A Physiological Analysis of Desiccation Stress in the Green Tide Species Ulva stenophylloides and Ulva uncialis in the South Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Seaweed Collection and Environmental Parameter Measurements
2.2. Species Identification for Sample Selection
2.3. Experimental Design
2.4. Influence of Desiccation on Specific Growth Rate
2.5. Influence of Desiccation on Lipid Peroxidation
2.6. Influence of Desiccation on Cell Viability
2.7. Data Analyses
3. Results
3.1. Environmental Parameters at the Sampling Site
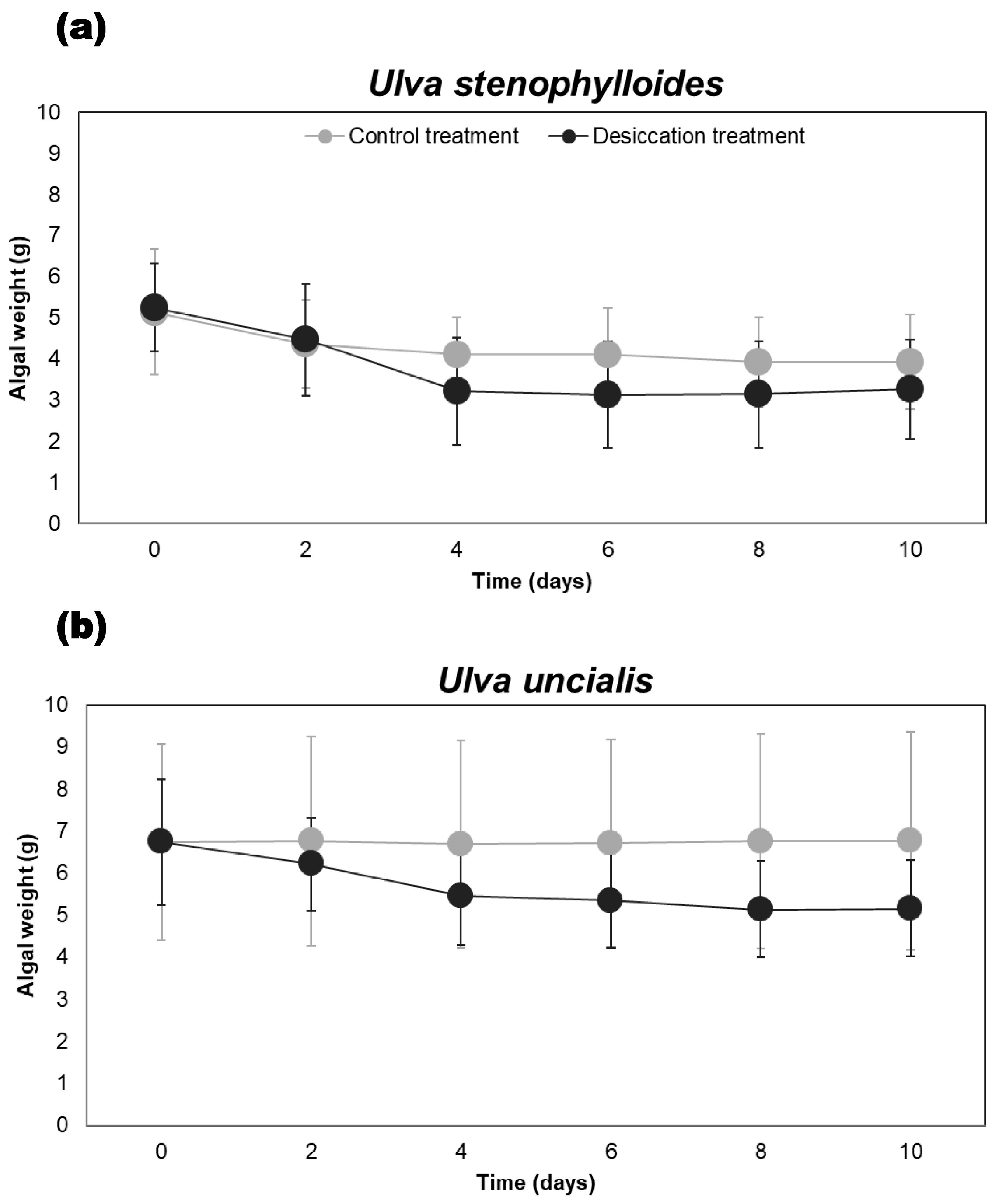
3.2. Influence of Desiccation on the Average Algal Weight
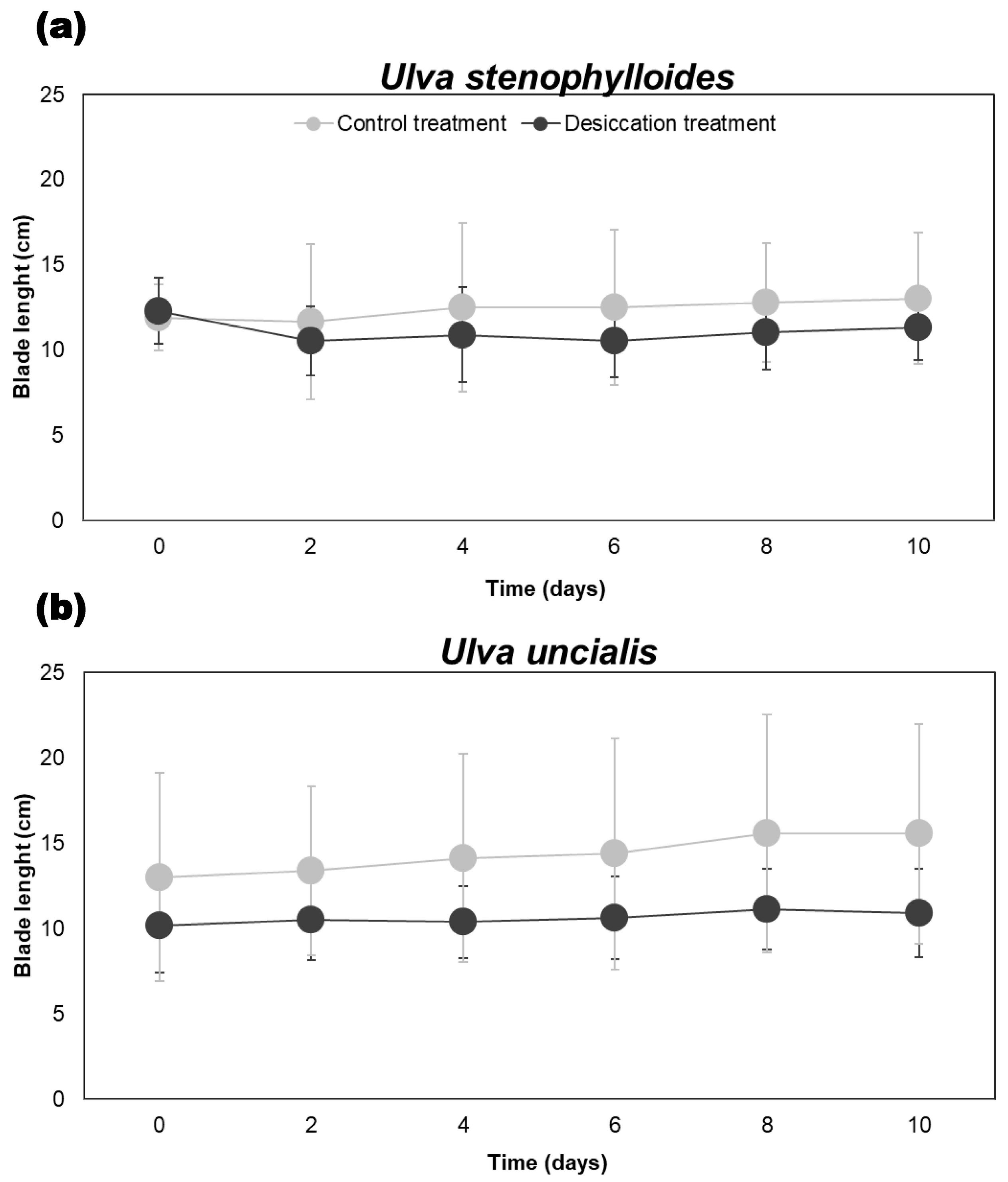
3.3. Influence of Desiccation on the Average Blade Length
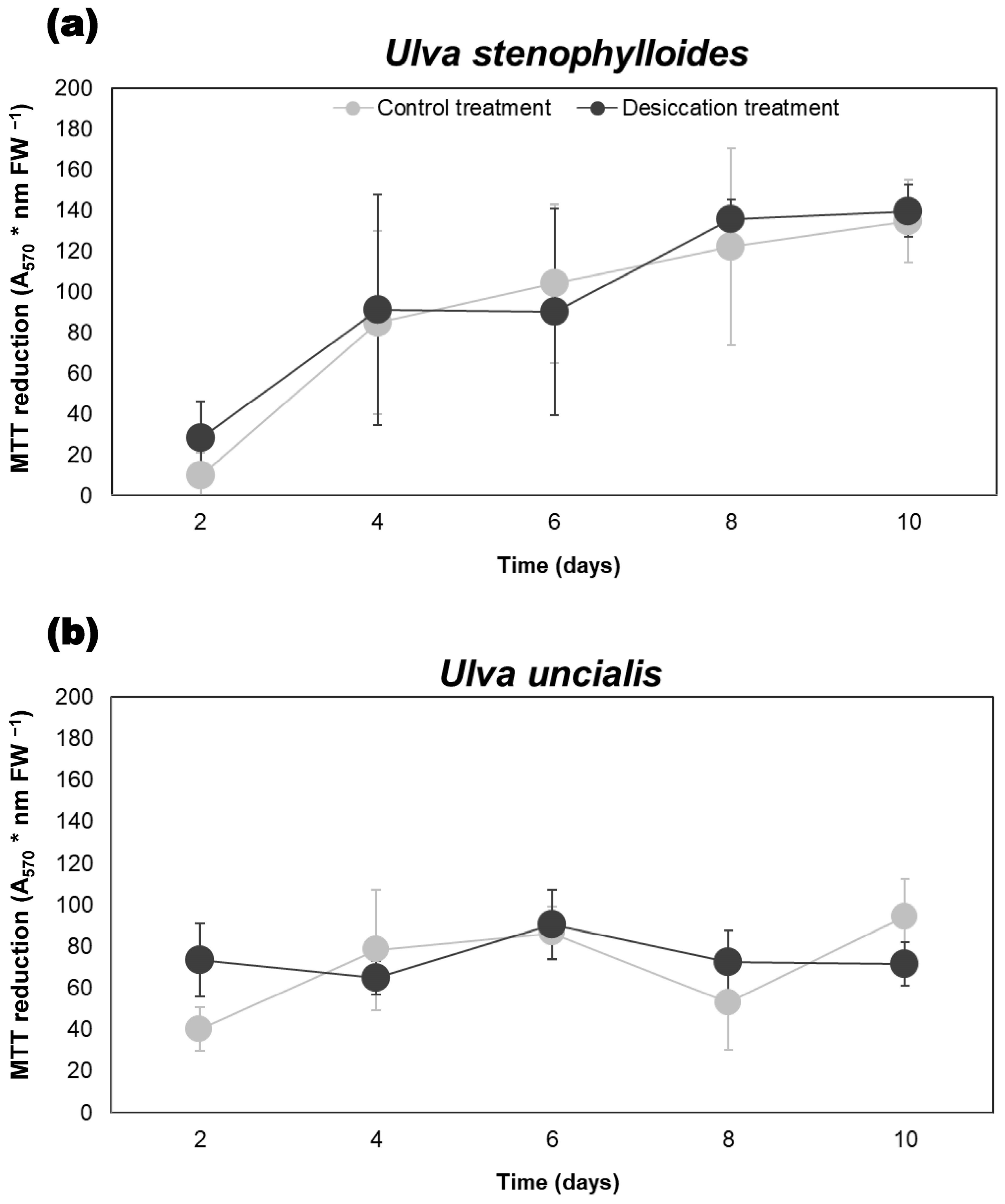
3.4. Influence of Desiccation on Cellular Viability Based on the MTT Assay
3.5. Influence of Desiccation on Lipid Peroxidation Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Salinity and desiccation induced oxidative stress acclimation in seaweeds. Adv. Bot. Res. 2014, 71, 91–123. [Google Scholar]
- Contreras-Porcia, L.; Meynard, A.; Piña, F.; Kumar, M.; Lovazzano, C.; Núñez, A.; Flores-Molina, M.R. Desiccation stress tolerance in Porphyra and Pyropia species: A latitudinal analysis along the Chilean coast. Plants 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2011, 62, 1815–1829. [Google Scholar] [CrossRef]
- Xie, X.; Gao, S.; Gu, W.; Pan, G.; Wang, G. Desiccation induces accumulations of antheraxanthin and zeaxanthin in intertidal macro-alga Ulva pertusa (Chlorophyta). PLoS ONE 2013, 8, e72929. [Google Scholar] [CrossRef]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, K.; Tanaka, J. Photosynthetic recovery of desiccated intertidal seaweeds after rehydration. Prog. Nat. Sci. 2005, 15, 689–693. [Google Scholar]
