Morphology, Molecular Genetics and Potential Importance for Mucilage Events of the New Coccolithophorid Ochrosphaera neapolitana in the Sea of Marmara
Abstract
1. Introduction
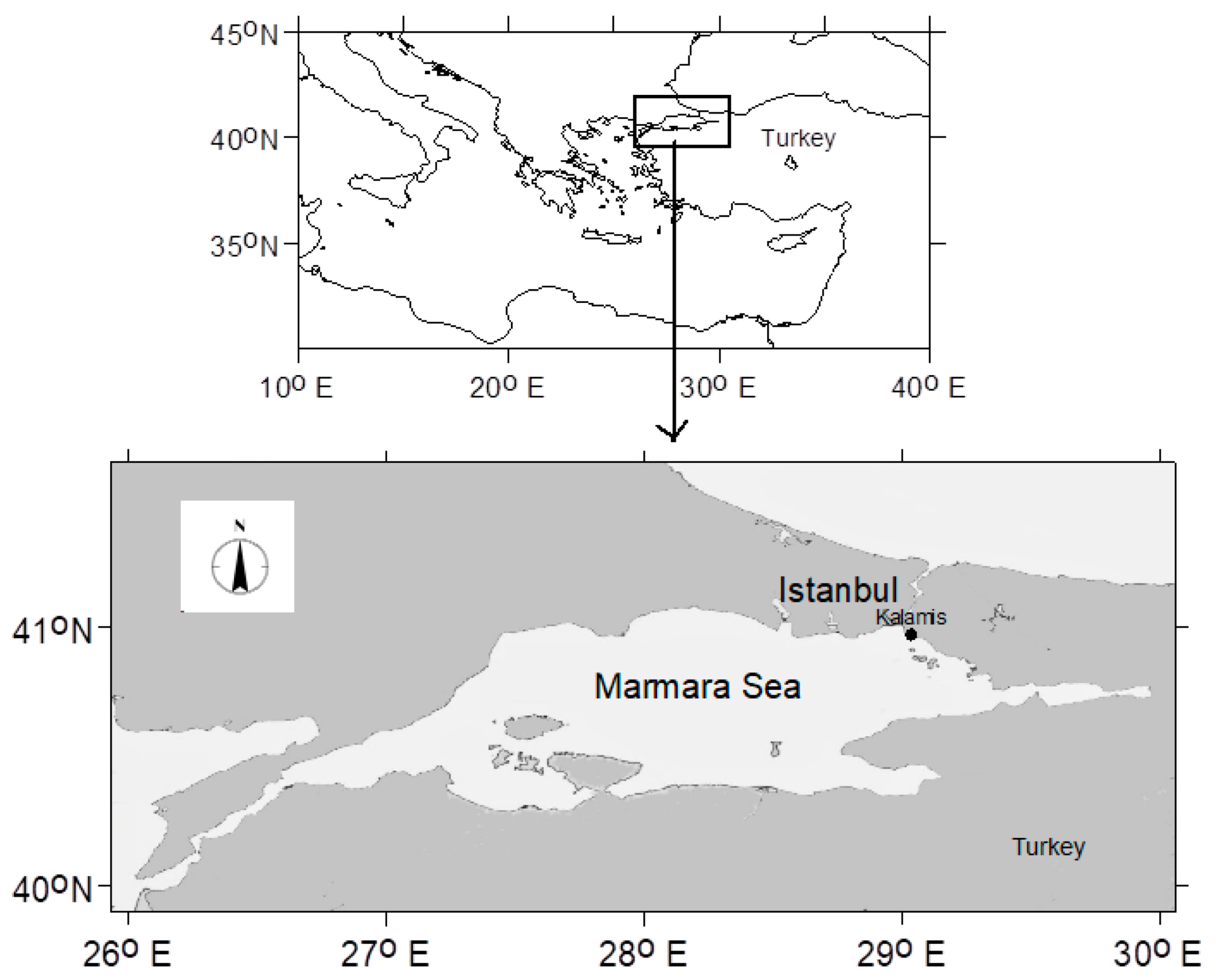
2. Materials and Methods
2.1. Microscopy
2.2. DNA Isolation, PCR Analysis, and Phylogenetic Tree Construction
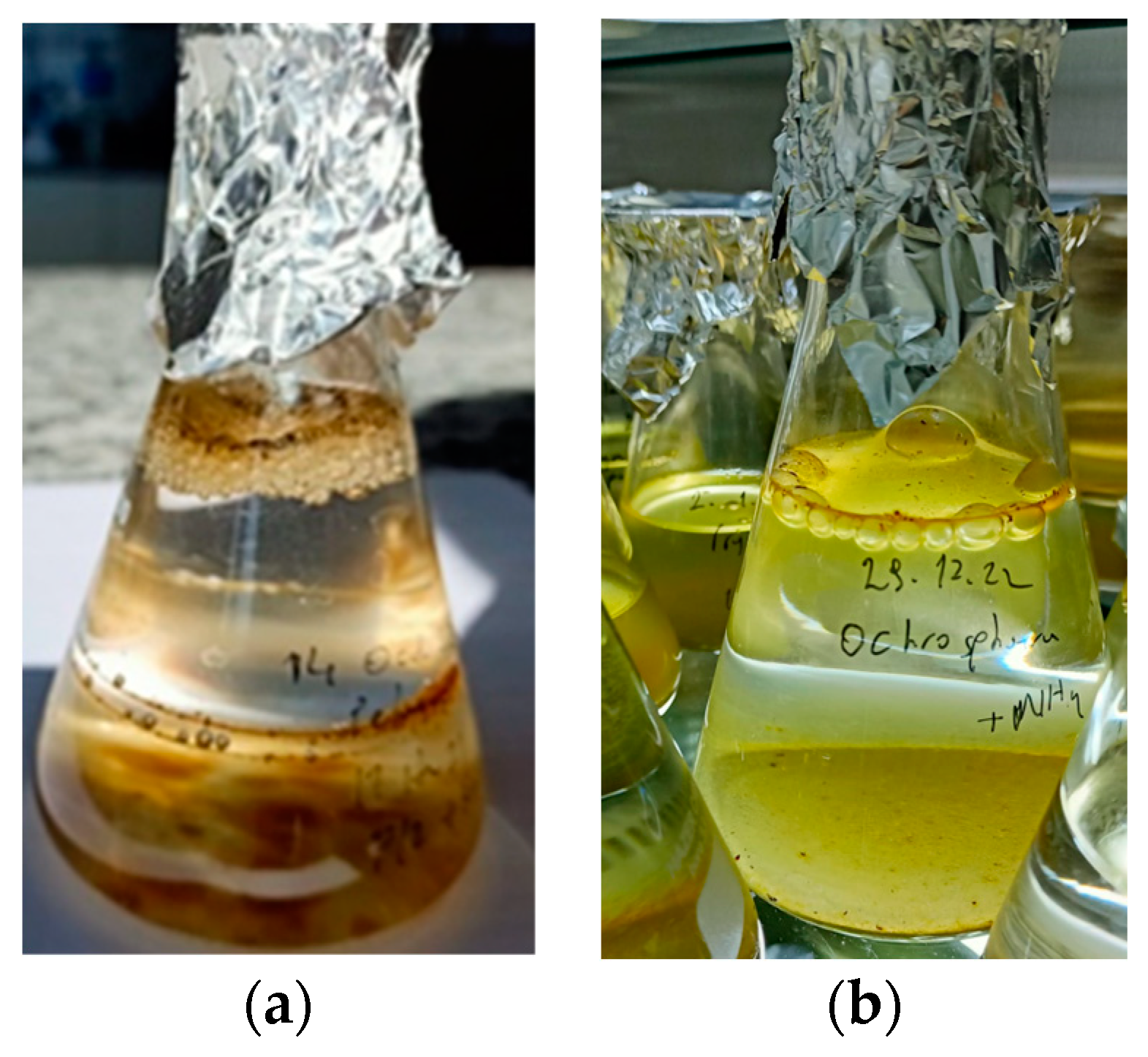
2.3. Mucilage Observation
3. Results
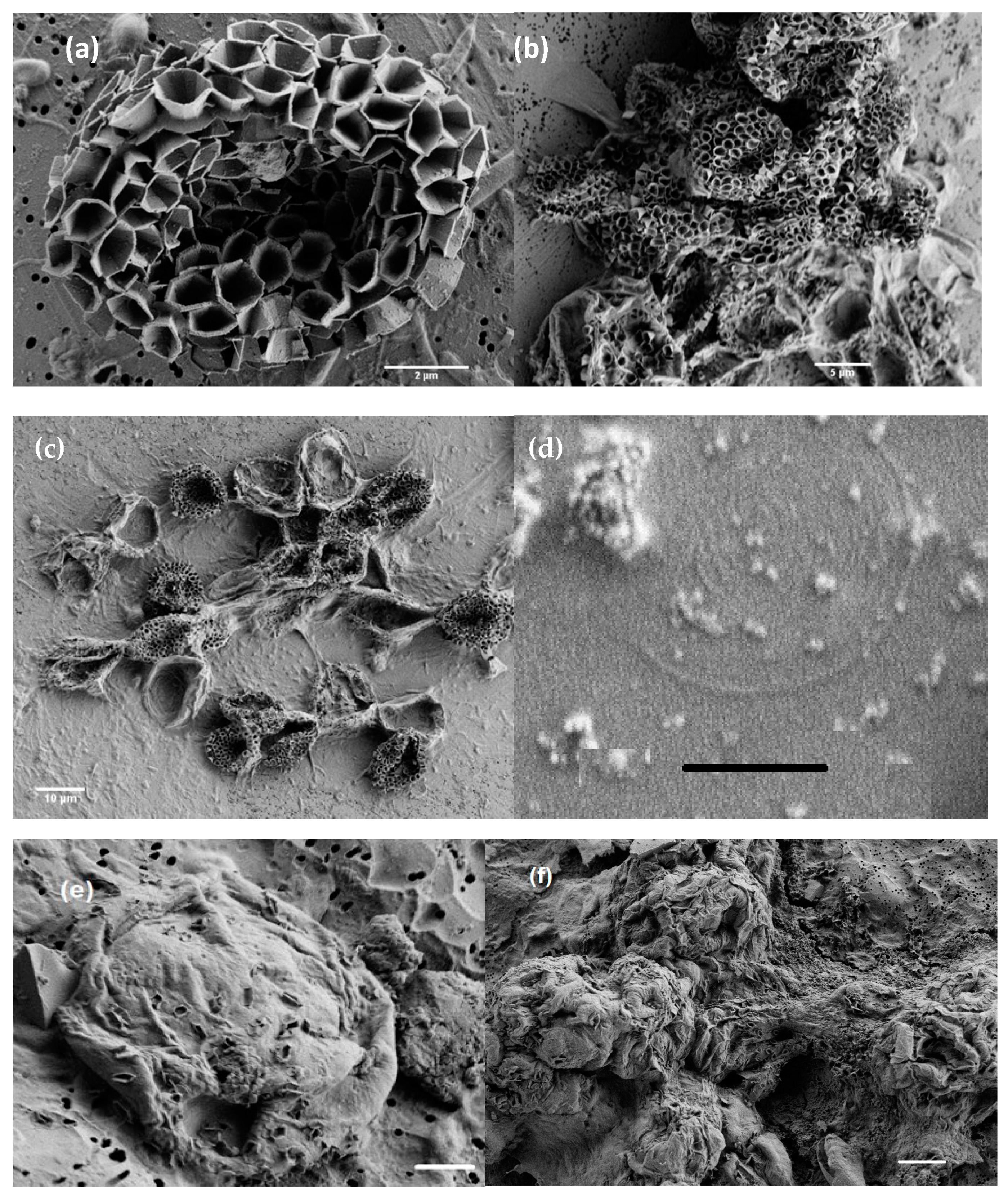
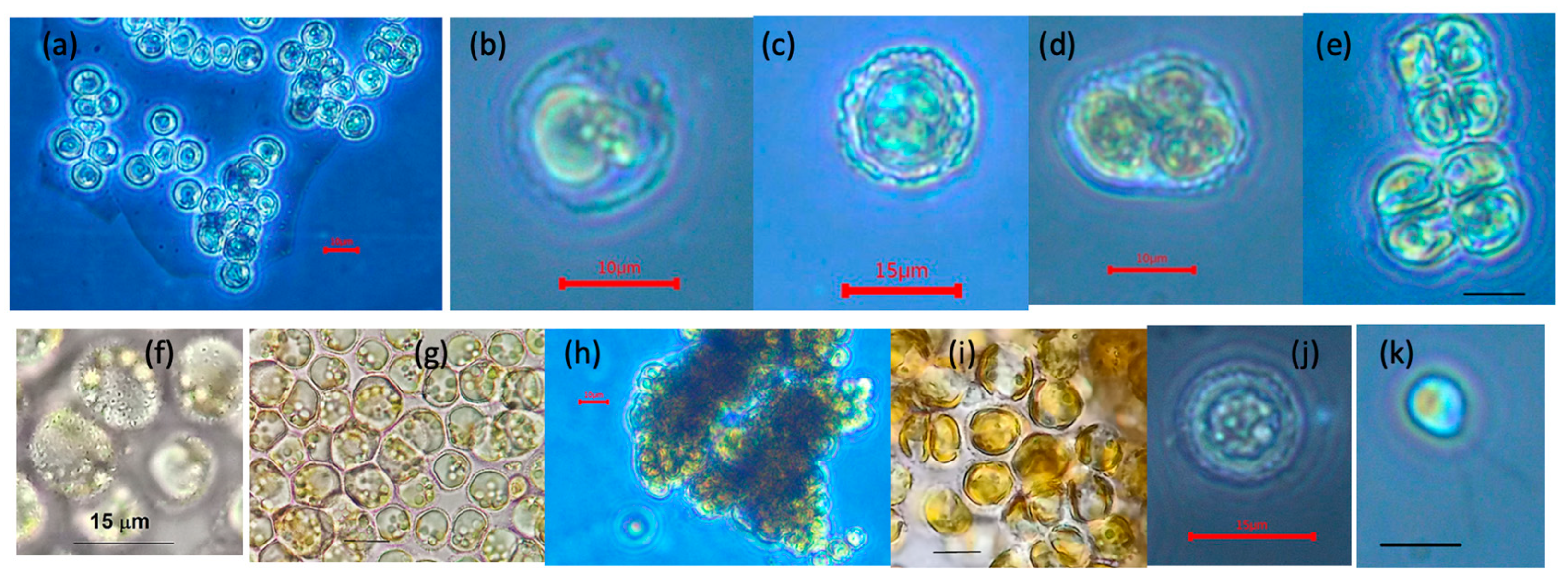
3.1. Morphology from Light and SEM Microscopy
3.2. Molecular Analysis
3.3. Mucilage Observation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degobbis, D.; Fonda-Umani, S.; Franco, P.; Malej, A.; Precali, R.; Smodlaka, N. Changes in the northern Adriatic ecosystem and hypertrophic appearance of gelatinous aggregates. Sci. Total Environ. 1995, 165, 43–58. [Google Scholar] [CrossRef]
