The Development of a Floating Mono-Particle “Sun Shield” to Protect Corals from High Irradiance during Bleaching Conditions
Abstract
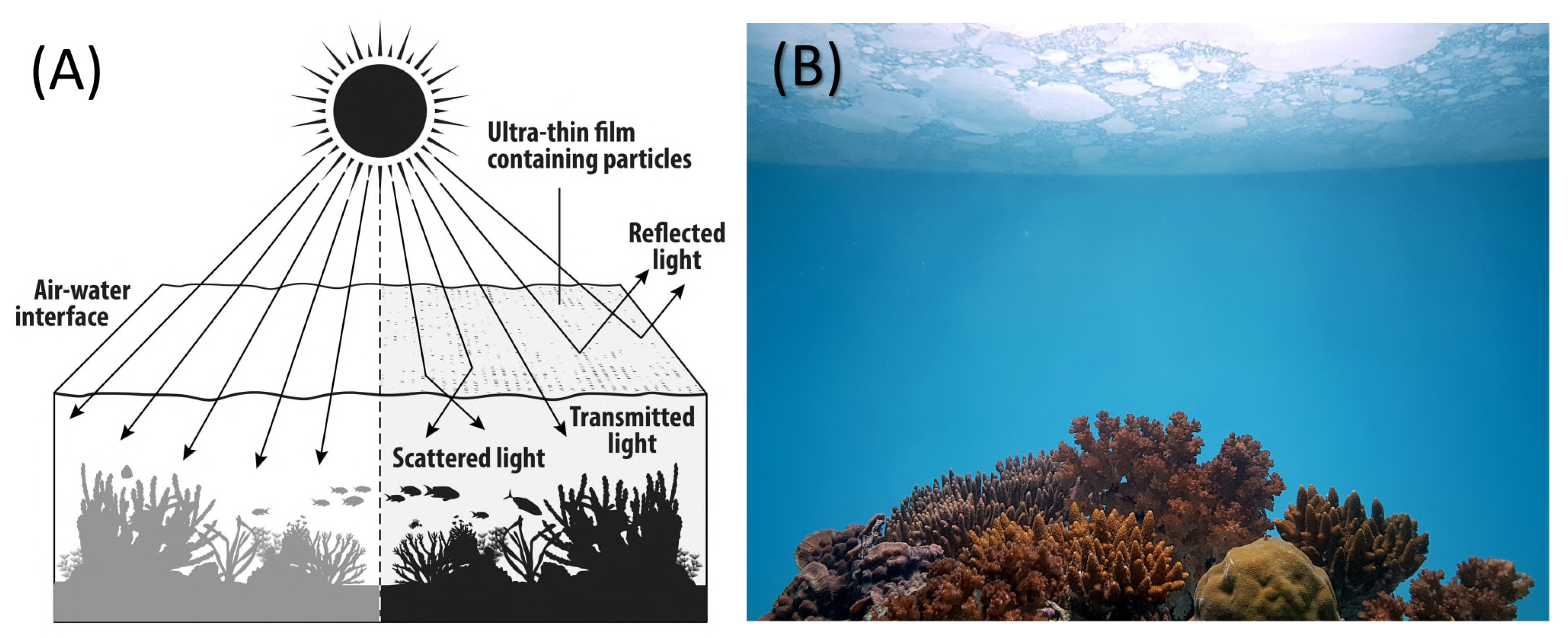
1. Introduction
1.1. Influence of Thermal Stress and Solar Radiation on Coral Bleaching
1.2. Shading Interventions to Reduce Bleaching
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mono-Particle Films
2.3. Light Microscope Imaging
2.4. Small-Scale Light Attenuation Measurements of Mono-Particle Films
2.5. Outdoor Small-Tank Trials
2.6. Outdoor Large-Tank Attenuation
2.7. Large-Scale Sea Trials
2.8. Modelling the Response of Coral Reefs to Shading
3. Results and Discussion
- Part 1: Proof of concept and initial development
- Mono-particle layer formation
- Influence of particle size on light attenuation properties
- Impact of particle surface coverage on light attenuation
- Influence of particle/stearic acid ratio
- Part 2: Development of solvent-free formulations
- Choice of monolayer material
- Film optimisation
- Part 3: Outdoor and larger scale trials
- Small-scale outdoor light attenuation trials
- Large-scale outdoor light attenuation trials
- Open-sea deployment of formulation
- Part 4. Modelling shading responses in corals
- Part 5. Potential benefits, issues, and future directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in coral reefs. In Ecosystems of the World, 25. Coral Reefs; Dubinsky, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 75–97. [Google Scholar]
- Suggett, D.J.; Smith, D.J. Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Change Biol. 2020, 26, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Cantin, N.; James, N.; Stella, J. Aerial Surveys of the 2024 Mass Coral Bleaching Event on the Great Barrier Reef. Australian Institute of Marine Science, Townsville. 2024. Available online: https://www.aims.gov.au/sites/default/files/2024-04/FINAL-Aerial%20Bleaching%20GBR2024Report_AIMS_Final_15Apr2024.pdf (accessed on 24 May 2024).
