Abstract
5G technology aims to satisfy several service requirements, leading to high data-rate connections and lower latency times than current ones. 5G systems use different frequency bands of the radio wave spectrum, taking advantage of higher frequencies than previous mobile radio generations. To guarantee capillary radio coverage, it will be necessary to install a huge number of repeaters since electromagnetic waves at higher frequencies, and especially microwaves at higher bands, exhibit lower capacity to propagate in free space. Since the introduction of this new technology, there has been growing concern about possible harmful effects on human health. The aim of this study was to investigate the possible short-term effects induced by 5G-millimeter waves on the embryonic development of zebrafish (Danio rerio). Fertilized eggs were exposed to 27 GHz using a non-commercial high-gain pyramidal horn antenna, and several endpoints were monitored every 24 h. As a result, exposure to electromagnetic fields at 27 GHz caused no significant impacts on mortality or on morphology since the exposed larvae showed normal detachment of the tail, the presence of a heartbeat, and well-organized somites. Exposure to 27 GHz caused an increase in the heart rate in exposed embryos compared to that in the control group at 48 h. However, this increase was not observed at 72 and 96 h. Finally, very weak positivity regarding exposed larvae was highlighted by immunohistochemical analysis.
1. Introduction
Nowadays, telecommunication systems are truly widespread in our society. The increasing demand for pervasive connectivity, low latency, and high-data rate transfers have given rise to the new fifth generation (5G) technology, which promises to have a deep impact on economy and society by improving wireless communications and transforming existing market sectors and industries [1]. Moreover, the 5G system will drive future economic resiliency by untethering more workers from physical workstations, by triggering the growth of new digital industries, and by increasing efficiency and productivity across a variety of other industries [2].
Electromagnetic waves are an integral part of the environment where we live and work, and their origin is partly artificial (radio waves, radar and telecommunications, etc.) and partly natural (visible light, UV, X-rays, gamma rays, etc.). Therefore, everybody is constantly surrounded by electromagnetic fields of various natures. The biological effects of electromagnetic waves essentially depend on their intensity and frequency. More specifically, the electromagnetic spectrum can be divided in two parts: ionizing radiation (i.e., UV, X and gamma rays) and non-ionizing radiation, such as radio waves, microwaves, and millimeter waves [3]. These two portions of the spectrum differ from each other due to their different abilities to interact with atoms and molecules. In particular, the main effect that non-ionizing radiation produces on molecules is field-driven oscillation, in turn producing friction and heat dissipation; thus, heating is precisely the main effect of non-ionizing radiation, but its biological effect strongly depends on its intensity [4,5].
However, regarding the effects of electromagnetic fields (EMFs), it is possible to distinguish between thermal and non-thermal effects [6]. The thermal effects of high-frequency fields are related to energy absorption by irradiated tissues and, consequently, to their temperature increase. Thermal effects are usually caused by short and intense exposures. To measure the radiative power absorbed by the human body per kilogram of body mass (W/kg), the specific absorption rate (SAR) has been introduced. The value of the SAR has a direct correspondence with the biological effects of electromagnetic exposure. In the presence of high absorption rates, small, particularly vascularized organs are at risk, i.e., those with poor blood circulation and therefore slower decongestion time, such as the eyes or testicles. In general, they heat up faster; hence, they are more exposed to risk than other areas of the body. In addition to thermal effects, electromagnetic radiation can cause biological effects on humans at lower SAR values (<0.01 W/kg), which cannot be explained by heating models alone, which is why they are usually referred to as non-thermal effects. Usually, these effects are related to long-term, low-intensity exposures [6]. There have been few studies in the literature shedding light on possible real consequences induced by non-thermal effects on human health; moreover, results have appeared often contradictory. In particular, studies of bioeffects related to frequency bands at 25–39 GHz are particularly scarce [4,5,7,8,9].
The primary scope of this research is to assess whether exposure to electromagnetic fields at 27 GHz (belonging to 5G “millimeter-wave bands”) is associated with developmental perturbations during zebrafish embryogenesis. This choice is motivated by 5G higher frequency bands being increasingly used in short/medium-range communications; thus, it is reasonable to expect higher exposure in next future [1]. To decrease the impact of experimental assays on living animals, the European Guidelines suggest using the zebrafish embryo toxicity test (ZFET) as a useful tool, instead of testing with adult fish [10,11]. Through this test, it has been possible to evaluate the effects induced by 5G-millimeter waves on the embryonic stages of zebrafish.
It is known that all freshwater and saltwater animals are under the constant influence of the earth’s geomagnetic field [12]. This geomagnetic field allows several animals (e.g., turtles, fish) to derive positional information during migration [13,14,15]. However, the level of the geomagnetic field can be disturbed by the presence of anthropogenic constructions, such as underwater cables that connect wind farms or marine and freshwater renewable energy devices and transport electric currents, usually over long distances [16,17].
Currently, little is known about whether EMF modifies biological systems. To date, a causal relationship and biological mechanisms for the potential effects of EMF on living animals have not yet been clearly identified.