- Fonda-Umani, S.; Milani, L.; Borme, D.; de Olazabal, A.; Parlato, S.; Precali, R.; Kraus, R.; Lučić, D.; Njire, J.; Totti, C.; et al. Inter-annual variations of planktonic food webs in the northern Adriatic Sea. Sci. Total Environ. 2005, 353, 218–231. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from microalgae and cyanobacteria: Diversity of strains, production strategies, and applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Svetličić, V.; Žutić, V.; Radić, T.M.; Pletikapić, G.; Zimmermann, A.H.; Urbani, R. Polymer networks produced by marine diatoms in the Northern Adriatic Sea. Mar. Drugs 2011, 9, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Penna, N.; Capellaci, S.; Penna, A. Potential environmental factors influencing mucilage formation in the northern Adriatic Sea. Chem. Ecol. 2014, 30, 364–375. [Google Scholar] [CrossRef]
- Ergul, H.A.; Balkis-Ozdelice, N.; Koral, M.; Aksan, S.; Durmus, T.; Kaya, M.; Kayal, M.; Ekmekci, F.; Canli, O. The early stage of mucilage formation in the Marmara Sea during spring. J. Black Sea/Mediterr. Environ. 2021, 27, 232–257. [Google Scholar]
- Turk, V.; Hagström, A.; Kova, N.; Faganeli, J. Composition and function of mucilage 42. macroaggregates in the northern Adriatic. Aquat. Microb. Ecol. 2010, 61, 279–289. [Google Scholar] [CrossRef]
- Danovaro, R.; Umani, S.F.; Pusceddu, A. Climate change and the potential spreading of marine mucilage and microbial pathogens in the Mediterranean Sea. PLoS ONE 2009, 4, e7006. [Google Scholar] [CrossRef]