- Emslie, M.J.; Ceccarelli, D.M.; Logan, M.; Blandford, M.I.; Bray, P.; Campili, A.; Jonker, M.J.; Parker, J.G.; Prenzlau, T.; Sinclair-Taylor, T.H. Changing dynamics of Great Barrier Reef hard coral cover in the Anthropocene. Coral Reefs 2024, 43, 747–762. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Lesser, M.P. Coral bleaching: Causes and mechanisms. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Berlin, Germany, 2011; pp. 405–419. [Google Scholar]
- Courtial, L.; Roberty, S.; Shick, J.M.; Houlbrèque, F.; Ferrier-Pagès, C. Interactive effects of ultraviolet radiation and thermal stress on two reef-building corals. Limnol. Oceanogr. 2017, 62, 1000–1013. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Glynn, P.W. Coral reef bleaching: Ecological perspectives. Coral Reefs 1993, 12, 1–17. [Google Scholar] [CrossRef]
- Hill, R.; Ralph, P.J. Photosystem II heterogeneity of in hospite zooxanthellae in scleractinian corals exposed to bleaching conditions. Photochem. Photobiol. 2006, 82, 1577–1585. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O.; Larkum, A.W.D.; Schreiber, U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998, 21, 1219–1230. [Google Scholar] [CrossRef]
- Lesser, M.P.; Farrell, J.H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 2004, 23, 367–377. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Johnson, J.A.; Smithers, S.G. Nearshore turbid-zone corals exhibit high bleaching tolerance on the Great Barrier Reef following the 2016 ocean warming event. Front. Mar. Sci. 2017, 4, 224. [Google Scholar] [CrossRef]
- Carlson, R.R.; Li, J.; Crowder, L.B.; Asner, G.P. Large-scale effects of turbidity on coral bleaching in the Hawaiian islands. Front. Mar. Sci. 2022, 9, 969472. [Google Scholar] [CrossRef]
- Fisher, R.; Bessell-Browne, P.; Jones, R. Synergistic and antagonistic impacts of suspended sediments and thermal stress on corals. Nat. Commun. 2019, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.C.; Lima, I.C.; Garcia, T.M.; Tavares, T.C.L.; Carneiro, P.B.M.; Teixeira, C.E.P.; Bejarano, S.; Rossi, S.; Soares, M.O. Turbidity buffers coral bleaching under extreme wind and rainfall conditions. Mar. Environ. Res. 2023, 192, 106215. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, C.; van Woesik, R. Climate-change refugia: Shading reef corals by turbidity. Glob. Change Biol. 2016, 22, 1145–1154. [Google Scholar] [CrossRef]
- Sully, S.; van Woesik, R. Turbid reefs moderate coral bleaching under climate-related temperature stress. Glob. Change Biol. 2020, 26, 1367–1373. [Google Scholar] [CrossRef]
- Mumby, P.J.; Chisholm, J.R.M.; Edwards, A.L.; Andrefouet, S.; Jaubert, J. Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Mar. Ecol. Prog. Ser. 2001, 222, 209–216. [Google Scholar] [CrossRef]
- Gonzalez-Espinosa, P.C.; Donner, S.D. Cloudiness reduces the bleaching response of coral reefs exposed to heat stress. Glob. Change Biol. 2021, 27, 3474–3486. [Google Scholar] [CrossRef]
- Hoogenboom, M.O.; Frank, G.E.; Chase, T.J.; Jurriaans, S.; Álvarez-Noriega, M.; Peterson, K.; Critchell, K.; Berry, K.L.; Nicolet, K.J.; Ramsby, B. Environmental drivers of variation in bleaching severity of Acropora species during an extreme thermal anomaly. Front. Mar. Sci. 2017, 4, 376. [Google Scholar] [CrossRef]
- van Woesik, R.; McCaffrey, K.R. Repeated thermal stress, shading, and directional selection in the Florida reef tract. Front. Mar. Sci. 2017, 4, 182. [Google Scholar] [CrossRef]
