Relationship between Submerged Marine Debris and Macrobenthic Fauna in Jeju Island, South Korea
Abstract
1. Introduction
2. Materials and Methods
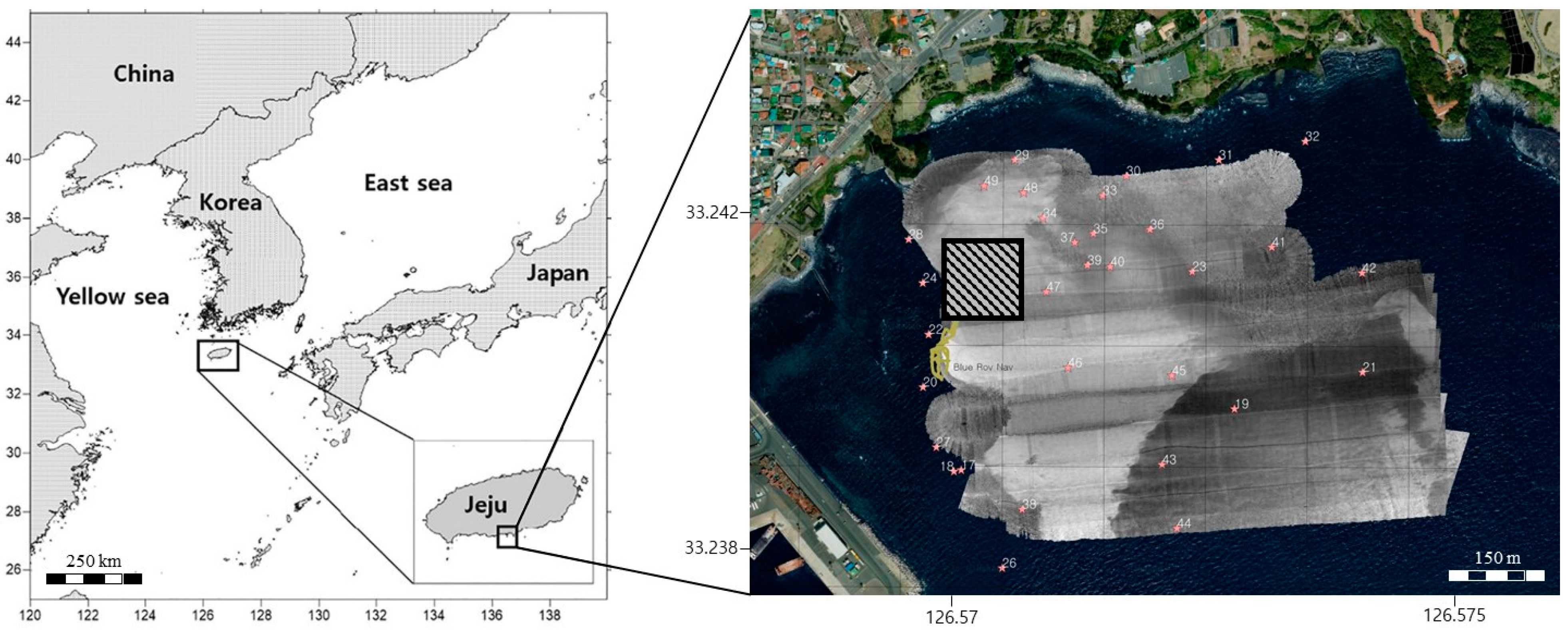
2.1. Study Area
2.2. Echo Sounding Technique
2.3. Macrobenthos Sampling Process
2.4. Data Processing
3. Results
3.1. Topography and Surface Geological Characteristic
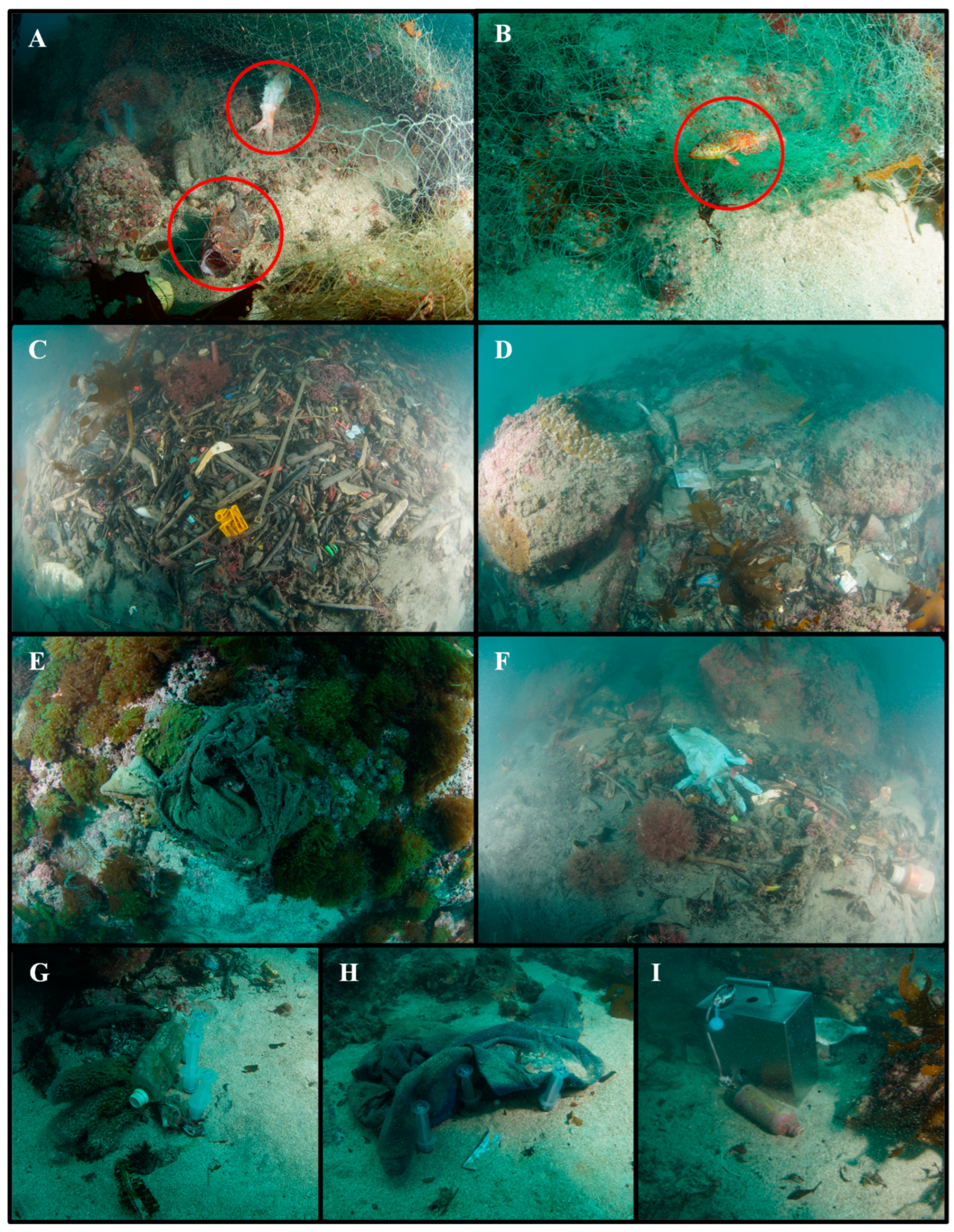
3.2. Submerged Marine Debris Distribution
3.3. Environmental Variability
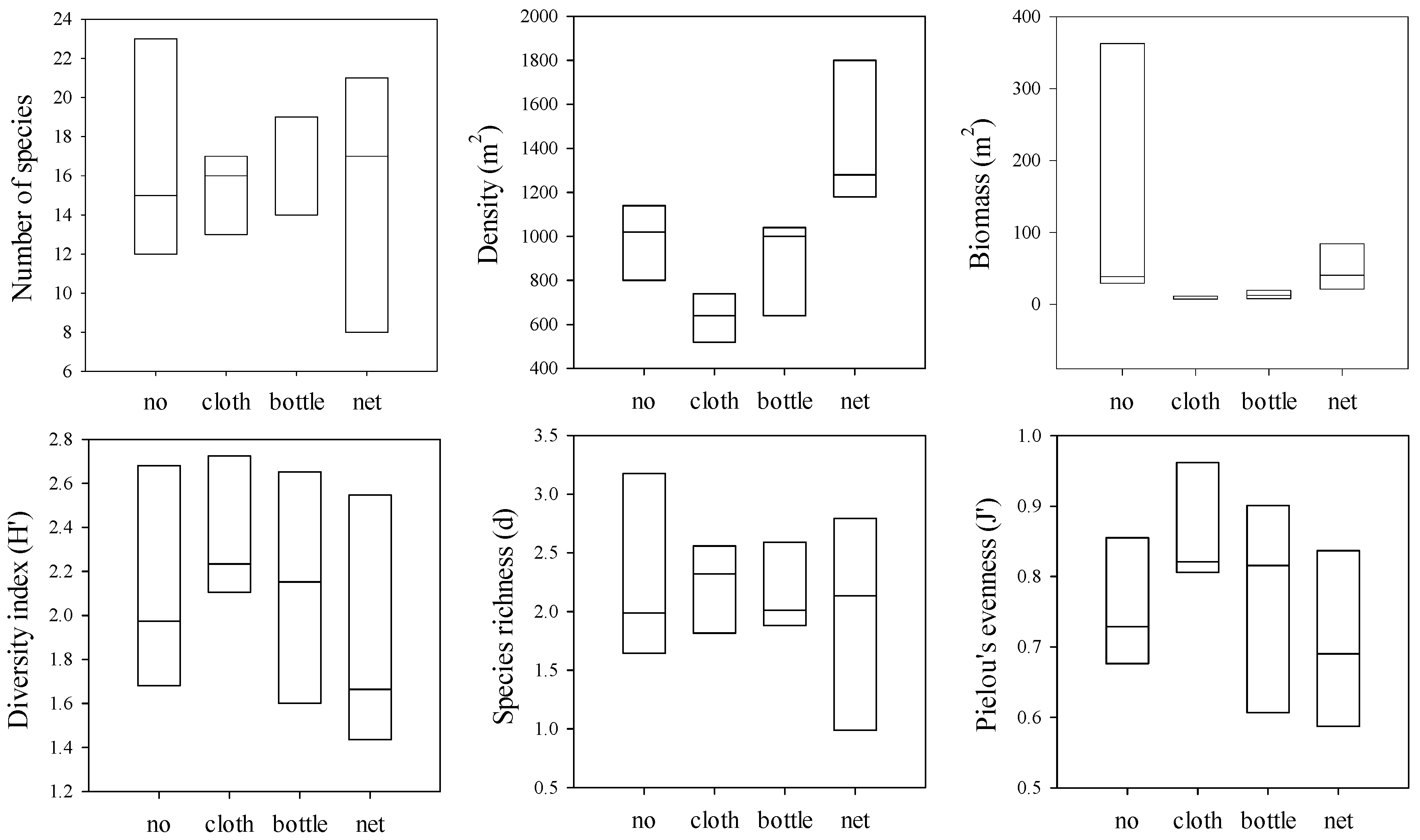
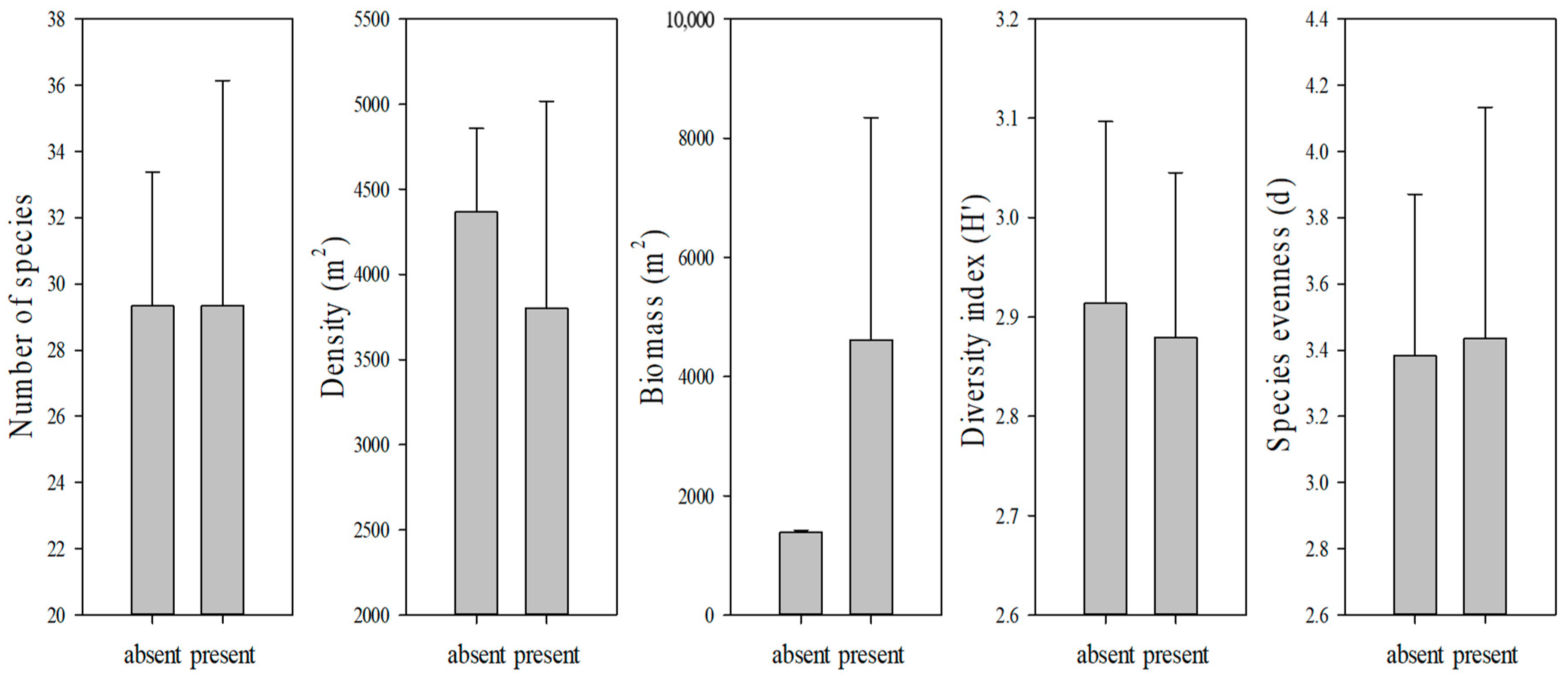
3.4. Macrobenthos Groups
3.5. Dominant Species
3.6. Correlation between Submerged Marine Debris and Macrobenthos
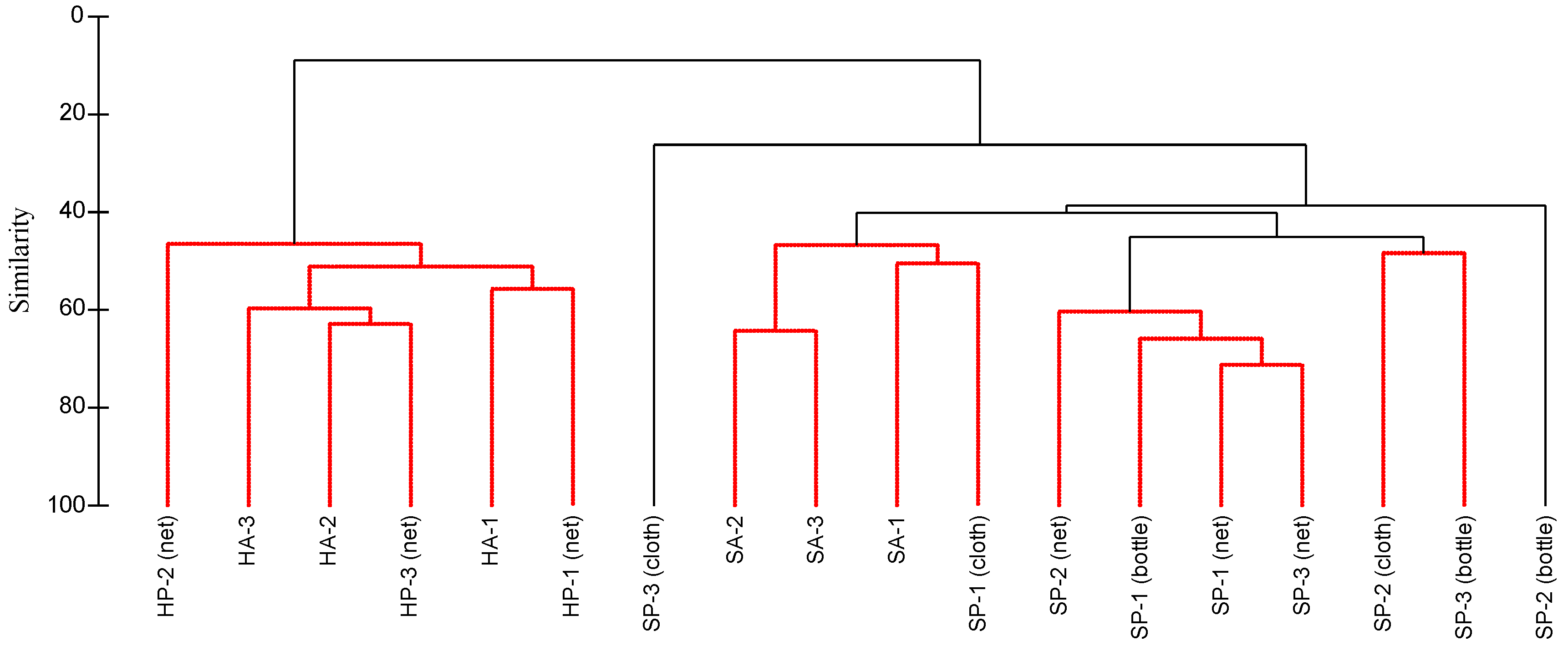
3.7. Macrobenthos Assemblages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheavly, S.B.; Register, K.M. Marine debris & plastics: Environmental concerns, sources, impacts and solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar] [CrossRef]
- Watters, D.L.; Yoklavich, M.M.; Love, M.S.; Schroeder, D.M. Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 2010, 60, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Damian, M.; Harris, A.; Aussage, J.; Fraser, G.S. Seasonal deposition of marine debris on an important marine turtle Nesting Beach in Costa Rica. Mar. Pollut. Bull. 2022, 177, 113525. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Roland, G.; Chris, W.; Theodore, R.S.; Miriam, P.; Anthony, A.; Ramani, N.; Lavender, L.K. Plastic waste inputs from land into the ocean. Science 2015, 347, 764–768. [Google Scholar] [CrossRef]
- Engler, R.E. Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 302–315. [Google Scholar]
- Nollkaemper, A. Land-based discharges of marine debris: From local to global regulation. Mar. Pollut. Bull. 1994, 28, 649–652. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Santos, I.R.; Friedrich, A.C.; Matthiensen, A.; Fillmann, G. Plastic Pollution at a Sea Turtle Conservation Area in NE Brazil: Contrasting Developed and Undeveloped Beaches. Estuaries Coasts 2011, 34, 814–823. [Google Scholar] [CrossRef]
- Rech, S.; Borrell, Y.; García-Vazquez, E. Marine litter as a vector for non-native species: What we need to know. Mar. Pollut. Bull. 2016, 113, 40–43. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; ISBN 9783319165103. [Google Scholar]
- Santos, I.R.; Friedrich, A.C.; do Sul, J.A.I. Marine debris contamination along undeveloped tropical beaches from northeast Brazil. Environ. Monit. Assess. 2009, 148, 455–462. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Mcilgorm, A.; Campbell, H.F.; Rule, M.J. The economic cost and control of marine debris damage in the Asia-Pacific region. Ocean Coast. Manag. 2011, 54, 643–651. [Google Scholar] [CrossRef]