- Uflaz, E.; Akyüz, E.; Bolat, F.; Bolat, P.; Arslan, Ö. Investigation of the effects of mucilage on maritime operation. J. Black Sea/Mediterr. Environ. 2021, 27, 140–153. [Google Scholar]
- Topçu, N.E.; Öztürk, B. The impact of the massive mucilage outbreak in the Sea of Marmara on gorgonians of Prince Islands: A qualitative assessment. J. Black Sea/Mediterr. Environ. 2021, 27, 270–278. [Google Scholar]
- Lancelot, C. The mucilage phenomenon in the continental coastal waters of the North Sea. Sci. Total Environ. 1995, 165, 83–102. [Google Scholar] [CrossRef]
- Najdek, M.; Blazina, M.; Djakovac, T.; Kraus, R. The role of the diatom Cylindrotheca closterium in a mucilage event in the northern Adriatic Sea: Coupling with high salinity water intrusions. J. Plankton Res. 2005, 27/9, 851–862. [Google Scholar] [CrossRef]
- Besiktepe, S.T.; Sur, H.İ.; Ozsoy, E.; Latif, M.A.; Oguz, T.; Unluata, U. The circulation and hydrography of the Marmara Sea. Prog. Oceanogr. 1994, 34, 285–334. [Google Scholar] [CrossRef]
- Orhon, D.; Uslu, O.; Meriç Salihoğlu, S.; Filibeli, I. Waste-water management for Istanbul: Basis for treatment and disposal. Environ. Pollut. 1994, 84, 167–178. [Google Scholar] [CrossRef]
- Burak, S. Evaluation of pollution abatement policies in the Marmara Sea with water quality monitoring. Asian J. Chem. 2008, 20, 4117–4128. [Google Scholar]
- TUIK. Municipality Wastewater Statistics 2018. 2018. Available online: https://biruni.tuik.gov.tr/medas/?kn=120&locale=tr (accessed on 14 August 2021).
