Abstract
Mapping the distribution and evaluating the impacts of marine non-indigenous species (NIS) are two fundamental tasks for management purposes, yet they are often time consuming and expensive. This case study focuses on the NIS gilthead seabream Sparus aurata escaped from offshore farms in Madeira Island in order to test an innovative, cost-efficient combined approach to risk assessment and georeferenced dispersal data collection. Species invasiveness was screened using the Aquatic Species Invasiveness Screening Kit (AS-ISK), and revealed a high invasion risk. Occurrences of S. aurata were assessed involving citizens in GIS participatory mapping and data from recreational fishing contests. A probability map showed that S. aurata is well dispersed around Madeira Island. This assessment proved to be a cost-efficient early warning method for detecting NIS dispersal, highlighting the urgent need for additional surveys that should search for sexually mature individuals and assess the direct and indirect impacts in the native ecosystem.
1. Introduction
During the past decades, the introduction and establishment of marine non-indigenous species (NIS) have increasingly become a global concern [1]. The potential impacts of NIS are multiple and can include the displacement or loss of native species, reduction in biodiversity, shifts in ecosystem functioning, and socio-economic issues [2,3,4,5,6,7]. Several tools and protocols have been developed to better assess the risks associated with the escape or the unintentional or unauthorised liberation of reared NIS and to improve informed management practices of potential negative effects [8]. Available risk assessment schemes vary widely in approach, objective, implementation, environments, and taxa covered [9], with the majority based on qualitative methods [8]. For example, some protocols were designed to be taxonomically generic (e.g., GB-NNRA), while others are specific for screening certain taxonomic groups (e.g., FISK), particular habitats (e.g., BINPAS), or vectors of introduction [10,11]. One of the most detailed and exhaustive risk identification decision-support tools is the Aquatic Species Invasiveness Screening Kit (AS-ISK), an electronic ready-to-use tool that provides a quantitative output (i.e., score) about the risk of a selected species to become invasive in a target introduction area under present and future (predicted) climate change conditions [12]. The added value of having a section dedicated to the possible impact due to climatic projections is extremely important when dealing with NIS. Climate change may directly or indirectly affect biological invasions, influencing species distribution, trophic interaction, and ease of management [13,14].
Besides using available tools to predict and assess invasion risk associated with NIS, management practices should also include regular, long-term monitoring programs targeting NIS distribution and inventorying local diversity [15,16]. However, regular comprehensive monitoring programs designed to compile georeferenced data are typically time consuming, laborious, expensive, and often require a high level of technical expertise [17]. In this context, researchers and managers are increasingly taking advantage of local knowledge from people living in the area and with close relationships with the natural environment, which can effectively reduce costs and enhance data collection (i.e., extension of the study area) [18]. To some extent, the exponential involvement of citizens in marine research since the late 2000s is linked to the growing availability of smartphones, Internet of Things (IoT) devices, and new communication technologies (e.g., high speed mobile internet and mobile applications) that close communication gaps, democratise data collection, and facilitate data curation [19]. In particular, the use of participatory mapping methods and citizen-science platforms like iNaturalist (www.inaturalist.org (accessed on 9 December 2022)), Globe Observer [20], and eBird [21] has greatly improved in recent years, with greater accessibility to geographic information system (GIS) software and the development of web-based GIS interfaces such as Google Maps, Google Earth, AquaMaps, or Sea Around Us [22,23,24]. Online mapping tools offer new interactive inputs to conventional cartography and provide key information for the conservation of marine ecosystems [18,25]. However, the use of GIS participatory mapping is not flawless and generally requires data verification and validation, where results gathered by volunteers are compared to those obtained by scientists [26,27,28,29]. This can be particularly important when a species’ identity can be mistaken or if it is required to distinguish an individual from a wild population or one in captivity, such as those bred in aquaculture facilities [30].
Madeira Island (Portugal) is located in the NE Atlantic, about 700 km off the Moroccan coast (Figure 1). Considering the lack of adequate terrestrial area and its oligotrophic and subtropical waters [31], Madeira presents optimal conditions for offshore fish aquaculture [32]. The first offshore fish farm was installed in 1996, as a public initiative of the local government to demonstrate the technical and commercial feasibility of the operation in the open and deep seas south of the island [33]. Stirling [34] determined the viability of locally cultured fish, such as the gilthead seabream (Sparus aurata), based on acceptability in the local market, the potential to export to the mainland, and the existing dealers willing to pay for quality. The success of the initiative prompted the interest of private investors, leading to the installation of three S. aurata offshore farms in the south of Madeira Island (Figure 1), accounting for 1565.7 tons produced in 2021 [35]. However, this important cultured species, whose natural habitat ranges from the coasts of southern England to Mauritania and includes the Mediterranean and Black Sea [36], is considered an NIS in the Madeira archipelago and the western Canaries [37,38,39].
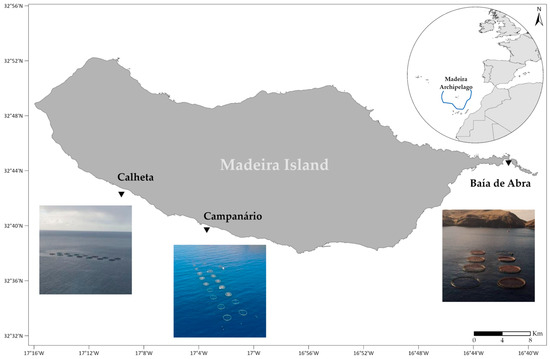
Figure 1.
