ Culture of Cystoseira s.l. to Restore Macroalgal Forests. Plants 2023, 12, 2615. [Google Scholar] [CrossRef] [PubMed]
- Ogorelec, B.; Faganeli, J.; Mišič, M.; Čermelj, B. Reconstruction of paleoenvironment in the Bay of Koper (Gulf of Trieste, northern Adriatic). Annales 1997, 11, e200. [Google Scholar]
- Ogrin, D. Podnebje in izredni vremenski dogodki ob Tržaškem zalivu pred letom 1841. Geogr. Obz. 2012, 3, 23–30. [Google Scholar]
- Boicourt, W.; Licer, M.; Li, M.; Vodopivec, M.; Malacic, V. Sea State: Recent Progress in the Context of Climate Change. In Coastal Ecosystems in Transition: A Comparative Analysis of the Northern Adriatic and Chesapeake Bay; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 21–48. [Google Scholar]
- Bailey, R.G. Ecoregions, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Bićanić, Z. Undersurface Salinity Minimum Participation in the Process of Making Deep Adriatic Sea-water. Hrvat. Geogr. Glas. 1998, 60, 123–134. [Google Scholar]
- Mihanović, H.; Orlić, M.; Pasarić, Z. Diurnal thermocline oscillations driven by tidal flow around an island in the Middle Adriatic. J. Mar. Syst. 2009, 78, S157–S168. [Google Scholar] [CrossRef]
- Cosoli, S.; Ličer, M.; Vodopivec, M.; Malačič, V. Surface circulation in the Gulf of Trieste (northern Adriatic Sea) from radar, model, and ADCP comparisons. J. Geophys. Res. Ocean. 2013, 118, 6183–6200. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wilcox, R.R.; Erceg-Hurn, D.M.; Clark, F.; Carlson, M. Comparing two independent groups via the lower and upper quantiles. J. Stat. Comput. Simul. 2014, 84, 1543–1551. [Google Scholar] [CrossRef]
- Mair, P.; Wilcox, R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Clark, J.S.; Poore, A.G.B.; Coleman, M.A.; Doblin, M.A. Local Scale Thermal Environment and Limited Gene Flow Indicates Vulnerability of Warm Edge Populations in a Habitat Forming Macroalga. Front. Mar. Sci. 2020, 7, 711. [Google Scholar] [CrossRef]
- Coleman, M.A.; Wernberg, T. Forgotten underwater forests: The key role of fucoids on Australian temperate reefs. Ecol. Evol. 2017, 7, 8406–8418. [Google Scholar] [CrossRef]
- Straub, S.C.; Wernberg, T.; Thomsen, M.S.; Moore, P.J.; Burrows, M.T.; Harvey, B.P.; Smale, D.A. Resistance, Extinction, and Everything in Between—The Diverse Responses of Seaweeds to Marine Heatwaves. Front. Mar. Sci. 2019, 6, 473578. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar] [CrossRef] [PubMed]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a canopy-forming alga based on recruitment enhancement: Methods and long-term success assessment. Front. Plant Sci. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garcon, J.; Bennett, S.; Marbà, N.; Vergés, A.; Arthur, R.; Alcoverro, T. Tropicalization shifts herbivore pressure from seagrass to rocky reef communities. Proc. R. Soc. B Biol. Sci. 2023, 290, 20221744. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140846. [Google Scholar] [CrossRef] [PubMed]
- Voosen, P. Climate change spurs global speedup of ocean currents. Science 2020, 367, 612–613. [Google Scholar] [CrossRef]
- Yang, H.; Lohmann, G.; Krebs-Kanzow, U.; Ionita, M.; Shi, X.; Sidorenko, D.; Gong, X.; Chen, X.; Gowan, E.J. Poleward Shift of the Major Ocean Gyres Detected in a Warming Climate. Geophys. Res. Lett. 2020, 47, e2019GL085868. [Google Scholar] [CrossRef]