- Kumar, M.; Gupta, V.; Trivedi, N.; Kumari, P.; Bijo, A.J.; Reddy, C.R.K.; Jha, B. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ. Exp. Bot. 2011, 72, 194–201. [Google Scholar] [CrossRef]
- Papathanasiou, V.; Kariofillidou, G.; Malea, P.; Orfanidis, S. Effects of air exposure on desiccation and photosynthetic performance of Cymodocea nodosa with and without epiphytes and Ulva rigida in comparison, under laboratory conditions. Mar. Environ. Res. 2020, 158, 104948. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, X.; Wang, Y.; Fan, X.; Miao, Y.; Ye, N.; Zhuang, Z. Responses of photosynthesis and nitrogen assimilation in the green-tide macroalga Ulva prolifera to desiccation. Mar. Biol. 2016, 163, 9. [Google Scholar] [CrossRef]
- Nelson, T.A.; Olson, J.; Imhoff, L.; Nelson, A.V. Aerial exposure and desiccation tolerances are correlated to species composition in “green tides” of the Salish Sea (northeastern Pacific). Bot. Mar. 2010, 53, 103–111. [Google Scholar] [CrossRef]
- Gao, S.; Shen, S.; Wang, G.; Niu, J.; Lin, A.; Pan, G. PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol. 2011, 52, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Bukhov, N.; Egorova, E.; Carpentier, R. Electron flow to photosystem I from stromal reductants in vivo: The size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 2002, 215, 812–820. [Google Scholar] [CrossRef]
- Gao, S.; Niu, J.; Chen, W.; Wang, G.; Xie, X.; Pan, G.; Gu, W.; Zhu, D. The physiological links of the increased photosystem II activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and re-hydration. Photosynth. Res. 2013, 116, 45–54. [Google Scholar] [CrossRef]
- Huan, L.; Xie, X.; Zheng, Z.; Sun, F.; Wu, S.; Li, M.; Gao, S.; Gu, W.; Wang, G. Positive correlation between PSI response and oxidative pentose phosphate pathway activity during salt stress in an intertidal macroalga. Plant Cell Physiol. 2014, 55, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Herburger, K.; Kaplan, F.; Lewis, L.A. Desiccation tolerance in the chlorophyte green alga Ulva compressa: Does cell wall architecture contribute to ecological success? Planta 2015, 242, 477–492. [Google Scholar] [CrossRef]