- Smith, H.A.; Prenzlau, T.; Whitman, T.; Fulton, S.E.; Borghi, S.; Logan, M.; Heron, S.F.; Bourne, D.G. Macroalgal canopies provide corals limited protection from bleaching and impede post-bleaching recovery. J. Exp. Mar. Biol. Ecol. 2022, 553, 151762. [Google Scholar] [CrossRef]
- Stewart, H.A.; Kline, D.I.; Chapman, L.J.; Altieri, A.H. Caribbean mangrove forests act as coral refugia by reducing light stress and increasing coral richness. Ecosphere 2021, 12, e03413. [Google Scholar] [CrossRef]
- Tagliafico, A.; Baker, P.; Kelaher, B.; Ellis, S.; Harrison, D. The Effects of Shade and Light on Corals in the Context of Coral Bleaching and Shading Technologies. Front. Mar. Sci. 2022, 9, 919382. [Google Scholar] [CrossRef]
- Coelho, V.; Fenner, D.; Caruso, C.; Bayles, B.; Huang, Y.; Birkeland, C. Shading as a mitigation tool for coral bleaching in three common Indo-Pacific species. J. Exp. Mar. Biol. Ecol. 2017, 497, 152–163. [Google Scholar] [CrossRef]
- Butcherine, P.; Tagliafico, A.; Ellis, S.L.; Kelaher, B.P.; Hendrickson, C.; Harrison, D. Intermittent shading can moderate coral bleaching on shallow reefs. Front. Mar. Sci. 2023, 10, 1162896. [Google Scholar] [CrossRef]
- Coles, S.L.; Jokiel, P.L. Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar. Biol. 1978, 49, 187–195. [Google Scholar] [CrossRef]
- Hendrickson, C.; Butcherine, P.; Tagliafico, A.; Ellis, S.L.; Harrison, D.P.; Kelaher, B.P. Combining shading and lipid-enriched diets as an adaption tool to reduce coral bleaching. J. Exp. Mar. Biol. Ecol. 2024, 572, 151988. [Google Scholar] [CrossRef]
- Harrison, D.P. An Overview of Environmental Engineering Methods for Reducing Coral Bleaching Stress. In Oceanographic Processes of Coral Reefs; CRC Press: Boca Raton, FL, USA, 2024; pp. 403–418. [Google Scholar]
- Seitz, R. Bright water: Hydrosols, water conservation and climate change. Clim. Change 2011, 105, 365–381. [Google Scholar] [CrossRef]
- Russell, L.; Sorooshian, A.; Seinfeld, J.; Albrecht, B.; Nenes, A.; Ahlm, L.; Chen, Y.; Coggon, M.; Craven, J.; Flagan, R. Eastern Pacific Emitted Aerosol Cloud Experiment (E-PEACE). Am. Meteorol. Soc. 2012, 94, 709–729. [Google Scholar] [CrossRef]
- Mazoyer, M.; Burnet, F.; Denjean, C. Experimental study on the evolution of droplet size distribution during the fog life cycle. Atmos. Chem. Phys. 2022, 22, 11305–11321. [Google Scholar] [CrossRef]
- Salter, S.; Sortino, G.; Latham, J. Sea-going hardware for the cloud albedo method of reversing global warming. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Latham, J.; Kleypas, J.; Hauser, R.; Parkes, B.; Gadian, A. Can marine cloud brightening reduce coral bleaching? Atmos. Sci. Lett. 2013, 14, 214–219. [Google Scholar] [CrossRef]
- Tollefson, J. Can artificially altered clouds save the Great Barrier Reef? Nature 2021, 596, 476–478. [Google Scholar] [CrossRef]
- Harrison, D.P.; Harrison, L.; Baird, M.; Utembe, S.; Schofield, R.; Escobar Correa, R.; Mongin, M.; Rizwi, F. Environmental Modelling of Large Scale Solar Radiation Management: A Report Provided to the Australian Government by the Reef Restoration and Adaptation Program. Australian Institute of Marine Science. 2019. Available online: https://gbrrestoration.org/wp-content/uploads/2020/09/T14-Environmental-Modelling-of-Large-Scale-SRM_v3.03-3.pdf (accessed on 24 May 2024).