By a multimarker approach, the following have been analyzed: hatching failure, four endpoints for ZFET (embryo coagulation, lack of somite formation, lack of detachment of the tail-bud from the yolk sac, and absence/presence of heartbeat), the heart rate by DanioScope™ software, intracellular reactive oxygen species (ROS) by 2,7-dichlorodihydrofluorescein diacetate (DCFH 2-DA) and heat shock protein 70 (HSP70) and P540 aromatase (Cyp-19b) expression by immunohistochemical assay. The cytochrome P450 (CYP) enzymes are a group of heme monooxygenases that have been studied in relation to their crucial roles in the metabolism of drugs and xenobiotics, as well as in the biosynthesis of sterols, fatty acids, eicosanoids, etc. [18]. Heat-shock proteins (HSPs), which are an evolutionally well-conserved protein family, are induced in response to a variety of stress conditions and metabolic insults [19]. HSP70 is widely distributed protein of the HSP family found in different organisms, and its expression is markedly induced in response to environmental stresses (e.g., heat shock, UV and γ-irradiation, chemical exposure) [20]. The amino acid sequences of zebrafish HSP70 are highly homologous to heat-inducible-type HSP70 proteins in other vertebrates, such as human HSP70-1 [21,22]. Supposing that vertebrate development may be impacted by EMF, this multimarker approach has been useful in understanding the aforementioned developmental effects.
2. Materials and Methods
2.1. Exposure Setup Description for Numerical Dosimetry
Experiments at 27 GHz were conducted using a non-commercial high-gain pyramidal horn antenna. The dimensions of the antenna aperture are were 2 × 6.02 cm, and the maximum gain was 24.9 dBi. Moreover, the antenna was fed by a RF signal generation (R&S SMB100A) with +23 dBm (200 mW) of output power through a coaxial cable that introduced an attenuation of 3 dB. The distance between the antenna aperture and the six well microplate sample holder was set to 15 cm, assuming normal incidence of the antenna’s main beam (Figure 1a), to ensure an incident power density level comparable with the exposure limits imposed by international guidelines [23]. A numerical simulation of the experimental setup was performed using the electromagnetic simulator CST Microwave Studio. The simulation was performed by considering a highly accurate model of the six-well microplate, of the antenna, and of the water bath used to control the temperature of the samples during the long-time exposure protocol, as well as to avoid also daily thermal excursion. The table used in the experimental setup as a rigid plane for the water bowl and the sample holder was not considered in the overall simulation since the water bath exponentially attenuates the electromagnetic field inside it; hence, no reflections from the table plane were expected to affect the field distribution inside the aqueous samples.
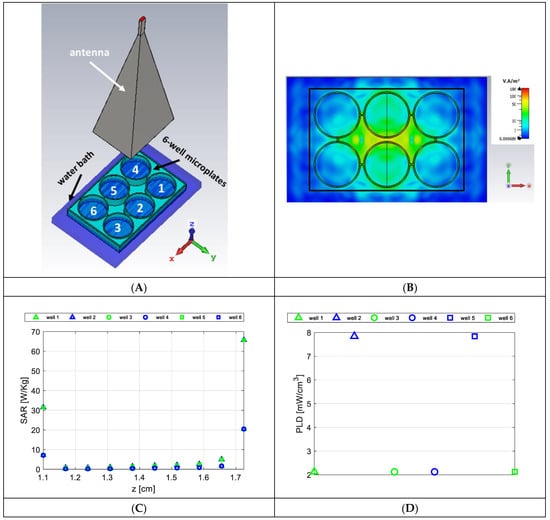
Figure 1.
Experimental setup for numerical dosimetry. (A) CAD model (six-well microplates numbered for understanding the following figures); (B) Incident power density at the water sample-air interface; (C) Average point SAR for each aqueous sample in the well; (D) Average PLD for each aqueous sample in the wells.
The electric parameters of the materials adopted for the simulation at the working frequency of 27 GHz are reported in Table 1.

Table 1.
Dielectric parameters adopted for the numerical dosimetry.
To evaluate the exposure condition, three complementary metrics were considered since the commonly used SAR indicator is mainly suggested for lower operating frequencies (less than 6 GHz) [23]:
- -
- electric field density power [W/m2];
- -
- local specific absorption rate, defined as [W/Kg]; and
- -
- power loss density (PLD) into exposed aqueous samples [W/m3].
2.2. Experimental Procedure
The zebrafish embryo toxicity (ZFET) test was performed according to the OECD (2013) guidelines for testing chemicals [24]. Fertilized eggs, derived from the Centre for Experimental Fish Pathology of Sicily (CISS) in the Department of Veterinary Sciences (University of Messina, Italy), were used for ecotoxicological assay. CISS is accredited for the production of aquatic animals to employ in experimental research (DM N°39/Marzo/2006). Adult zebrafish were kept in a stand-alone facility (ZebTec, Tecniplast) in controlled conditions (14-h light/10-h dark regimen, temperature of 27–28 °C, pH 7.2, conductivity of 600 μS/cm). Moreover, adult animals were fed twice per day with Artemia sp. nauplii (JBL ArtemioPur®, BL GmbH & Co., KG, Germany). No authorization is required for experiments with larvae before yolk sac resorption. Following mating, the fertilized eggs were collected by Pasteur pipettes under a stereomicroscope, while unfertilized eggs were not selected. The exposure was performed by a water bath used to control the temperature of the samples during the 96 h to maintain a daily constant temperature. Healthy embryos were placed in six-well microplates (five embryos/well) with 5 mL of embryo medium/well inside the water bath on a special plane adapted for this purpose. A group of embryos called “exposed” were placed under the antenna to receive the electromagnetic field. Another group of embryos incubated only with embryo medium and placed far from the antenna were considered the “control” (negative control). A third group, considered the “positive control,” were exposed to 3,4-dichloroaniline (3,4-DCA). A total of 90 eggs were used for each group (three replicates, with 30 larvae for each group). Moreover, a controlled lab-room temperature was also maintained (27 ± 1 °C).