- Dwivedi, D.N.; Patil, G. Climate change: Prediction of solar radiation using advanced machine learning techniques. In Visualization Techniques for Climate Change with Machine Learning and Artificial Intelligence; Srivastav, A., Dubey, A., Kumar, A., Kumar Narang, S., Ali Khan, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 335–358. [Google Scholar]
- Jurgens, L.J.; Ashlock, L.W.; Gaylord, B. Facilitation alters climate change risk on rocky shores. Ecology 2022, 103, e03596. [Google Scholar] [CrossRef]
- Kaleb, S.; Sánchez de Pedro, R.; Bañares-España, E.; Alboresi, A.; Savonitto, G.; Natale, S.; Bevilacqua, S.; Falace, A. Cultivation of Gongolaria barbata (Fucales, Phaeophyceae) with a seaweed-derived biostimulant in order to improve photophysiological fitness and promote fertility to advance the restoration of marine macroalgal forests. J. Appl. Phycol. 2023, 35, 2337–2350. [Google Scholar] [CrossRef]
- Savonitto, G.; Alongi, G.; Falace, A. Reproductive phenology, zygote embryology and germling development of the threatened Carpodesmia barbatula (=Cystoseira barbatula) (Fucales, Phaeophyta) towards its possible restoration. Webbia 2019, 74, 317–323. [Google Scholar] [CrossRef]
- Falace, A.; Bressan, G. Seasonal Variations of Cystoseira barbata (Stackhouse) C. Agardh Frond Architecture. Hydrobiologia 2006, 555, 193–206. [Google Scholar] [CrossRef]
- Albritton, D.; Allen, M.; Alfons, P.; Baede, J.; Church, U.; Xiaosu, D.; Yihui, D.; Ehhalt, D.; Folland, C.; Giorgi, F. Climate Change 2001: The Scientific Basis, Summary for Policymakers. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. 2001. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/WGI_TAR_full_report.pdf (accessed on 10 February 2024).
- Malačič, V.; Celio, M.; Čermelj, B.; Bussani, A.; Comici, C. Interannual evolution of seasonal thermohaline properties in the Gulf of Trieste (northern Adriatic) 1991–2003. J. Geophys. Res. Ocean. 2006, 111. [Google Scholar] [CrossRef]
- Raicich Fabio, C.R.R. Trieste 1899-2015 near-surface sea temperature. SEANOE 2021, 11, 761–768. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Iñiguez, C.; Heinrich, S.; Harms, L.; Gordillo, F.J.L. Increased temperature and CO2 alleviate photoinhibition in Desmarestia anceps: From transcriptomics to carbon utilization. J. Exp. Bot. 2017, 68, 3971–3984. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.N.; Poong, S.W.; Gachon, C.; Brodie, J.; Sade, A.; Lim, P.E. Impact of elevated temperature on the physiological and biochemical responses of Kappaphycus alvarezii (Rhodophyta). PLoS ONE 2020, 15, e0239097. [Google Scholar] [CrossRef]
- Nati, J.J.H.; Lindström, J.; Halsey, L.G.; Killen, S.S. Is there a trade-off between peak performance and performance breadth across temperatures for aerobic scope in teleost fishes? Biol. Lett. 2016, 12, 20160191. [Google Scholar] [CrossRef]
- Eggert, A. Seaweed Responses to Temperature. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–66. [Google Scholar]
- Gouvêa, L.P.; Schubert, N.; Martins, C.D.L.; Sissini, M.; Ramlov, F.; Rodrigues, E.R.d.O.; Bastos, E.O.; Freire, V.C.; Maraschin, M.; Carlos Simonassi, J.; et al. Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnol. Oceanogr. 2017, 62, 2056–2075. [Google Scholar] [CrossRef]