- Donohue, M.J.; Boland, R.C.; Sramek, C.M.; Antonelis, G.A. Derelict fishing gear in the Northwestern Hawaiian Islands: Diving surveys and debris removal in 1999 confirm threat to Coral Reef ecosystems. Mar. Pollut. Bull. 2001, 42, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.; Thompson, R. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.C.; Lee, J.S. The willingness to pay for removing the microplastics in the ocean—The case of Seoul metropolitan area, South Korea. Mar. Policy 2018, 93, 93–100. [Google Scholar] [CrossRef]
- Jin, S.J.; Kwon, Y.J.; Yoo, S.H. Economic valuation of reducing submerged marine debris in South Korea. Appl. Sci. 2020, 10, 6086. [Google Scholar] [CrossRef]
- Li, W.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Corcoran, P.L. Benthic plastic debris in marine and fresh water environments. Environ. Sci. Process. Impacts 2015, 17, 1363–1369. [Google Scholar] [CrossRef]
- Kumar, A.A.; Sivakumar, R.; Reddy, Y.S.R.; Bhagya Raja, M.V.; Nishanth, T.; Revanth, V. Preliminary study on marine debris pollution along Marina beach, Chennai, India. Reg. Stud. Mar. Sci. 2016, 5, 35–40. [Google Scholar] [CrossRef]
- Debrot, A.O.; Vinke, E.; van der Wende, G.; Hylkema, A.; Reed, J.K. Deepwater marine litter densities and composition from submersible video-transects around the ABC-islands, Dutch Caribbean. Mar. Pollut. Bull. 2014, 88, 361–365. [Google Scholar] [CrossRef]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.J.; Huang, H.W. Strategy for mitigation of marine debris: Analysis of sources and composition of marine debris in northern Taiwan. Mar. Pollut. Bull. 2014, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Guitart, R.; Mateo, R.; Raga, J.A. Marine debris ingestion in loggerhead sea turtles, Caretta caretta, from the Western Mediterranean. Mar. Pollut. Bull. 2002, 44, 211–216. [Google Scholar] [CrossRef]
- Melli, V.; Angiolillo, M.; Ronchi, F.; Canese, S.; Giovanardi, O.; Querin, S.; Fortibuoni, T. The first assessment of marine debris in a Site of Community Importance in the north-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 2017, 114, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Bilkovic, D.M.; Havens, K.; Stanhope, D.; Angstadt, K. Derelict fishing gear in Chesapeake Bay, Virginia: Spatial patterns and implications for marine fauna. Mar. Pollut. Bull. 2014, 80, 114–123. [Google Scholar] [CrossRef]