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef] [PubMed]
- Aktan, Y.; Dede, A.; Çiftçi, P.S. Mucilage event associated with diatoms and dinoflagellates in Sea of Marmara, Turkey. Harmful Algae News 2008, 36, 1–3. [Google Scholar]
- Tüfekçi, V.; Balkıs, N.; Beken, C.P.; Ediger, D.; Mantıkçı, M. Phytoplankton composition and environmental conditions of a mucilage event in the Sea of Marmara. Turk. J. Biol. 2010, 34, 199–210. [Google Scholar] [CrossRef]
- Balkis, N.; Atabay, H.; Türetgen, I.; Albayrak, S.; Balkis, H.; Tüfekçi, V. Role of single-celled organisms in mucilage formation on the shores of Büyükada Island (the Marmara Sea). J. Mar. Biol. Assoc. United Kingd. 2011, 91, 771–781. [Google Scholar] [CrossRef]
- İşinibilir-Okyar, M.; Üstün, F.; Orun, D.A. Changes in abundance and community structure of zooplankton population during the 2008 mucilage event in the northeastern Marmara Sea. Turk. J. Zool. 2015, 39, 28–38. [Google Scholar] [CrossRef]
- Balkis-Ozdelice, N.; Durmus, T.; Balci, M. A preliminary study on the intense pelagic and benthic mucilage phenomenon observed in the Sea of Marmara. Int. J. Environ. Geoinformatics 2021, 8, 414–422. [Google Scholar] [CrossRef]
- Karadurmuş, U.; Sarı, M. Marine mucilage in the Sea of Marmara and its effects on the marine ecosystem: Mass deaths. Turk. J. Zool. 2022, 46, 6. Available online: https://journals.tubitak.gov.tr/zoology/vol46/iss1/6 (accessed on 18 January 2023). [CrossRef]
- Taş, S.; Kus, D.; Yilmaz, N. Temporal variations in phytoplankton composition in the northeastern Sea of Marmara: Potentially toxic species and mucilage event. Mediterr. Mar. Sci. 2020, 21, 668–683. [Google Scholar] [CrossRef]
- Durmuş, T.; Balkıs-Ozdelıce, N.; Taş, S.; Bayram Partal, F.; Balcı, M.; Dalyan, C.; Sarı, M. New Records for Microalgae Species of the Turkish Seas Under the Effect of Intense Mucilage in the Sea of Marmara. Eur. J. Biol. 2022, 81, 144–162. [Google Scholar] [CrossRef]
- Eker-Develi, E.; Kiydes, A.E. First record of the diatom Nitzschia navis-varingica in the Sea of Marmara. Mar. Sci. Tech. Bull. 2022, 11, 231–235. [Google Scholar]
- Svetlichny, L.; Isinibilir, M.; Kideys, A.E. First finding of live specimens of non-native copepod Calanus finmarchicus (Gunnerus, 1770) in the Sea of Marmara. Aquat. Sci. Eng. 2023. [Google Scholar] [CrossRef]
- İmamoğlu, E.; Demirel, Z.; Dalay, M.C. Evaluation of different microalgae strains for biomass production. In Proceedings of the First National Workshop on Marine Biotechnology and Genomics, Bodrum, Mugla, Turkey, 24–25 May 2012; Turan, C., Ed.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2012; Volume 36, pp. 107–112. [Google Scholar]
- Fresnel, J.; Probert, I. The ultrastructure and life cycle of the coastal coccolithophorid Ochrosphaera neapolitana (Prymnesiophyceae). Eur. J. Phycol. 2005, 40, 105–122. [Google Scholar] [CrossRef]
- Inouye, I.; Pienaar, R.N. Light, and electron microscope observations of the type species of Syracosphaera, S. pulchra (Prymnesiophyceae). Br. Phycol. J. 1988, 23, 205–217. [Google Scholar] [CrossRef]
- Schwartz, E. Beitra ge zur Entwicklungsgeschichte der Protophyten. Arch. Protistenk. 1932, 77, 434–462. [Google Scholar]
- Gastineau, R.; Hansen, G.; Davidovich, N.A.; Davidovich, O.; Bardeau, J.F.; Kaczmarska, I.; Mouget, J.L. A new blue-pigmented haploid diatom, Haslea provincialis, from the Mediterranean Sea. Eur. J. Phycol. 2016, 51, 156–170. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Ochrosphaera Schussnig, 1930. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=601148 (accessed on 12 January 2023).