- Jones, R.; Bessell Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Myers, L.E.; Frasier, G.W. Evaporation reduction with floating granular materials. J. Irrig. Drain. Div. 1970, 96, 425–436. [Google Scholar] [CrossRef]
- Prime, E.L.; Tran, D.N.H.; Plazzer, M.; Sunartio, D.; Leung, A.H.M.; Yiapanis, G.; Baoukina, S.; Yarovsky, I.; Qiao, G.G.; Solomon, D.H. Rational design of monolayers for improved water evaporation mitigation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 47–58. [Google Scholar] [CrossRef]
- Barnes, G.T. The potential for monolayers to reduce the evaporation of water from large water storages. Agric. Water Manag. 2008, 95, 339–353. [Google Scholar] [CrossRef]
- Machida, S.; Mineta, S.; Fujimori, A.; Nakahara, H. Retardation of water evaporation by less-defective mixed monolayers spread from bulk solids onto water surface. J. Colloid Interface Sci. 2003, 260, 135–141. [Google Scholar] [CrossRef]
- Brink, G.N.; Wandel, A.P.; Hancock, N.H.; Pather, S. Spreading rate and dispersion behavior of evaporation-suppressant monolayer on open water surfaces: Part 1—At zero wind stress. Exp. Therm. Fluid Sci. 2017, 87, 182–190. [Google Scholar] [CrossRef]
- Lange, P.; Hühnerfuss, H. Drift Response of Monomolecular Slicks to Wave and Wind Action. J. Phys. Oceanogr. 1978, 8, 142–150. [Google Scholar] [CrossRef]
- Deo, A.V.; Kulkarni, S.B.; Gharpurey, M.K.; Biswas, A.B. Rate of spreading of long-chain n-alcohols and n-alkoxyethanols from solid into monolayer on water. J. Colloid Sci. 1964, 19, 820–830. [Google Scholar] [CrossRef]
- Baird, M.; Mongin, M.; Rizwi, F.; Bay, L.; Cantin, N.; Soja Woźniak, M.; Skerratt, J. A mechanistic model of coral bleaching due to temperature-mediated light-driven reactive oxygen build-up in zooxanthellae. A Ecol. Model. 2018, 386, 20–37. [Google Scholar] [CrossRef]
- Steven, A.D.L.; Baird, M.; Brinkman, R.; Car, N.; Cox, S.; Herzfeld, M.; Hodge, J.; Jones, E.; King, E.; Margvelashvili, N.; et al. eReefs: An operational information system for managing the Great Barrier Reef. J. Oper. Oceanogr. 2019, 12, S12–S28. [Google Scholar] [CrossRef]
- Baird, M.E.; Green, R.; Lowe, R.; Mongin, M.; Bougeot, E. Optimising cool-water injections to reduce thermal stress on coral reefs of the Great Barrier Reef. PLoS ONE 2020, 15, e0239978. [Google Scholar] [CrossRef]
- Balch, W.M.; Kilpatrick, K.A.; Holligan, P.; Harbour, D.; Fernandez, E. The 1991 coccolithophore bloom in the central North Atlantic. 2. Relating optics to coccolith concentration. Limnol. Oceanogr. 1996, 41, 1684–1696. [Google Scholar] [CrossRef]
- Baker, E.T.; Lavelle, J.W. The effect of particle size on the light attenuation coefficient of natural suspensions. J. Geophys. Res. Ocean. 1984, 89, 8197–8203. [Google Scholar] [CrossRef]
- Lockwood, D.J. Rayleigh and Mie Scattering. In Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–12. [Google Scholar]