- Lee, D.I.; Cho, H.S.; Jeong, S.B. Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Estuar. Coast. Shelf Sci. 2006, 70, 187–194. [Google Scholar] [CrossRef]
- Consoli, P.; Andaloro, F.; Altobelli, C.; Battaglia, P.; Campagnuolo, S.; Canese, S.; Castriota, L.; Cillari, T.; Falautano, M.; Pedà, C.; et al. Marine litter in an EBSA (Ecologically or Biologically Significant Area) of the central Mediterranean Sea: Abundance, composition, impact on benthic species and basis for monitoring entanglement. Environ. Pollut. 2018, 236, 405–415. [Google Scholar] [CrossRef]
- Gilman, E.; Musyl, M.; Suuronen, P.; Chaloupka, M.; Gorgin, S.; Wilson, J.; Kuczenski, B. Highest risk abandoned, lost and discarded fishing gear. Sci. Rep. 2021, 11, 7195. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, H.W.; Kim, J.N.; Jeong, J.M.; Ji, H.S.; Jo, H.S.; Kim, D.H.; Park, C. Frist observation and effect of fishery of seabed litter on sea bed by trawl survey Korea waters. Mar. Pollut. Bull. 2021, 170, 112228. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Yang, J.S.; Park, K.; Lebrato, M.; Wang, Y.V.; Tseng, L.C.; Achterberg, E.P.; Chen, X.G.; Molinero, J.C.; Bremer, K.; et al. Distribution of microcystins in environmental multimedia and their bioaccumulation characteristics in marine benthic organisms in the Geum River Estuary, South Korea. Environ. Int. 2019, 19, 143815. [Google Scholar] [CrossRef]
- Kim, J.N.; Kang, M.; Jo, H.-S. The distribution and composition of seabed litter in the exclusive economic zone of the West Sea of South Korea. J. Korean Soc. Fish. Technol. 2017, 53, 437–445. [Google Scholar] [CrossRef]
- Sruthy, S.; Ramasamy, E.V. Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India. Environ. Pollut. 2017, 222, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Schlining, K.; von Thun, S.; Kuhnz, L.; Schlining, B.; Lundsten, L.; Jacobsen Stout, N.; Chaney, L.; Connor, J. Debris in the deep: Using a 22-year video annotation database to survey marine litter in Monterey Canyon, central California, USA. Deep. Res. Part I Oceanogr. Res. Pap. 2013, 79, 96–105. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Jang, Y.C.; Kim, Y.J.; Kim, H.J.; Han, D.; Hong, S.H.; Kang, D.; Shim, W.J. Impacts of marine debris on wild animals in the coastal area of Korea. Mar. Pollut. Bull. 2013, 66, 117–124. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; De Mol, B.; Company, J.B.; Coll, M.; Sardà, F. Effects of natural and anthropogenic processes in the distribution of marine litter in the deep Mediterranean Sea. Prog. Oceanogr. 