2.3. Observation of Endpoints
During the exposure period, which started within 180 min from fertilization of the eggs and was stopped at 96 h, as suggested by European guidelines, four endpoints were analyzed every 24 h by stereomicroscope: embryo coagulation, lack of somite formation, lack of detachment of the tail-bud from the yolk sac, and lack of heartbeat. Hatching failure and post-hatching death were also recorded, which is why a failure in hatching is considered an important sub-lethal effect.
2.4. Cardiology Measurements
Cardiac measurements were recorded using DanioScope™ (Noldus) software. This software is able to analyze videos to provide quantitative data about the investigated endpoint, such as heart rate and intervals between beats. Daily, after the observation of acute endpoints, the embryos were immobilized on a small dish of agarose and acclimated for a few minutes before recording videos. The videos were recorded with the E200 MV-R LED microscope (Nikon) equipped with CMOS camera (Nikon). After selecting the heart area, the cardiac activity was automatically detected by DanioScope™ software. The software applies an algorithm that detects changes in pixel density during ventricular contractions, and these changes are directly correlated with cardiac muscle contraction. DanioScope™ (Noldus) software provides the number of beats per second (BPS) and beats per minute (BPM). Only beats per minute (BPM) values of the control group and embryos exposed to 27 GHz obtained by DanioScopeTM software are shown.
2.5. Immunohistochemical Analysis and Evaluation of Intracellular Reactive Oxygen Species (ROS)
The immunofluorescence protocol was performed according to Pecoraro et al. (2017) [11] on larvae exposed to 5G-millimeter waves, including negative controls, to detect positivity of HSP70 and P540 biomarkers. Three larvae from each group were incubated with anti-rabbit-HSP70 (GeneTex®, 1:1000) and anti-rabbit-P540 aromatase polyclonal (Creative Diagnostics®, 1:1000) primary antibodies; the secondary antibodies was goat anti-rabbit IgG antibody pre-adsorbed (rhodamine) (GeneTex®, 1:1000). Samples were mounted with DAPI (Bioptica) and sealed with rubber cement. A fluorescence microscope (NIKON ECLIPSE Ci) was used to observe the larvae, and images were captured by a NIKON DS-Qi2 camera. Intracellular ROS content in zebrafish larvae after exposure to electromagnetic fields was detected by the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (DCFH 2-DA), which is useful for measuring reactive oxygen species (ROS). DCFH 2-DA is able to pass through the cell membrane, and it changes into DCFH-2 by action of intracellular esterase. DCFH-2 reacts with intracellular ROS, yielding the fluorescent compound DCF, which measured by fluorescent microscopy [25]. At 96 hpf, larvae exposed and not exposed to electromagnetic fields were stained with ROS-detection solution as described by Mugoni et al. [26]. The embryos were washed twice with Hank’s Balanced Salt Solution (HBSS) (ThermoFisher Scientific) for 2 min each in small tubes. ROS-detection solution (5 µM DCFH 2-DA in HBSS) was added to each tube and then incubated in the dark for 15 min at 28 °C. At the end of the incubation period, the ROS-detection solution was removed, and the larvae washed twice for 2 min with HBSS. Finally, they were placed on a glass slide. The fluorescence was observed using a fluorescence microscope (NIKON ECLIPSE Ci) equipped with a NIKON DS-Qi2 camera.
2.6. Statistical Analyses
Regarding on the cardiac activity acquired by the DanioScope™ (Noldus) software, the mean values were compared with GraphPad Software by two-way ANOVA to detect significant differences between the control and exposed groups at different times of exposure (p < 0.05). To process the images obtained by fluorescence microscopy, Image J software [27] was used. This software calculates the mean value (the sum of the values at all pixels divided by the number of pixels) of a specified area. In each photo, for control and exposed larvae, the same area (macro) was set to obtain density histograms. The mean values were compared with GraphPad Software using Student’s t-test to detect significant differences between the photos of exposed larvae and the photos of control groups (p < 0.05).
3. Results
3.1. Numerical Dosimetry Analyses
The resulting density power flow is reported in Figure 1b; as can be seen, although the power density was not uniform above the six-well microplates since the far-field condition was not satisfied [23], it reached maximum values (around 60 W/m2) in a small area between the two central wells. However, the power density decreased very fast in correspondence with the outermost wells and was comparable to 10 mW/cm2 (i.e., 100 W/m2), which was set by the international guidelines as the exposure limit greater than 6 GHz; see Table 2 in [23]. As far as SAR calculation is concerned, it is worth underlining that the specific absorption rate (SAR) averaged over a 10-g cubic volume, considered by international guidelines for frequencies less than 6 GHz, cannot be considered here since the total mass of the samples (5 g) was smaller than the average mass considered by ICNIRP guidelines [23]. For this reason, we report the local SAR as numerically evaluated in each cell of the grid used to discretize the samples in the wells (point SAR). The computed SAR level averaged in each layer of the aqueous samples is shown in Figure 1c. The SAR distribution was nonhomogeneous in the different aqueous samples since the largest values were reached in the central wells (2 and 5) due to the near-field condition exposure. Moreover, as expected, the bottom and top layers of the aqueous samples showed the largest SAR values due to a strong electromagnetic material discontinuity and small penetration depth in water at 27 GHz. Finally, the PLD also showed that the power deposition into the central samples is about four times larger than that in the peripheral samples. However, as fish moved randomly inside the sample, they experienced an average PLD value of about 2 mW/cm3 in the outermost samples (well numbers 1, 3, 4, and 6) and of about 8 mW/cm3 in the innermost ones (wells 2 and 5); see Figure 1d. Finally, it is worth noting that only numerical dosimetry was performed because continuous thermal monitoring during the exposure protocol was not viable due to the invasiveness of most common temperature probes (thermocouple or optical fiber) at these frequencies. However, temperature measurements of the aqueous sample during the water refilling increased by only 0.2 °C with respect to sham samples because of the thermally controlled water bath. This very low temperature increase cannot be the result of any thermal effect in the study at hand. Therefore, the overall dosimetric analysis suggests that no relevant thermal energy was delivered to the system by the radiation at millimeter waves.