- Jalal, I.M.; Zografi, G.; Rakshit, A.K.; Gunstone, F.D. Monolayer properties of fatty acids. I. Thermodynamics of spreading. J. Colloid Interface Sci. 1980, 76, 146–156. [Google Scholar] [CrossRef]
- Boyd, G.E.; Schubert, J. Energy Relations in Unimolecular Film Formation: The Spreading of Cetyl Alcohol and Palmitic Acid on Aqueous Surfaces. J. Phys. Chem. 1957, 61, 1271–1275. [Google Scholar] [CrossRef]
- He, Y.; Sun, C.; Li, W.; Yang, G.-P.; Ding, H. Degradation of lipids in seasonal hypoxic seawater under different oxygen saturation. J. Oceanol. Limnol. 2018, 36, 1570–1585. [Google Scholar] [CrossRef]
- Mudge, S.M. Fatty Alcohols: Anthropogenic and Natural Occurrence in the Environment; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Mongin, M.; Baird, M.; Hadley, S.; Lenton, A. Optimising reef-scale CO2 removal by seaweed to buffer ocean acidification. Environ. Res. Lett. 2016, 11, 034023. [Google Scholar] [CrossRef]
- Dickey, T.; Ma, K. Inherent optical properties and irradiance. In Elements of Physical Oceanography: A Derivative of the Encyclopedia of Ocean Sciences, 1st ed.; Steele, J.H., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 1990, 8, 225. [Google Scholar] [CrossRef]
- Zhang, Z.; Jones, A.; Crabbe, M.J.C. Impacts of stratospheric aerosol geoengineering strategy on Caribbean coral reefs. Int. J. Clim. Change Strateg. Manag. 2018, 10, 523–532. [Google Scholar] [CrossRef]
- McDonald, J.; McGee, J.; Brent, K.; Burns, W. Governing geoengineering research for the Great Barrier Reef. Clim. Policy 2019, 19, 801–811. [Google Scholar] [CrossRef]
- Ricardo, G.; Jones, R.; Clode, P.; Humanes, A.; Giofre, N.; Negri, A.P. Sediment characteristics influence the fertilisation success of the corals Acropora tenuis and Acropora millepora. Mar. Pollut. Bull. 2018, 135, 941–953. [Google Scholar] [CrossRef]
- Yarlett, R.T.; Perry, C.T.; Wilson, R.W. Quantifying production rates and size fractions of parrotfish-derived sediment: A key functional role on Maldivian coral reefs. Ecol. Evol. 2021, 11, 16250–16265. [Google Scholar] [CrossRef]
- Sanderson, H.; Belanger, S.; Fisk, P.; Schäfers, C.; Veenstra, G.; Nielsen, A.; Kasai, Y.; Willing, A.; Dyer, S.; Stanton, K.; et al. An overview of hazard and risk assessment of the OECD high production volume chemical category—Long chain alcohols [C6–C22] (LCOH). Ecotoxicol. Environ. Saf. 2009, 72, 973–979. [Google Scholar] [CrossRef]
- Kamaya, Y.; Kurogi, Y.; Suzuki, K. Acute toxicity of fatty acids to the freshwater green algaSelenastrum capricornutum. Environ. Toxicol. 2003, 18, 289–294. [Google Scholar] [CrossRef]
- Scott, M.; Jones, M. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- Belanger, S.; Sanderson, H.; Fisk, P.; Schaefers, C.; Mudge, S.; Willing, A.; Kasai, Y.; Nielsen, A.; Dyer, S.; Toy, R. Assessment of the environmental risk of long-chain aliphatic alcohols. Ecotoxicol. Environ. Saf. 2009, 72, 1006–1015. [Google Scholar] [CrossRef]