2013, 118, 273–287. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Xue, B.; Huang, B.; Chen, G.; Li, H.; Wei, W. Deep-sea debris identification using deep convolutional neural networks. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 8909–8921. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.L.; Kang, S.M.; Lee, H.G.; Yu, O.H. Effect of submerged marine debris on the species compositions and community characteristics of the macrobenthos in the subtidal zone of Jeju Island, Korea. J. Sea Res. 2023, 192, 102347. [Google Scholar] [CrossRef]
- Hess, N.A.; Ribic, C.A.; Vining, I. Benthic marine debris, with an emphasis on fishery-related items, surrounding Kodiak Island, Alaska, 1994–1996. Mar. Pollut. Bull. 1999, 38, 885–890. [Google Scholar] [CrossRef]
- Backhurst, M.K.; Cole, R.G. Subtidal benthic marine litter at Kawau Island, north-eastern New Zealand. J. Environ. Manag. 2000, 60, 227–237. [Google Scholar] [CrossRef]
- Moore, S.L.; Allen, M.J. Distribution of anthropogenic and natural debris on the mainland shelf of the Southern California Bight. Mar. Pollut. Bull. 2000, 40, 83–88. [Google Scholar] [CrossRef]
- Spengler, A.; Costa, M.F. Methods applied in studies of benthic marine debris. Mar. Pollut. Bull. 2008, 56, 226–230. [Google Scholar] [CrossRef]
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef]
- Woodall, L.C.; Robinson, L.F.; Rogers, A.D.; Narayanaswamy, B.E.; Paterson, G.L.J. Deep-sea litter: A comparison of seamounts, banks and a ridge in the Atlantic and Indian Oceans reveals both environmental and anthropogenic factors impact accumulation and composition. Front. Mar. Sci. 2015, 2, 3. [Google Scholar] [CrossRef]
- Grøsvik, B.E.; Prokhorova, T.; Eriksen, E.; Krivosheya, P.; Horneland, P.A.; Prozorkevich, D. Assessment of marine litter in the Barents Sea, a part of the joint Norwegian-Russian ecosystem survey. Front. Mar. Sci. 2018, 5, 72. [Google Scholar] [CrossRef]
- Jang, S.W.; Park, J.M.; Chung, Y.H.; Kim, D.H.; Yoon, H.J. A Study on the Inflow and Seasonal Characteristics of Foreign Marine Debris in the Coastal Area of the West Sea. J. Korean Soc. Mar. Environ. Energy 2012, 15, 89–100. [Google Scholar] [CrossRef]
- Kim, S.; Kang, W. Distribution Characteristics and Cost Estimation of Collection and Treatment of Deposited Marine Debris in Coastal Fisheries around the Southwestern Islands of Korea. J. Korean Soc. Mar. Environ. Eng. 2012, 15, 330–336. [Google Scholar] [CrossRef]