3.2. Endpoints and Biomarkers Analysis
The evaluation of the endpoints set by the ZFET test was useful for investigating the effect of 27 GHz on embryonic development. Exposure to 27 GHz did not cause the coagulation of eggs. Both exposed and unexposed embryos had complete embryonic development; in fact, normal development of the head, notochord, fin, pigmentation, heart, and eyes was observed (Figure 2) [28].
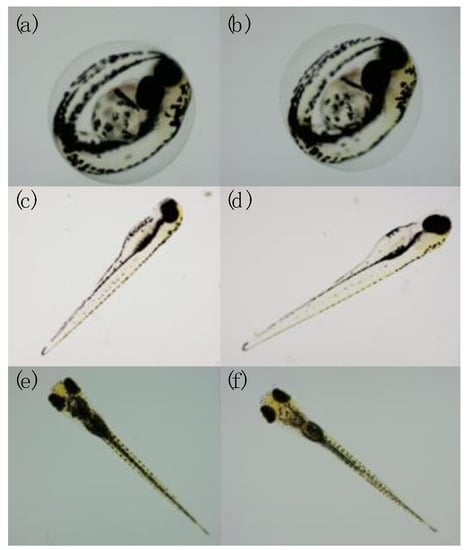
Figure 2.
Phenotypes of larvae unexposed (a,c,e) and exposed (b,d,f) to 27 GHz at 48 hpf (a,b); 72 hpf (c,d), and 96 hpf (e,f). No morphological defects were found.
Regarding the hatching of the larvae, there was no statistically significant difference (p > 0.05) between the controls and the exposed larvae (Figure 3).
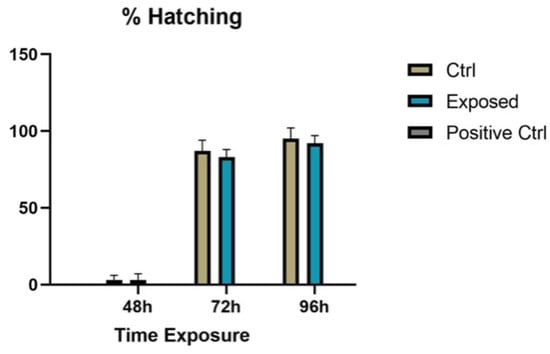
Figure 3.
Hatching rates of controls, embryos exposed to 27 GHz, and positive control exposed to 3,4-DCA. There are no values at 24 h since no hatching occurred physiologically at this stage.
Instead, a statistically significant difference was observed for heart rate. Thanks to the DanioScopeTM (Noldus) software, it was highlighted that the exposure to 27 GHz caused an increase in heart rate of the exposed embryos at 48 h compared to the control group, but this increase was no longer seen at 72 h and 96 h (shown in Figure 4), as supported by statistical analysis that was not significant. Heart rate variability is the most significant parameter for the study of cardiac function; in zebrafish, the heart rate is physiologically around 120–180 bpm, but its alteration is associated with cardiotoxicity [28,29].
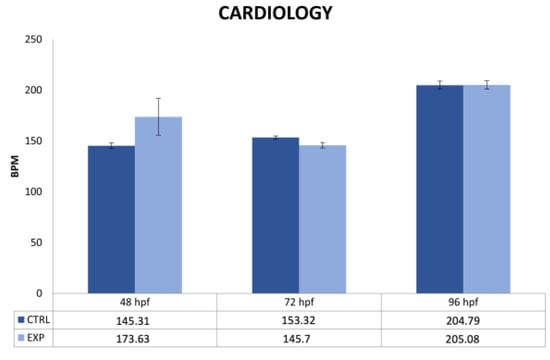
Figure 4.
Beats per minute (BPM) values of the control group and embryos exposed to 27 GHz obtained by DanioScope™ software. Under the histograms, the number of BPM found at each stage (expressed as a mean) is shown.
Nevertheless, post-hatching death was not observed. All embryos were vital until the end of ZFET.
By immunohistochemical investigation, we observed higher expression of P540 biomarker in the exposed larvae compared to the controls, but concerning HSP70, the expression was not increased in exposed larvae compared to the controls (Figure 5 and Figure 6).
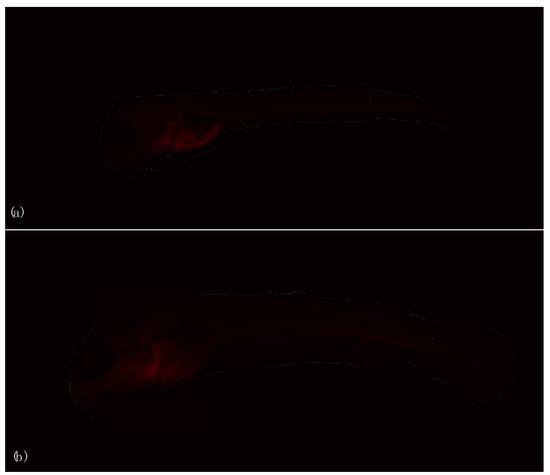
Figure 5.