- Schussnig, B. Über einige neue Protophyten aus der Adria. Arch. Protistenk. 1940, 93, 317–330. [Google Scholar]
- Norris, R.E. Microalgae in enrichment cultures from Puerto Penasco, Sonora, Mexico. Bull. South Calif. Acad. Sci. 1967, 66, 233–250. [Google Scholar]
- Degobbis, D.; Malej, A.; Fonda Umani, S. The mucilage phenomenon in the northern Adriatic Sea: A critical review of the present scientific hypotheses. Ann. Dell’istituto Super. Di Sanità 1999, 35, 373d381. [Google Scholar]
- McKenzie, L.; Sims, I.; Beuzenberg, V.; Gillespie, P. Mass accumulation of mucilage caused by dinoflagellate polysaccharide exudates in Tasman Bay, New Zeland. Harmful Algae 2002, 1, 69–83. [Google Scholar] [CrossRef]
- Malej, A.; Mozetič, P.; Turk, V.; Terzič, S.; Ahel, M.; Cauwet, G. Changes in particulate and dissolved organic matter in nutrient-enriched enclosures from an area influenced by mucilage: The northern Adriatic Sea. J. Plankton Res. 2003, 25, 949–966. [Google Scholar] [CrossRef]
- Cozzi, S.; Ivancic, I.; Catalano, G.; Djakovac, T.; Degobbis, D. Dynamics of the oceanographic properties during mucilage appearance in the Northern Adriatic Sea: Analysis of the 1997 event in comparison to earlier events. J. Mar. Syst. 2004, 50, 223–241. [Google Scholar] [CrossRef]
- Fukao, T.; Kimoto, K.; Yamatogi, T.; Yamamoto, K.; Yoshida, Y.; Kotani, Y. Marine mucilage in Ariake Sound, Japan, is composed of transparent exopolymer particles produced by the diatom Coscinodiscus granii. Fish. Sci. 2009, 75, 1007e1014. [Google Scholar] [CrossRef]
- Schiaparelli, S.; Castellano, M.; Povero, P.; Sartoni, G.; Cattaneo-Vietti, R. A benthic mucilage event in North-Western Mediterranean Sea and its possible relationships with the summer 2003 European heatwave: Short term effects on littoral rocky assemblages. Mar. Ecol. 2007, 28, 1–13. [Google Scholar] [CrossRef]
- Fernandes, L.F.; Frassão-Santos, E.K. Mucilaginous species of Thalassiosira Cleve emend. Hasle (Diatomeae) in South Brazilian waters. Acta Bot. Bras. 2011, 25, 31–42. [Google Scholar] [CrossRef]
- Lowenstein, D.P.; Mayers, K.; Fredricks, H.F.; Van Mooy, B.A.S. Targeted and untargeted lipidomic analysis of haptophyte cultures reveals novel and divergent nutrient-stress adaptations. Org. Geochem. 2021, 161, 104315. [Google Scholar] [CrossRef]
- Dimiza, M.D.; Triantaphyllou, M.V.; Malinverno, E.; Psarra, S.; Karatsolis, B.T.; Mara, P.; Lagaria, A.; Gogou, A. The composition and distribution of living coccolithophores in the Aegean Sea (NE Mediterranean). Micropaleontology 2015, 61, 521–540. [Google Scholar] [CrossRef]
- Fresnel, J. Les Coccolithophorides (Prymnesiophyceae) du Littoral. Genres: Cricosphaera, Cruciplacolithus, Hymenomonas et Ochrosphaera, Ultrastructure, Cycle Biologique, Syste´Matique. Ph.D. Thesis, Universite´ de Caen, Caen, France, 1989; p. 281. [Google Scholar]
- Bonomo, S.; Cascella, A.; Alberico, I.; Lirer, F.; Vallefuoco, M.; Marsella, E.; Ferraro, E. Living and thanatocoenosis coccolithophore communities in a neritic area of the central Tyrrhenian Sea. Mar. Micropaleontol. 2018, 142, 67–91. [Google Scholar] [CrossRef]
- Karatsolis, B.-T.; Triantaphyllou, M.; Dimiza, M.; Malinverno, E.; Lagaria, A.; Mara, P.; Archontikis, O.; Psarra, S. Coccolithophore assemblage response to Black Sea Water inflow into the North Aegean Sea (NE Mediterranean). Cont. Shelf Res. 2017, 149, 138–150. [Google Scholar] [CrossRef]