- Yang, S.-C.; Yang, S.-K.; Kim, Y.-S. Mean Velocity Distribution of Natural Stream using Entropy Concept in Jeju. J. Environ. Sci. Int. 2019, 28, 535–544. [Google Scholar] [CrossRef]
- Ko, J.-C.; Koo, J.-H.; Yang, M.-H. Characteristics of Ocean Environmental Factors and Community Structure of Macrobenthos around Munseom, Jeju Island, Korea. Korean J. Malacol. 2008, 24, 215–228. [Google Scholar]
- Cho, I.Y.; Kang, D.W.; Kang, J.; Hwang, H.; Won, J.H.; Paek, W.K.; Seo, S.Y. A study on the biodiversity of benthic invertebrates in the waters of Seogwipo, Jeju Island, Korea. J. Asia Pac. Biodivers. 2014, 7, e11–e18. [Google Scholar] [CrossRef]
- Folk, R.; Ward, W. Brazos river bar: A study in the significance of grain-size parameters. J. Sediment Pet. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Mees, J.; Janssen, C.R. Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Pawar, P.R.; Shirgaonkar, S.S.; Patil, R.B. Plastic marine debris: Sources, distribution and impacts on coastal and ocean biodiversity. Pencil Publ. Biol. Sci. 2016, 3, 40–54. [Google Scholar]
- Kim, M.S.; Kim, S.K.; Kim, J.K.; Jeong, S.B.; Jo, H.J. A study on the distribution of marine litters in the eastern part area of the southern sea mainly on discarded pots. Bull. J. Korean Soc. Fish. Technol. 1999, 35, 386–390. [Google Scholar]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the sea floor along European coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Pasternak, G.; Ribic, C.A.; Spanier, E.; Ariel, A.; Mayzel, B.; Ohayon, S.; Zviely, D. Nearshore survey and cleanup of benthic marine debris using citizen science divers along the Mediterranean coast of Israel. Ocean Coast. Manag. 2019, 175, 17–32. [Google Scholar] [CrossRef]
- Manickavasagam, S.; Kumar, S.; Kumar, K.; Rathi Bhuvaneswari, G.; Paul, T.; Shukla, S.P. Quantitative assessment of influx and efflux of marine debris in a water channel of South Juhu creek, Mumbai, India. Reg. Stud. Mar. Sci. 2020, 34, 101095. [Google Scholar] [CrossRef]
- Ruiz-Orejón, L.F.; Sardá, R.; Ramis-Pujol, J. Floating plastic debris in the Central and Western Mediterranean Sea. Mar. Environ. Res. 2016, 120, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Perroca, J.F.; Giarrizzo, T.; Azzurro, E.; Rodrigues-Filho, J.L.; Silva, C.V.; Arcifa, M.S.; Azevedo-Santos, V.M. Negative effects of ghost nets on Mediterranean biodiversity. Aquat. Ecol. 2022. [Google Scholar] [CrossRef]
- de Oliveira Leis, M.; Devillers, R.; Medeiros, R.P.; Chuenpagdee, R. Mapping fishers’ perceptions of marine conservation in Brazil: An exploratory approach. Ocean Coast. Manag. 2019, 167, 32–41. [Google Scholar] [CrossRef]