- Murthy, M.S.; Sharma, C.L.N.S. Peroxidase Activity in Ulva lactuca under Desiccation. Bot. Mar. 1989, 32, 511–513. [Google Scholar] [CrossRef]
- Flores-Molina, M.R.; Thomas, D.; Lovazzano, C.; Nuñez, A.; Zapata, J.; Kumar, M.; Correa, J.A.; Contreras-Porcia, L. Desiccation stress in intertidal seaweeds: Effects on morphology, antioxidant responses and photosynthetic performance. Aquat. Bot. 2014, 113, 90–99. [Google Scholar] [CrossRef]
- Yoshida, G.; Uchimura, M.; Hiraoka, M. Persistent occurrence of floating Ulva green tide in Hiroshima Bay, Japan: Seasonal succession and growth patterns of Ulva pertusa and Ulva spp. (Chlorophyta, Ulvales). Hydrobiologia 2015, 758, 223–233. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, J.; Zhao, S.; Sun, Y.; Cui, Q.; Wu, L.; Gao, S.; Zhang, J.; He, P. Review of the development of the green tide and the process of control in the southern Yellow Sea in 2022. Estuar. Coast. Shelf Sci. 2024, 302, 108772. [Google Scholar] [CrossRef]
- Mutizabal-Aros, J.; Ramírez, M.E.; Haye, P.A.; Meynard, A.; Pinilla-Rojas, B.; Núñez, A.; Latorre-Padilla, N.; Search, F.V.; Tapia, F.J.; Saldías, G.S.; et al. Morphological and Molecular Identification of Ulva spp. (Ulvophyceae; Chlorophyta) from Algarrobo Bay, Chile: Understanding the Composition of Green Tides. Plants 2024, 13, 1258. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2024; Available online: https://www.algaebase.org (accessed on 16 August 2024).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Caroca-Valencia, S.; Rivas, J.; Araya, M.; Núñez, A.; Piña, F.; Toro-Mellado, F.; Contreras-Porcia, L. Indoor and Outdoor Cultures of Gracilaria chilensis: Determination of Biomass Growth and Molecular Markers for Biomass Quality Evaluation. Plants 2023, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, M.; Lee, T. 2, 3, 5-Triphenyltetrazolium reduction in the viability assay of Ulva fasciata (Chlorophyta) in response to salinity stress. Bot. Bull. Acad. Sin. 1999, 40, 207–212. [Google Scholar]