- Burns, K.; Brinkman, D. Organic biomarkers to describe the major carbon inputs and cycling of organic matter in the central Great Barrier Reef region. Estuar. Coast. Shelf Sci. 2011, 93, 132–141. [Google Scholar] [CrossRef]
- Blank, M.; Roughton, F.J.W. The permeability of monolayers to carbon dioxide. Trans. Faraday Soc. 1960, 56, 1832. [Google Scholar] [CrossRef]
- Board, O.S.; National Academies of Sciences, E. A Research Review of Interventions to Increase the Persistence and Resilience of Coral Reefs; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Fidelman, P.; McGrath, C.; Newlands, M.; Dobbs, K.; Jago, B.; Hussey, K. Regulatory implications of coral reef restoration and adaptation under a changing climate. Environ. Sci. Policy 2019, 100, 221–229. [Google Scholar] [CrossRef]
- Condie, S.A.; Anthony, K.R.; Babcock, R.C.; Baird, M.E.; Beeden, R.; Fletcher, C.S.; Gorton, R.; Harrison, D.; Hobday, A.J.; Plagányi, É.E. Large-scale interventions may delay decline of the Great Barrier Reef. R. Soc. Open Sci. 2021, 8, 201296. [Google Scholar] [CrossRef]
- Suggett, D.; Warner, M.; Smith, D.; Davey, P.; Hennige, S.; Baker, N.R. Photosynthesis and production of hydrogen peroxide by Symbiodinium (pyrrhophyta) phylotypes with different thermal tolerances. J. Phycol. 2008, 44, 948–956. [Google Scholar] [CrossRef]
- Baird, M.E.; Cherukuru, N.; Jones, E.; Margvelashvili, N.; Mongin, M.; Oubelkheir, K.; Ralph, P.J.; Rizwi, F.; Robson, B.J.; Schroeder, T. Remote-sensing reflectance and true colour produced by a coupled hydrodynamic, optical, sediment, biogeochemical model of the Great Barrier Reef, Australia: Comparison with satellite data. Environ. Model. Softw. 2016, 78, 79–96. [Google Scholar] [CrossRef]
- Jones, E.M.; Baird, M.E.; Mongin, M.; Parslow, J.; Skerratt, J.; Lovell, J.; Margvelashvili, N.; Matear, R.J.; Wild-Allen, K.; Robson, B. Use of remote-sensing reflectance to constrain a data assimilating marine biogeochemical model of the Great Barrier Reef. Biogeosciences 2016, 13, 6441–6469. [Google Scholar] [CrossRef]
- Baird, M.E.; Wild-Allen, K.A.; Parslow, J.; Mongin, M.; Robson, B.; Skerratt, J.; Rizwi, F.; Soja-Woźniak, M.; Jones, E.; Herzfeld, M.; et al. CSIRO Environmental Modelling Suite (EMS): Scientific description of the optical and biogeochemical models (vB3p0). Geosci. Model Dev. 2020, 13, 4503–4553. [Google Scholar] [CrossRef]





| Experiment | Particle Diameter (µm) | Ratio Particle: Monolayer | Particle Loading (g/m2) (% Surface Coverage) | Attenuation (% at ʎ = 500 nm) |
|---|---|---|---|---|
| 1: Particle diameter | 0.15 | 5.1:1 | 0.081 (30%) | 12 ± 4.0 |
| 1.0 | 34:1 | 0.54 (30%) | 50 ± 6.5 | |
| 1.8 | 62:1 | 0.97 (30%) | 65 ± 11 | |
| 3.5 | 121:1 | 1.89 (30%) | 82 ± 5.5 | |