Immunohistochemical investigation of P540 biomarker in larvae unexposed (a) (CTRL) and exposed (b) (EXP) to 27 GHz at the end of exposure.
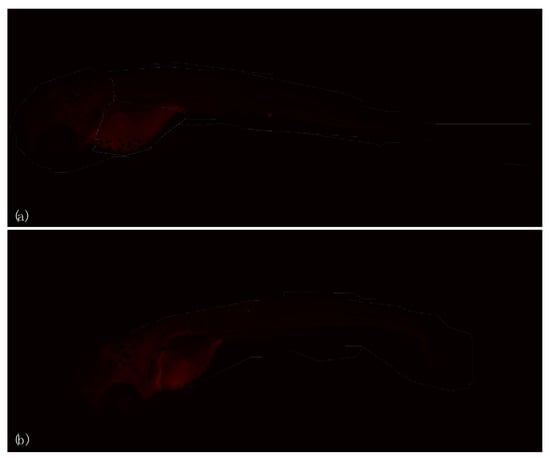
Figure 6.
Immunohistochemical investigation of HSP70 biomarker in larvae unexposed (a) (CTRL) and exposed (b) (EXP) to 27 GHz at the end of exposure.
The analysis did not confirm a statistically significant difference (p < 0.05) for P540 biomarker between the control group and the exposed larvae response (Figure 7). Relating to HSP70 biomarkers, a statistically significant difference was not obtained (p > 0.05) (Figure 8). Regarding ROS production, no statistically significant difference among the control group, larvae exposed to 27 GHz, and the positive control group was found (images not shown).
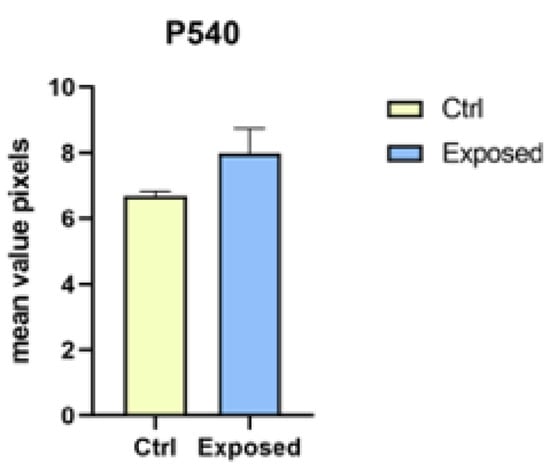
Figure 7.
Statistical analysis for P540 biomarker between the control group and larvae exposed to 27 GHz (no statistically significant difference was found for p < 0.05).
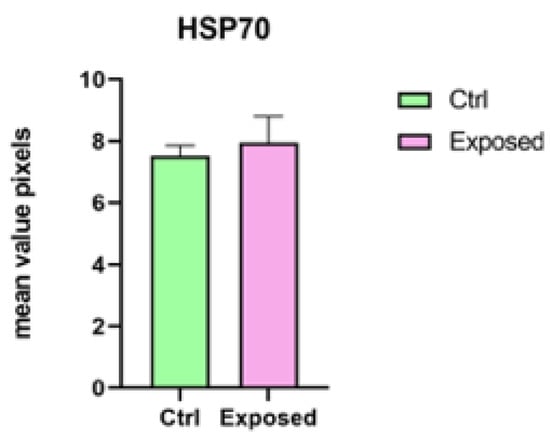
Figure 8.
Statistical analysis for HSP70 biomarker between the control group and larvae exposed to 27 GHz (no statistically significant difference was found at p < 0.05).
4. Discussion
The new fifth-generation technology (5G) should promote high data speed connections and latency times shorter than current ones since it is able to work at different frequency bands of the radio wave spectrum (700 MHz, 3.6–3.8 GHz and 26.5–27.5 GHz), thus also exploiting higher frequencies than previous generations of mobile radios (1G–4G). On the other hand, the higher frequency waves have less ability to propagate in free space; therefore, to ensure adequate radio coverage for high reliability applications, it will be necessary to install a large number of repeaters.
The introduction of this new technology gave rise to growing concerns about possible adverse effects on human health. Generally, exposure to electromagnetic fields (EMFs) has been linked to the production of reactive oxygen species (ROS) [30,31]. Some in vitro studies have shown that the production of ROS leads to cellular or systemic oxidative stress [32,33]; similarly, oxidative stress has been revealed in laboratory animal brains after EMF exposure [34].
In another study [35], the authors evaluated electromagnetic wave shielding performances of nonwoven textile surfaces of zebrafish embryos exposed to electromagnetic waves at frequencies between 15 and 3000 MHz. Their results showed that electromagnetic shielding fabrics produced as a conductive nonwoven fabric-influenced oxidant-antioxidant system (increased lipid peroxidation and nitric oxide levels, decreased superoxide dismutase activity, and increased glutathione S-transferase activity) in electromagnetic-wave-exposed zebrafish embryos. Additionally, tp53 expression was impaired in electromagnetic-wave-exposed zebrafish embryos, and electromagnetic shielding fabrics decreased apoptosis-related gene casp3a expression, while it increased in electromagnetic wave-exposed zebrafish embryos. These authors concluded that, thanks to the development of textile materials produced using conductive fibers and yarns, it is possible to shield a large part of impinging electromagnetic waves to protect the health of living organisms against the effects of electromagnetic radiation.