- Burritt, D.J.; Larkindale, J.; Hurd, C.L. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 2002, 215, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Gao, G.; Beardall, J.; Bao, M.; Wang, C.; Ren, W.; Xu, J. Ocean acidification and nutrient limitation synergistically reduce growth and photosynthetic performances of a green tide alga Ulva linza. Biogeosciences 2018, 15, 3409–3420. [Google Scholar] [CrossRef]
- Sun, J.; Dai, W.; Zhao, S.; Liu, J.; Zhang, J.; Xu, J.; He, P. Response to the CO2 concentrating mechanisms and transcriptional time series analysis of Ulva prolifera under inorganic carbon limitation. Harmful Algae 2024, 139, 102727. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, Z.; Zhou, X.; Xu, J. Changes in morphological plasticity of Ulva prolifera under different environmental conditions: A laboratory experiment. Harmful Algae 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J.; Huo, Y.; Zhou, L.; Wu, Q.; Chen, L.; Yu, K.; He, P. Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar. Pollut. Bull. 2015, 101, 660–666. [Google Scholar] [CrossRef]
- Largo, D.B.; Sembrano, J.; Hiraoka, M.; Ohno, M. Taxonomic and ecological profile of ‘green tide’ species of Ulva (Ulvales, Chlorophyta) in central Philippines. Hydrobiologia 2004, 512, 247–253. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Mao, Y.; Liang, C.; Zhuang, Z.; Wang, Q.; Ye, N. Somatic cells serve as a potential propagule bank of Enteromorpha prolifera forming a green tide in the Yellow Sea, China. J. Appl. Phycol. 2010, 22, 173–180. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Mao, Y.; Li, Y.; Xue, S.; Zou, J.; Lian, W.; Liang, C.; Zhuang, Z.; Wang, Q.; et al. Settlement of vegetative fragments of Ulva prolifera confirmed as an important seed source for succession of a large-scale green tide bloom. Limnol. Oceanogr. 2011, 56, 233–242. [Google Scholar] [CrossRef]