| 2: Particle loading | 1.8 | 20.5:1 | 0.32 (10%) | 26 ± 0.7 |
| 1.8 | 41:1 | 0.65 (20%) | 46 ± 3.4 | |
| 1.8 | 61.6:1 | 0.97 (30%) | 65 ± 11 | |
| 1.8 | 82.1:1 | 1.30 (40%) | 69 ± 2.0 | |
| 3: Ratios | 1.8 | 123:1 | 0.97 (30%) | 69 ± 3.0 |
| 1.8 | 61.6:1 | 0.97 (30%) | 65 ± 10 | |
| 1.8 | 30.8:1 | 0.97 (30%) | 57 ± 3.9 | |
| 1.8 | 15.4:1 | 0.97 (30%) | 55 ± 11 |
| Experiment | Particle Diameter (µm) | Monolayer Material | Ratio Particle: Monolayer | Solvent | Particle Loading (g/m2) (% Coverage) | Attenuation (% at ʎ = 500 nm) |
|---|---|---|---|---|---|---|
| 4: Solvent-free | 1.8 | Cetyl alcohol | 25:1 | None | 7.1 | 30 * |
| 1.8 | Cetyl alcohol | 50:1 | None | 7.1 | 41 ± 3.5 | |
| 1.8 | Cetyl alcohol | 100:1 | None | 7.1 | 40 ± 1.7 | |
| 1.8 | Cetyl alcohol | 200:1 | None | 7.1 | 41 * | |
| 1.8 | Cetyl alcohol | 300:1 | None | 7.1 | 30 ± 3.2 | |
| 5: Alternate setup | 1.8 | Stearic acid | 61.6:1 | Ethanol | 0.97 (30%) | 18.6 ± 2.3 |
| Experiment | Deployment Method | No. Tests | Attenuation (%) |
|---|---|---|---|
| Small-scale tank trials | Coarse powder | 2 | 4.2–7.3 * |
| Fine sprinkling | 6 | 23.4 ± 8.7 | |
| Suspension in seawater | 3 | 20.7 ± 3.8 | |
| Large-scale tank trials | Suspension in seawater | 4 | 17.2 ± 3.1 |
| Open sea trial: AIMS jetty | Suspension in seawater | 3 | 18.8 ± 3.4 |
| Open sea trial: Red Rock Bay | Suspension in seawater | 3 | 23.1 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scofield, J.M.P.; Prime, E.L.; Flores, F.; Severati, A.; Mongin, M.; Bougeot, E.; Baird, M.E.; Negri, A.P.; Qiao, G.G. The Development of a Floating Mono-Particle “Sun Shield” to Protect Corals from High Irradiance during Bleaching Conditions. J. Mar. Sci. Eng. 2024, 12, 1809. https://doi.org/10.3390/jmse12101809
Scofield JMP, Prime EL, Flores F, Severati A, Mongin M, Bougeot E, Baird ME, Negri AP, Qiao GG. The Development of a Floating Mono-Particle “Sun Shield” to Protect Corals from High Irradiance during Bleaching Conditions. Journal of Marine Science and Engineering. 2024; 12(10):1809. https://doi.org/10.3390/jmse12101809
Chicago/Turabian StyleScofield, Joel M. P., Emma L. Prime, Florita Flores, Andrea Severati, Mathieu Mongin, Elodie Bougeot, Mark E. Baird, Andrew P. Negri, and Greg G. Qiao. 2024. "The Development of a Floating Mono-Particle “Sun Shield” to Protect Corals from High Irradiance during Bleaching Conditions" Journal of Marine Science and Engineering 12, no. 10: 1809. https://doi.org/10.3390/jmse12101809
APA StyleScofield, J. M. P., Prime, E. L., Flores, F., Severati, A., Mongin, M., Bougeot, E., Baird, M. E., Negri, A. P., & Qiao, G. G. (2024). The Development of a Floating Mono-Particle “Sun Shield” to Protect Corals from High Irradiance during Bleaching Conditions. Journal of Marine Science and Engineering, 12(10), 1809. https://doi.org/10.3390/jmse12101809