In our study, no production of ROS was detected by DCFH 2-DA assay in embryos after 96 h of exposure to electromagnetic fields at 27 GHz (data not shown). This outcome is in contrast with some of the data in the literature since this indicator is an important tool for highlighting the toxicity of many pollutants, as demonstrated by studies of neurons or neural cells in which ROS formation and the impairment of antioxidative protection measures occurred after exposure to electromagnetic fields (EMFs) [31,32,33].
In addition to oxidative stress, other radiofrequency exposure endpoints can help to assess biological effects. In this context, the best animal model is the zebrafish (Danio rerio). Commonly, D. rerio is the gold standard for evaluating the harmful effects of many xenobiotics, such as chemical compounds or engineered nanomaterials [36], but recently, it has also served as a reliable model to investigate the biological effects of radiofrequency waves. For example, Kim et al. [37] investigated the pro-pigmentation effect of pulsed electromagnetic fields (PEMFs) in a zebrafish model. Their results suggested that PEMFs, at an optimal intensity and frequency, promote pigmentation and are a useful tool for treating gray hair with reduced melanin synthesis in the hair shaft or hypopigmentation-related skin disorders.
The biological effects of extremely low-frequency magnetic fields (ELF-MF) (<300 Hz) on the development of zebrafish embryos were investigated by Li et al. [38]. Embryos in experimental groups were continuously exposed to 50-Hz sinusoidal MF with intensities of 30, 100, 200, 400 and 800 μT for 96 h. The results showed that ELF-MF exposure caused delayed hatching (the hatching rate was measured at 48, 54, 60, 72 and 96 h post-fertilization) in the groups exposed to high intensity of ELF-MF (200, 400, 800 μT) compared to that of the controls at 48, 54 and 60 h post-fertilization. Furthermore, they investigated the influence of ELF-MF exposure on the heart rate of zebrafish embryos (with the heart rate expressed as beats/15 s checked at 36, 48, 60 and 72 h post-fertilization). The results showed no significant differences between the embryos in the groups treated with low intensity of ELF-MF (30 and 100 μT) and the controls; however, significant bradycardia (low heart rate) was observed in embryos in the groups exposed to the relatively high intensity of ELF-MF (200, 400 and 800 μT) at 36, 48 and 60 h post-fertilization. Finally, no significant differences in embryo mortality were observed.
In our study, we investigated changes in heart rate using DanioScope™ (Noldus) software to measure the number of beats per minute (BPM) in the control group and embryos exposed to 27 GHz at 48, 72, and 96 hpf. We found that exposure to 27 GHz caused an increase in the heart rate of exposed embryos compared to that in the control group at 48 h, but this increase was not shown at 72 and 96 h (as shown by statistical analysis).
According to Piccinetti and colleagues [39], biological effects were no more visible at hatching time because of the activation of specific detoxification biological pathways.
Dasgupta and colleagues [40] attempted to assess whether exposure to 3.5-GHz radio frequency radiations (RFR) is associated with any developmental perturbations during embryogenesis of zebrafish. Their results did not reveal any large-scale effects of RFR exposure on embryonic survival or development but revealed a modest depression of sensorimotor function.
In another study, Fey and colleagues [41] used eggs and larvae of rainbow trout (Oncorhynchus mykiss) under experimental conditions to study the impact of a static magnetic field (MF) of 10 mT and a 50-Hz electromagnetic field (EMF) of 1 mT for a period of 36 days. They found that neither MF nor EMF had significant effects on embryonic or larval mortality, hatching time, larval growth, or the time of larvae swimming up from the bottom. However, both MF and EMF enhanced the yolk-sac absorption rate. Although it was not related directly to the magnetic field effect, it was also shown that larvae with absorbed yolk-sacs by the time of swimming up were less efficient in taking advantage of available food at first feeding, indicating the importance of processes affecting the yolk-sac absorption rate.
Our results are aligned with some studies’ insights [39,40,41] since, even at the higher frequency used for 96 h of exposure, no alterations were obtained in embryonic development or in mortality after hatching.
Regarding biomarkers used in our research, we found by immunohistochemical investigation a higher expression of P540 biomarker in the exposed larvae compared to controls, but regarding HSP70, the expression was not increased in exposed larvae compared to the controls. Electromagnetic field exposures have been shown to induce heat shock proteins (HSPs) in chick embryos continuously exposed to EMFs (8 mT) for 4 days and to radio-frequency (RF, 3.5 mW incident power) repeated daily (20, 30, or 60 min once or twice daily for 4 days) [42]. In a previous study, the same authors reported that short-term exposure of chick embryos to extremely low frequency 60 Hz or radio-frequency (915 MHz)-induced protection against hypoxia. In another study [43], a modulation of kinetic parameters, such as CYP-450 and other enzymes, was demonstrated in response to extremely low frequency (ELF)-EMF, indicating an interaction between ELF-EMF and enzyme systems. These results confirmed that the ELF-EMF can affect not only ROS production but also the enzymatic activity influencing metabolism and ROS signaling.