- Cao, J.; Liu, J.; Zhao, S.; Tong, Y.; Li, S.; Xia, Z.; Hu, M.; Sun, Y.; Zhang, J.; He, P. Advances in the research on micropropagules and their role in green tide outbreaks in the Southern Yellow Sea. Mar. Pollut. Bull. 2023, 188, 114710. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, Y.; Zeng, Y.; Sun, Y.; Cui, Q.; Chen, Z.; Liu, J.; Zhang, J.; He, P. Temporal succession of micropropagules during accumulation and dissipation of green tide algae: A case study in Rudong coast, Jiangsu Province. Mar. Environ. Res. 2024, 202, 106719. [Google Scholar] [CrossRef]
- Taylor, R.; Fletcher, R.L.; Raven, J.A. Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 2001, 44, 327–336. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, C.Y.; Cho, K.H. The fitness trade-off between growth and stress resistance determines the phenotypic landscape. BMC Biol. 2024, 22, 62. [Google Scholar] [CrossRef]
- Zou, D.; Gao, K. The photosynthetic and respiratory responses to temperature and nitrogen supply in the marine green macroalga Ulva conglobata (Chlorophyta). Phycologia 2014, 53, 86–94. [Google Scholar] [CrossRef]
- Bandehagh, A.; Taylor, N.L. Can alternative metabolic pathways and shunts overcome salinity induced inhibition of central carbon metabolism in crops? Front. Plant Sci. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Gupta, V.; Kushwaha, H.R. Metabolic regulatory oscillations in intertidal green seaweed Ulva lactuca against tidal cycles. Sci. Rep. 2017, 7, 16430. [Google Scholar] [CrossRef]


| Species | Treatment | Response Variable | R2 | F | Probability (p) | Slope |
|---|---|---|---|---|---|---|