- Meyer, A.; Todt, C.; Mikkelsen, N.T.; Lieb, B. Fast evolving 18S rRNA sequences from Solenogastres (Mollusca) resist standard PCR amplification and give new insights into mollusk substitution rate heterogeneity. BMC Evol. Biol. 2010, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Godrijan, J.; Drapeau, D.; Balch, W.M. Mixotrophic uptake of organic compounds by coccolith-ophores. Limnol. Oceanogr. 2020, 65, 1410–1421. [Google Scholar] [CrossRef]
- Pompei, M.; Mazziotti, C.; Guerrini, F.; Cangini, M.; Pigozzi, S.; Benzib, M.; Palamidesic, S.; Bonic, L.; Pistocchi, R. Correlation between the presence of Gonyaulax fragilis (Dinophyceae) and the mucilage phenomena of the Emilia-Romagna coast (northern Adriatic Sea). Harmful Algae 2003, 2, 301–316. [Google Scholar] [CrossRef]
- Pistocchi, R.; Cangini, M.; Totti, C.; Urbani, R.; Guerrini, F.; Romagnoli, T.; Sist, P.; Palamidesi, S.; Boni, L.; Pompei, M. Relevance of the dinoflagellate Gonyaulax fragilis in mucilage formations of the Adriatic Sea. Sci. Total Environ. 2005, 353, 307–316. [Google Scholar] [CrossRef]
- Shaika, N.A.; Alhomaidi, E.; Sarker, M.M.; An Nur, A.; Sadat, M.A.; Awal, S.; Mostafa, G.; Hasan, S.J.; Mahmud, Y.; Khan, S. Winter bloom of marine cyanobacterium, Trichodesmium erythraeum and its relation to environmental factors. Sustainability 2023, 15, 1311. [Google Scholar] [CrossRef]
- Bolinesi, F.; Saggiomo, M.; Aceto, S.; Cordone, A.; Serino, E.; Valoroso, M.C.; Mangoni, O. On the relationship between a novel Prorocentrum sp. and colonial Phaeocystis antarctica under iron and vitamin B12 limitation: Ecological implications for Antarctic waters. Appl. Sci. 2020, 10, 6965. [Google Scholar] [CrossRef]
- Escalera, L.; Italiano, A.; Pistocchi, R.; Montresor, M.; Zingone, A. Gonyaulax hyalina and Gonyaulax fragilis (Dinoflagellata), two names associated with ‘mare sporco’, indicate the same species. Phycologia 2018, 57, 453–464. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eker-Develi, E.; Tekdal, D.; Demet, A.E.; Yıldız, H.B.; Kideys, A.E. Morphology, Molecular Genetics and Potential Importance for Mucilage Events of the New Coccolithophorid Ochrosphaera neapolitana in the Sea of Marmara. J. Mar. Sci. Eng. 2023, 11, 468. https://doi.org/10.3390/jmse11030468
Eker-Develi E, Tekdal D, Demet AE, Yıldız HB, Kideys AE. Morphology, Molecular Genetics and Potential Importance for Mucilage Events of the New Coccolithophorid Ochrosphaera neapolitana in the Sea of Marmara. Journal of Marine Science and Engineering. 2023; 11(3):468. https://doi.org/10.3390/jmse11030468
Chicago/Turabian StyleEker-Develi, Elif, Dilek Tekdal, Atıf Emre Demet, Hüseyin Bekir Yıldız, and Ahmet Erkan Kideys. 2023. "Morphology, Molecular Genetics and Potential Importance for Mucilage Events of the New Coccolithophorid Ochrosphaera neapolitana in the Sea of Marmara" Journal of Marine Science and Engineering 11, no. 3: 468. https://doi.org/10.3390/jmse11030468
APA StyleEker-Develi, E., Tekdal, D., Demet, A. E., Yıldız, H. B., & Kideys, A. E. (2023). Morphology, Molecular Genetics and Potential Importance for Mucilage Events of the New Coccolithophorid Ochrosphaera neapolitana in the Sea of Marmara. Journal of Marine Science and Engineering, 11(3), 468. https://doi.org/10.3390/jmse11030468