Finally, it is reasonable to think that the resulting discrepancies between several analyzed studies may be explained by the different conditions of set-up exposure and by the wide range of frequency bands adopted to study the impact of electromagnetic waves. In light of these results and data from the literature, it will be needed and reasonable to investigate the EMF dose-response effects more to have a clearer overview of the electromagnetic impact on living beings.
5. Conclusions
Using embryos and larvae of zebrafish as a model organism, we have shown that exposure to the 27 GHz frequency does not interfere with embryonic development, although an increase in heart rate was observed at 48 hpf (increase not shown at 72 and 96 h), confirming that the first stages of development are always particularly sensitive. These results demonstrate that the zebrafish is an efficient in vivo model animal for studying EMF effects on embryonic development. This study could be useful for investigating the potential ecological impact of EMFs on aquatic animals, which currently are poorly investigated. Further experimental studies should enrich our knowledge about EMF effects and also reveal whether they can be hazardous to human health.
Author Contributions
Conceptualization, L.D.D. and M.V.B.; methodology, R.P., E.M.S., F.C., C.I., S.C.P. and G.S.; investigation, R.P., S.I. (Sara Ignoto), C.S. and S.I. (Stefania Indelicato); data curation, R.P., S.I. (Sara Ignoto) and E.M.S.; writing—original draft preparation, R.P., L.D.D. and M.V.B.; writing—review and editing, R.P., S.C.P., E.M.S. and M.V.B.; visualization, A.S.; supervision, L.D.D., R.P. and M.V.B.; funding acquisition, L.D.D. and M.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Programma ricerca di Ateneo UNICT 2020-22 linea 2”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattsson, M.O.; Simkó, M.; Foster, K.R. 5G new radio requires the best possible risk assessment studies: Perspective and recommended guidelines. Front. Comms. Net. 2021, 49, 724772. [Google Scholar] [CrossRef]
- Attaran, M. The impact of 5G on the evolution of intelligent automation and industry digitization. J. Ambient Intell. Human. Comput. 2021, 1–17. [Google Scholar] [CrossRef]
- Miller, A.B.; Sears, M.E.; Morgan, L.L.; Davis, D.L.; Hardell, L.; Oremus, M.; Soskolne, C.L. Risks to Health and Well-Being from Radio-Frequency Radiation Emitted by Cell Phones and Other Wireless Devices. Front. Public Health. 2019, 7, 223. [Google Scholar] [CrossRef]
- Sienkiewicz, Z.; Calderón, C.; Broom, K.A.; Addison, D.; Gavard, A.; Lundberg, L.; Maslanyj, M. Are Exposures to Multiple Frequencies the Key to Future Radiofrequency Research? Front. Public Health 2017, 5, 328. [Google Scholar] [CrossRef]
- Foster, K.R.; Vijayalaxmi. Needed: More Reliable Bioeffects Studies at “High Band” 5G Frequencies. Front. Comms. Net. 2021, 2, 721925. [Google Scholar] [CrossRef]
- Larsen, A.I.; Skotte, J. Hazardous health effects of microwaves and radio waves. Ugeskr Laeger. 1994, 156, 1618–1623. [Google Scholar] [PubMed]
- Simkó, M.; Remondini, D.; Zeni, O.; Scarfi, R.M. Quality Matters: Systematic Analysis of Endpoints Related to ‘Cellular Life’ In Vitro Data of Radiofrequency Electromagnetic Field Exposure. Int. J. Environ. Res. Public Health. 2016, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, D. Physiological Effects of Millimeter-Waves on Skin and Skin Cells: An Overview of the To-Date Published Studies. Rev. Environ. Health 2020, 35, 493–515. [Google Scholar] [CrossRef]
- Wood, A.; Mate, R.; Karipidis, K. Meta-Analysis of In Vitro and In Vivo Studies of the Biological Effects of Low-Level Millimetre Waves. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 606–613. [Google Scholar] [CrossRef]
- Brundo, M.V.; Pecoraro, R.; Marino, F.; Salvaggio, A.; Tibullo, D.; Saccone, S.; Bramanti, V.; Buccheri, M.A.; Impellizzeri, G.; Scuderi, V.; et al. Toxicity evaluation of new engineered nanomaterials in zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef]
- Pecoraro, R.; Salvaggio, A.; Marino, F.; Caro, G.D.; Capparucci, F.; Lombardo, B.M.; Messina, G.; Scalisi, E.M.; Tummino, M.; Loreto, F.; et al. Metallic nano-composite toxicity evaluation by zebrafish embryo toxicity test with identification of specific exposure biomarkers. Curr. Protoc. Toxicol. 2017, 74, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hulot, G.; Finlay, C.C.; Constable, C.G.; Olsen, N.; Mandea, M. The magnetic field of planet Earth. Space Sci. Rev. 2010, 152, 159–222. [Google Scholar] [CrossRef]
- Krylov, V.V.; Izyumov, Y.G.; Izvekov, E.I.; Nepomnyashchikh, V.A. Magnetic fields and fish behavior. Biol. Bull. Rev. 2014, 4, 222–231. [Google Scholar] [CrossRef]
- Putman, N.F.; Lohmann, K.J.; Putman, E.M.; Quinn, T.P.; Klimley, A.P.; Noakes, D.L. Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr. Biol. 2013, 23, 312–316. [Google Scholar] [CrossRef]