| Ulva stenophylloides | Control | Algal weight | 0.07698 | 5.42 | 0.02383 * | −0.10563 |
| Ulva stenophylloides | Desiccation | Algal weight | 0.2074 | 14.87 | 0.0003193 * | −0.19892 |
| Ulva uncialis | Control | Algal weight | −0.01922 | 0.0002951 | 0.9864 | 0.01635 |
| Ulva uncialis | Desiccation | Algal weight | 0.1859 | 13.11 | 0.0006672 * | −0.17054 |
| Species & Parameter | Source of Variation | df | MS | F | Probability (p) |
|---|---|---|---|---|---|
| Ulva stenophylloides Algal weight | Time (days) | 1 | 29.218 | 19.650 | 2.31 × 10−5 *** |
| Treatment | 1 | 7.048 | 4.740 | 0.0317 * | |
| Treatment × Time | 1 | 2.741 | 1.844 | 0.1775 | |
| Error | 104 | 1.487 | |||
| Ulva uncialis Algal weight | Time (days) | 1 | 8.987 | 2.530 | 0.11474 |
| Treatment | 1 | 28.882 | 8.131 | 0.00525 * | |
| Treatment × Time | 1 | 9.338 | 2.629 | 0.10797 | |
| Error | 104 | 3.552 | |||
| Ulva stenophylloides Blade length | Time (days) | 1 | 1.93 | 0.178 | 0.6742 |
| Treatment | 1 | 44.98 | 4.145 | 0.0443 * | |
| Treatment × Time | 1 | 10.12 | 0.932 | 0.3365 | |
| Error | 104 | 10.85 | |||
| Ulva uncialis Blade length | Time (days) | 1 | 41.0 | 1.977 | 0.163 |
| Treatment | 1 | 374.8 | 18.055 | 4.69 × 10−5 *** | |
| Treatment × Time | 1 | 12.2 | 0.590 | 0.444 | |
| Error | 104 | 20.8 |
| Species | Treatment | Response Variable | R2 | F | Probability (p) | Slope |
|---|---|---|---|---|---|---|
| Ulva stenophylloides | Control | Blade length | −0.0073 | 0.6155 | 0.436 | 0.1287 |
| Ulva stenophylloides | Desiccation | Blade length | 0.0065 | 0.3385 | 0.563 | −0.0505 |
| Ulva uncialis | Control | Blade length | 0.0068 | 1.3620 | 0.249 | 0.2790 |
| Ulva uncialis | Desiccation | Blade lenght | −0.0044 | 0.7691 | 0.385 | 0.0819 |
| Species | Treatment | Response Variable | R2 | F | Probability (p) | Slope |
|---|---|---|---|---|---|---|
| Ulva stenophylloides | Control | MTT | 0.5604 | 18.85 | 0.0007997 * | 14.382 |