- Andrades, R.; Trindade, P.A.A.; Giarrizzo, T. A novel facet of the impact of plastic pollution on fish: Silver croaker (Plagioscion squamosissimus) suffocated by a plastic bag in the Amazon estuary, Brazil. Mar. Pollut. Bull. 2021, 166, 112197. [Google Scholar] [CrossRef]
- Vitorino, H.; Ferrazi, R.; Correia-Silva, G.; Tinti, F.; Belizário, A.C.; Amaral, F.A.; Ottoni, F.P.; Silva, C.V.; Giarrizzo, T.; Arcifa, M.S.; et al. New treaty must address ghost fishing gear. Science 2022, 376, 1169. [Google Scholar] [CrossRef]
- Kim, S.L.; Lee, H.G.; Yu, O.H. Correlation between rocky reefs and surrounding benthic habitats: Distribution and diversity patterns of polychaetes in the macrobenthic community in the East Sea of South Korea. J. Sea Res. 2021, 174, 102083. [Google Scholar] [CrossRef]
- Kanaya, G.; Nakamura, Y.; Koizumi, T.; Yamada, K. Seasonal changes in infaunal community structure in a hypertrophic brackish canal: Effects of hypoxia, sulfide, and predator-prey interaction. Mar. Environ. Res. 2015, 108, 14–23. [Google Scholar] [CrossRef]
- Owada, M. Functional morphology and phylogeny of the rock-boring bivalves Leiosolenus and Lithophaga (Bivalvia: Mytilidae): A third functional clade. Mar. Biol. 2007, 150, 853–860. [Google Scholar] [CrossRef]
- Graham, E.R.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- López-López, L.; Preciado, I.; González-Irusta, J.M.; Arroyo, N.L.; Muñoz, I.; Punzón, A.; Serrano, A. Incidental ingestion of meso- and macro-plastic debris by benthic and demersal fish. Food Webs 2018, 14, 1–4. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Verriopoulos, G.; Nicolaidou, A.; Thessalou-Legaki, M. Effect of marine litter on the benthic megafauna of coastal soft bottoms: A manipulative field experiment. Mar. Pollut. Bull. 2007, 54, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Ivar do Sul, J.A.; Fillmann, G. Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Mar. Pollut. Bull. 2010, 60, 396–401. [Google Scholar] [CrossRef] [PubMed]
| No | Site | Latitude | Longitude | Depth (m) | Temperature (°C) | Salinity (psu) | Dissolved Oxygen (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | SP-1(net) | 33 14.344 | 126 34.278 | 11.2 | 18.0 | 34.2 | 4.0 |
| 2 | SP-2(net) | 33 14.363 | 126 34.288 | 13.6 | 18.0 | 34.2 | 4.0 |
| 3 | SP-3(net) | 33 14.363 | 126 34.288 | 13.6 | 18.0 | 34.2 | 4.0 |
| 4 | SP-1(cloth) | 33 14.344 | 126 34.282 | 10.4 | 18.2 | 34.0 | 3.9 |
| 5 | SP-2(cloth) | 33 14.344 | 126 34.282 | 10.4 | 18.2 | 34.0 | 3.9 |