- Brothers, J.R.; Lohmann, K.J. Evidence that magnetic navigation and geomagnetic imprinting shape spatial genetic variation in sea turtles. Curr. Biol. 2018, 28, 1325–1329. [Google Scholar] [CrossRef]
- Andrulewicz, E.; Napierska, D.; Otremba, Z. The environmental effects of the installation and functioning of the submarine SwePol Link HVDC transmission line: A case study of the Polish Marine Area of the Baltic Sea. J. Sea Res. 2003, 49, 337–345. [Google Scholar] [CrossRef]
- Lin, L.; Yu, H. Offshore wave energy generation devices: Impacts on ocean bio-environment. Acta Ecol. Sin. 2012, 32, 117–122. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Yamashita, M.; Yabu, T.; Ojima, N. Stress protein HSP70 in fish. Aqua-BioScience Monogr. 2010, 3, 111–141. [Google Scholar] [CrossRef]
- Morimoto, R.I.; Tissieres, A.; Georgopoulos, C.P. The stress response, function of the proteins, and perspectives. In Stress Proteins in Biology and Medicine; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1990; pp. 1–36. [Google Scholar]
- Yamashita, M.; Hojo, M. Generation of a transgenic zebrafish model overexpressing heat shock protein HSP70. Mar. Biotechnol. 2004, 6, S1–S7. [Google Scholar]
- Ojima, N.; Yamashita, M.; Watabe, S. Comparative expression analysis of two paralogous Hsp70s in rainbow trout cells exposed to heat stress. Biochim. Biophys. Acta Gene Struct. Expr. 2005, 1681, 99–106. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- OECD. Guideline for the Testing of Chemicals. Fish Embryo Toxicity (FET); N.236; OECD: Paris, France, 2013. [Google Scholar]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of Oxidative Stress in Zebrafish Embryos. J. Vis. Exp. 2014, 89, 51328. [Google Scholar]
- Bankhead, P. Analyzing fluorescence microscopy images with ImageJ. ImageJ 2014, 1, 10–1109. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- Wei., Y.; Meng, Y.; Huang, Y.; Liu, Z.; Zhong, K.; Ma, J.; Zhang, W.; Li, Y.; Lu, H. Development toxicity and cardiotoxicity in zebrafish from exposure to iprodione. Chemosphere 2021, 263, 127860. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Margaritis, L.H. Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects. Int. J. Mol. Sci. 2021, 22, 10041. [Google Scholar] [CrossRef]
- Gu, J.; Wang, H.; Zhou, L.; Fan, D.; Shi, L.; Ji, G.; Gu, A. Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci. Total Environ. 2020, 731, 139190. [Google Scholar] [CrossRef]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. Int. 2018, 25, 18286–18296. [Google Scholar] [CrossRef] [PubMed]
- Alkis, M.E.; Bilgin, H.M.; Akpolat, V.; Dasdag, S.; Yegin, K.; Yavas, M.C.; Akdag, M.Z. Effect of 900-, 1800, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn. Biol. Med. 2019, 38, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ, Ü.V.; Özen, M.S.; Ünal, İ.; Ateş, P.S.; Alturfan, A.A.; Akalın, M.; Sancak, E.; Emekli-Alturfan, E. Oxidative stress and apoptosis in electromagnetic waves exposed Zebrafish embryos and protective effects of conductive nonwoven fabric. Cell. Mol. Biol. 2020, 66, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Nel, A.E.; Lin, S. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lim, H.M.; Ro, H.S.; Ki, G.E.; Seo, Y.K. Pulsed electromagnetic fields increase pigmentation through the p-ERK/p-p38 pathway in zebrafish (Danio rerio). Int. J. Mol. Sci. 2018, 19, 3211. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Liu, K.; Miao, W.; Zhou, C.; Li, Y.; Wu, H. Extremely low-frequency magnetic fields induce developmental toxicity and apoptosis in zebrafish (Danio rerio) embryos. Biol. Trace Elem. Res. 2014, 162, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Piccinetti, C.C.; De Leo, A.; Cosoli, G.; Scalise, L.; Randazzo, B.; Cerri, G.; Olivotto, I. Measurement of the 100 MHz EMF radiation in vivo effects on zebrafish D. rerio embryonic development: A multidisciplinary study. Ecotoxicol. Environ. Saf. 2018, 154, 268–279. [Google Scholar] [CrossRef]
- Dasgupta, S.; Wang, G.; Simonich, M.T.; Zhang, T.; Truong, L.; Liu, H.; Tanguay, R.L. Impacts of high dose 3.5 GHz cellphone radiofrequency on zebrafish embryonic development. PLoS ONE 2020, 15, e0235869. [Google Scholar] [CrossRef]
- Fey, D.P.; Jakubowska, M.; Greszkiewicz, M.; Andrulewicz, E.; Otremba, Z.; Urban-Malinga, B. Are magnetic and electromagnetic fields of anthropogenic origin potential threats to early life stages of fish? Aquat. Toxicol. 2019, 209, 150–158. [Google Scholar] [CrossRef]
- Di Carlo, A.; White, N.; Guo, F.; Garrett, P.; Litovitz, T. Chronic electromagnetic field exposure decreases HSP70 levels and lowers cytoprotection. J. Cell. Biochem. 2022, 84, 447–454. [Google Scholar] [CrossRef]
- Patruno, A.; Tabrez, S.; Pesce, M.; Shakil, S.; Kamal, M.A.; Reale, M. Effects of extremely low frequency electromagnetic field (ELF-EMF) on catalase, cytochrome P450 and nitric oxide synthase in erythro-leukemic cells. Life Sci. 2015, 121, 117–123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).