| Ulva stenophylloides | Desiccation | MTT | 0.5457 | 17.82 | 0.000999 * | 13.376 |
| Ulva uncialis | Control | MTT | 0.1895 | 4.274 | 0.05921 | 4.132 |
| Ulva uncialis | Desiccation | MTT | −0.0761 | 0.009928 | 0.9221 | 0.1781 |
| Especie | Treatment | Response Variable | R2 | F | Probability (p) | Slope |
|---|---|---|---|---|---|---|
| Ulva stenophylloides | Control | Lipoperoxides | −0.07062 | 0.07651 | 0.7864 | −4.88 × 10−5 |
| Ulva stenophylloides | Desiccation | Lipoperoxides | 0.147 | 3.413 | 0.08757 | −0.000191 |
| Ulva uncialis | Control | Lipoperoxides | −0.04339 | 0.478 | 0.5293 | −6.54 × 10−5 |
| Ulva uncialis | Desiccation | Lipoperoxides | 0.05249 | 1.775 | 0.2056 | −1.11 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutizabal-Aros, J.; Meynard, A.; Contreras-Porcia, L. A Physiological Analysis of Desiccation Stress in the Green Tide Species Ulva stenophylloides and Ulva uncialis in the South Pacific. J. Mar. Sci. Eng. 2024, 12, 1893. https://doi.org/10.3390/jmse12111893
Mutizabal-Aros J, Meynard A, Contreras-Porcia L. A Physiological Analysis of Desiccation Stress in the Green Tide Species Ulva stenophylloides and Ulva uncialis in the South Pacific. Journal of Marine Science and Engineering. 2024; 12(11):1893. https://doi.org/10.3390/jmse12111893
Chicago/Turabian StyleMutizabal-Aros, Javiera, Andrés Meynard, and Loretto Contreras-Porcia. 2024. "A Physiological Analysis of Desiccation Stress in the Green Tide Species Ulva stenophylloides and Ulva uncialis in the South Pacific" Journal of Marine Science and Engineering 12, no. 11: 1893. https://doi.org/10.3390/jmse12111893
APA StyleMutizabal-Aros, J., Meynard, A., & Contreras-Porcia, L. (2024). A Physiological Analysis of Desiccation Stress in the Green Tide Species Ulva stenophylloides and Ulva uncialis in the South Pacific. Journal of Marine Science and Engineering, 12(11), 1893. https://doi.org/10.3390/jmse12111893