| 6 | SP-3(cloth) | 33 14.358 | 126 34.291 | 14.5 | 18.2 | 34.0 | 3.9 |
| 7 | SP-1(bottle) | 33 14.358 | 126 34.285 | 13 | 18.1 | 34.2 | 4.5 |
| 8 | SP-2(bottle) | 33 14.345 | 126 34.290 | 13 | 18.1 | 34.2 | 4.5 |
| 9 | SP-3(bottle) | 33 14.368 | 126 34.295 | 14 | 18.1 | 34.2 | 4.5 |
| 10 | SA-1 | 33 14.358 | 126 34.290 | 11.5 | 18.2 | 33.6 | 4.5 |
| 11 | SA-2 | 33 14.358 | 126 34.290 | 11.5 | 18.2 | 33.6 | 4.5 |
| 12 | SA-3 | 33 14.358 | 126 34.290 | 11.5 | 18.2 | 33.6 | 4.5 |
| 13 | HP-1(net) | 33 14.351 | 126 34.275 | 10.8 | 17.9 | 34.0 | 4.2 |
| 14 | HP-2(net) | 33 14.351 | 126 34.275 | 10.8 | 17.9 | 34.0 | 4.2 |
| 15 | HP-3(net) | 33 14.353 | 126 34.273 | 11.1 | 17.9 | 34.0 | 4.2 |
| 16 | HA-1 | 33 14. 358 | 126 34.280 | 14 | 18.1 | 34.1 | 4.5 |
| 17 | HA-2 | 33 14.358 | 126 34.280 | 14 | 18.1 | 34.1 | 4.5 |
| 18 | HA-3 | 33 14.358 | 126 34.280 | 14 | 18.1 | 34.1 | 4.5 |
| Site | Gravel (%) | Sand (%) | Silt (%) | Clay (%) | Mz (Phi) | TOC (%) |
|---|---|---|---|---|---|---|
| SP-1(bottle) | 0.0 | 100.0 | 0.0 | 0.0 | 1.1 | 0.878 |
| SP-2(cloth) | 0.0 | 100.0 | 0.0 | 0.0 | 1.7 | 1.286 |
| SP-3(net) | 0.0 | 100.0 | 0.0 | 0.0 | 1.9 | 0.171 |
| SA | 0.0 | 100.0 | 0.0 | 0.0 | 1.8 | 0.750 |
| Groups | Site | Average Density (m2) | Average Biomass (m2) |
|---|---|---|---|
| Polychaeta | SP-1 (bottle) | 26.7 | 2.6 |
| SP-2 (cloth) | 93.3 | 0.6 | |
| SP-3 (net) | 50.0 | 82.7 | |
| SA | 100.0 | 131.8 | |
| HP-1 (net) | 220.0 | 160.5 | |
| HA | 518.3 | 379.1 | |
| Arthropoda | SP-1 (bottle) | 26.7 | 2.6 |
| SP-2 (cloth) | 93.3 | 0.6 | |
| SP-3 (net) | 0.0 | 0.0 | |
| SA | 116.7 | 160.1 | |
| HP-1 (net) | 233.3 | 214.1 | |
| HA | 20.8 | 0.2 | |
| Echinoderms | SP-1 (bottle) | 75.0 | 54.6 |
| SP-2 (cloth) | 0.0 | 0.0 | |
| SP-3 (net) | 120.0 | 124.3 | |
| SA | 233.3 | 214.1 | |
| HP-1 (net) | 31.3 | 0.2 | |
| HA | 50.0 | 0.1 | |
| Mollusk | SP-1 (bottle) | 40.0 | 2.6 |
| SP-2 (cloth) | 46.7 | 1.0 | |
| SP-3 (net) | 0.0 | 0.0 | |
| SA | 103.3 | 82.2 | |
| HP-1 (net) | 100.0 | 131.8 | |
| HA | 220.0 | 160.5 | |
| Others | SP-1 (bottle) | 46.7 | 1.0 |
| SP-2 (cloth) | 6.7 | 0.5 | |
| SP-3 (net) | 40.0 | 2.6 | |
| SA | 53.3 | 0.6 | |
| HP-1 (net) | 116.7 | 160.1 | |
| HA | 233.3 | 214.1 |
| Bottom Type | Rank | Groups | Species | Average Density (m2) | % of Density | Frequency (%) |
|---|---|---|---|---|---|---|
| Sandy | 1 | Polychaeta | Armandia lanceolata Wiley, 1905 | 293 | 30 | 100 |
| 2 | Polychaeta | Dorvillea matsushimaensis (Okuda in Ikuda and Yamada, 1954) | 207 | 21 | 67 | |