- Jobling, M. Temperature tolerance and the final preferendum—Rapid methods for the assessment of optimum growth temperatures. J. Fish Biol. 1981, 19, 439–455. [Google Scholar] [CrossRef]
| 2-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Factor | Df | Sum Sq | Mean Sq | F Value | p-Value | Significance | |
| Temperature | 3 | 6,041,970 | 2,013,990 | 2274.1 | <0.0001 | *** | |
| Day | 12 | 490,429 | 40,869 | 46.15 | <0.0001 | *** | |
| Temperature × Day | 36 | 5,258,925 | 146,081 | 164.95 | <0.0001 | *** | |
| Residuals | 1742 | 1,542,751 | 886 | ||||
| Pairwise comparison | |||||||
| Day 2 | Day 5 | ||||||
| 15 °C | 18 °C | 24 °C | 15 °C | 18 °C | 24 °C | ||
| 18 °C | . | 18 °C | *** | ||||
| 24 °C | . | NS | 24 °C | *** | *** | ||
| 28 °C | NS | *** | ** | 28 °C | *** | *** | . |
| Day 7 | Day 12 | ||||||
| 15 °C | 18 °C | 24 °C | 15 °C | 18 °C | 24 °C | ||
| 18 °C | . | 18 °C | ** | ||||
| 24 °C | *** | *** | 24 °C | *** | *** | ||
| 28 °C | *** | *** | *** | 28 °C | *** | *** | . |
| Day 14 | Day 16 | ||||||
| 15 °C | 18 °C | 24 °C | 15 °C | 18 °C | 24 °C | ||
| 18 °C | * | 18 °C | *** | ||||
| 24 °C | *** | *** | 24 °C | *** | *** | ||
| 28 °C | *** | *** | NS | 28 °C | *** | *** | NS |
| Day | Temperature | Average Size [μm] ± SD | %Alive | %Deformed | %Dead |
|---|---|---|---|---|---|
| 2 | 15 | 93.4 ± 8.6 | 92 | 8 | 8 |
| 18 | 99.1 ± 15.4 | 58 | 0 | 42 | |
| 24 | 106.6 ± 22. 8 | 42 | 20 | 58 | |
| 28 | 90.5 ± 10.0 | 19 | 45 | 81 | |
| 5 | 15 | 130.0 ± 33.8 | 100 | 0 | 0 |
| 18 | 120.2 ± 35.8 | 100 | 0 | 0 | |
| 24 | 107.7 ± 20.2 | 52 | 38 | 48 | |
| 28 | 96.2 ± 11.0 | 21 | 10 | 79 | |
| 7 | 15 | 196.7 ± 24.9 | 100 | 0 | 0 |
| 18 | 182.6 ± 19.4 | 96 | 0 | 4 | |
| 24 | 102.4 ± 24.2 | 40 | 13 | 60 | |
| 28 | 98.2 ± 45.4 | 0 | 0 | 100 | |
| 12 | 15 | 252.5 ± 36.7 | 100 | 0 | 0 |
| 18 | 212.9 ± 35.3 | 100 | 0 | 0 | |
| 24 | 198.6 ± 58.8 | 15 | 18 | 75 | |
| 28 | 0 | 0 | 0 | 100 | |
| 14 | 15 | 367.9 ± 62.5 | 100 | 0 | 0 |
| 18 | 235.6 ± 42.7 | 100 | 0 | 0 | |
| 24 | 0 | 0 | 0 | 100 | |
| 28 | 0 | 0 | 0 | 100 | |
| 16 | 15 | 367.9 ± 62.5 | 100 | 0 | 0 |
| 18 | 235.6 ± 42.7 | 86 | 9 | 14 | |
| 24 | 0 | 0 | 0 | 100 | |
| 28 | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokovšek, A.; Pitacco, V.; Falace, A.; Trkov, D.; Orlando-Bonaca, M. Too Hot to Handle: Effects of Water Temperature on the Early Life Stages of Gongolaria barbata (Fucales). J. Mar. Sci. Eng. 2024, 12, 514. https://doi.org/10.3390/jmse12030514
Lokovšek A, Pitacco V, Falace A, Trkov D, Orlando-Bonaca M. Too Hot to Handle: Effects of Water Temperature on the Early Life Stages of Gongolaria barbata (Fucales). Journal of Marine Science and Engineering. 2024; 12(3):514. https://doi.org/10.3390/jmse12030514
Chicago/Turabian StyleLokovšek, Ana, Valentina Pitacco, Annalisa Falace, Domen Trkov, and Martina Orlando-Bonaca. 2024. "Too Hot to Handle: Effects of Water Temperature on the Early Life Stages of Gongolaria barbata (Fucales)" Journal of Marine Science and Engineering 12, no. 3: 514. https://doi.org/10.3390/jmse12030514
APA StyleLokovšek, A., Pitacco, V., Falace, A., Trkov, D., & Orlando-Bonaca, M. (2024). Too Hot to Handle: Effects of Water Temperature on the Early Life Stages of Gongolaria barbata (Fucales). Journal of Marine Science and Engineering, 12(3), 514. https://doi.org/10.3390/jmse12030514