| 3 | Mollusk | Jactellina clathrata (Deshayes, 1835) | 50 | 5 | 92 | |
| 4 | Mollusk | Callista pilsbryi Habe, 1960 | 38 | 4 | 92 | |
| 5 | Arthropoda | Bubocorophium exolitus (Hirayama, 1984) | 35 | 4 | 33 | |
| 6 | Polychaeta | Aricidea sp. | 33 | 3 | 67 | |
| 7 | Mollusk | Scintilla sp. | 27 | 3 | 58 | |
| 8 | Polychaeta | Sthenelais fusca Johnson, 1897 | 25 | 3 | 58 | |
| 9 | Polychaeta | Notomastus latericeus Sars, 1851 | 20 | 2 | 58 | |
| 10 | Polychaeta | Prionospio sp. | 20 | 2 | 83 | |
| Rocky | 1 | Mollusk | Leiosolenus lischkei M. Huber, 2010 | 758 | 19 | 100 |
| 2 | Mollusk | Cerithium alutaceum (A. Gould, 1861) | 325 | 8 | 83 | |
| 3 | Balanomorpha | Cantellius arcuatus (Hiro, 1938) | 300 | 7 | 67 | |
| 4 | Polychaeta | Lysidice collaris Grube, 1868 | 250 | 6 | 100 | |
| 5 | Polychaeta | Nereis sp. | 225 | 6 | 100 | |
| 6 | Polychaeta | Syllis (Typosyllis) sp. | 208 | 5 | 100 | |
| 7 | Mollusk | Onithohiton sp. | 142 | 3 | 83 | |
| 8 | Polychaeta | Eunice indica Kinberg, 1865 | 125 | 3 | 83 | |
| 9 | Mollusk | Chama limbula Lamarck, 1819 | 100 | 2 | 50 | |
| 10 | Mollusk | Cardita leana Dunker, 1860 | 92 | 2 | 100 |
| Number of Species | Density (ind./m2) | Diversity Index (H’) | ||
|---|---|---|---|---|
| Bottom type (sandy/rocky) | F value | 0.0619 | 100.667 | 12.513 |
| p value | 0.807 | <0.001 | 0.807 | |
| Debris (presence/absence) | F value | 29.207 | 0.855 | 0.00246 |
| p value | <0.001 | 0.371 | 0.378 | |
| Bottom x debris | F value | 0.0619 | 0.828 | 0.0111 |
| p value | 0.003 | 0.961 | 0.917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.L.; Lee, H.G.; Park, Y.; Yu, O.H. Relationship between Submerged Marine Debris and Macrobenthic Fauna in Jeju Island, South Korea. J. Mar. Sci. Eng. 2023, 11, 1427. https://doi.org/10.3390/jmse11071427
Kim SL, Lee HG, Park Y, Yu OH. Relationship between Submerged Marine Debris and Macrobenthic Fauna in Jeju Island, South Korea. Journal of Marine Science and Engineering. 2023; 11(7):1427. https://doi.org/10.3390/jmse11071427
Chicago/Turabian StyleKim, Sang Lyeol, Hyung Gon Lee, Yosup Park, and Ok Hwan Yu. 2023. "Relationship between Submerged Marine Debris and Macrobenthic Fauna in Jeju Island, South Korea" Journal of Marine Science and Engineering 11, no. 7: 1427. https://doi.org/10.3390/jmse11071427
APA StyleKim, S. L., Lee, H. G., Park, Y., & Yu, O. H. (2023). Relationship between Submerged Marine Debris and Macrobenthic Fauna in Jeju Island, South Korea. Journal of Marine Science and Engineering, 11(7), 1427. https://doi.org/10.3390/jmse11071427