Geographic setting and map with locations of the three existing aquaculture fish farms in Madeira Island.
A few years after the beginning of S. aurata farming in Madeira Island, individuals of this species were sighted outside and in the vicinity of the first pilot off-shore fish farm [37] and have been commonly captured by recreational fishers since 2004 [40,41].
Considering the ability of S. aurata to quickly disperse and survive in the wild for long periods, as has been previously reported in other geographic areas [42], understanding the current spatial distribution of S. aurata introduced in Madeira through aquaculture and assessing its invasiveness is essential for management, conservation, planning, and forecasting purposes [43]. This is especially true in an Island coastal ecosystem. Besides the first detection in the wild in 2000 [37], no data are available on S. aurata distribution along the Madeiran coasts. Moreover, risk assessments of the potential environmental and ecological impacts of this species’ introduction and the eventual establishment of wild populations have never been conducted. Applying a multidisciplinary approach, this study addresses some of the most critical knowledge gaps by assessing the following: (1) the dispersal ability of S. aurata in the coastal waters of Madeira by combining data from GIS participatory mapping with data from recreational fisheries contests and commercial landings and (2) S. aurata environmental/ecological risks in Madeira under current and future climatic conditions using the AS-ISK tool.
Overall, this study was designed to provide a cost-efficient management tool that can promptly assess a targeted NIS distribution and the associated invasiveness risk, detecting the current spread and high-risk invasive NIS, and thus supporting managers and policy makers to improve conservation strategies and marine spatial planning.
2. Materials and Methods
2.1. Survey Questionnaire and GIS Participatory Mapping
To assess occurrences of S. aurata in the coastal waters of Madeira Island, an online questionnaire survey addressed to citizens who practice marine activities (i.e., SCUBA diving, recreational angling, spearfishing, and freediving) was developed using the platform “maptionnaire” (www.maptionnaire.com (accessed on 9 December 2022)). This platform has the advantage of being accessible from different devices (i.e., computers, tablets, and phones) without the need to download a separate application. Moreover, this online questionnaire survey uses a novel technique that combines GIS participatory mapping methods with classical interviews to collect multiple levels of information.
The questionnaire was structured in two parts. The first part was designed to assess the participants’ familiarity with S. aurata, including a picture of the species and a mix of open-ended questions, Likert-scale, and fixed-choice questions. The second part of the questionnaire presented a Google maps interface designed to collect georeferenced data on S. aurata sightings during the respondents’ maritime activities (i.e., where the species was sighted while SCUBA diving, recreational angling, or spearfishing/freediving). With that intent, the form instructed each participant to select an activity (i.e., SCUBA diving, recreational angling, or spearfishing/freediving) and drag and drop the matching digital icon (hereafter referred to as “markers”) onto a map of Madeira Island. To ensure spatial precision in icon placement, the respondent was able to zoom the map interface to an approximate resolution of 0.016 m/pixel. After dropping the marker on the site where S. aurata was sighted, the participant needed to respond to four fixed-choice questions: (1) the frequency of site visits (i.e., always, frequently, occasionally, or rarely); (2) the frequency of S. aurata sightings (i.e., always, frequently, occasionally, or rarely); (3) the approximate number of S. aurata individuals sighted (i.e., 1–5, 6–10, 11–20, or >20); and (4) the frequency of S. aurata sightings of individuals larger than 30 cm (i.e., always, frequently, occasionally, rarely, or never). In addition, respondents were asked to name and report fish species that had been sighted together with S. aurata. After responding to these questions, the respondent was asked to continue reporting additional sightings (i.e., by selecting once again an activity, dropping a new pin, and answering the follow-up questions) or to submit the report.
To test this approach, prototype versions of the questionnaire were piloted on a random group of people (n = 20). This preliminary study suggested small modifications on phrasing to prevent misunderstandings and to confirm that the online questionnaire worked effectively. After the initial testing and improvement, the survey questionnaire was made available online for one month (from July to August 2020), and it was regularly disseminated through scientific and social networks and virtual word-of-mouth.
For the purpose of this research, all data were collected, stored, and analysed anonymously, and all participants were informed about the objective of the study and agreed to participate. Submitted questionnaires were examined and validated for completeness and consistency of responses prior to analysis. Incomplete questionnaires and respondents that were not able to correctly identify a picture of S. aurata (i.e., writing its scientific or common name) were excluded from the analysis.
At the end of the data collection period, spatial data (markers) and non-spatial data (responses to the survey) were downloaded from the webserver for analysis in ArcGIS and SPSS software.
2.2. Spatial Analysis
Spatial data obtained with the questionnaire survey were converted in a GIS database using the software ArcGIS Pro version 2.9.0 [44]. Preliminary spatial validation of reported sightings was carried out by generating a 1 km buffer around each reported marker location. Markers placed inland at more than 1 km from the shore were considered invalid and discarded prior to further analysis. Markers placed inland within 1 km from the shore were considered unintentional misplacements intended to report sightings in water and were moved to seawater in the closest proximity and considered valid. To estimate the probability of S. aurata sightings (PSa) in the coastal water of Madeira Island, valid data from the fixed-choice questions associated with each of the markers placed on the Madeira map were considered. Namely: (Q1) the frequency of site visits, (Q2) the frequency of successful S. aurata sightings, and (Q3) the approximate number of S. aurata individuals sighted. Answers for each of these three questions were transformed into values (i.e., always = 4, often = 3, sometimes = 2, and rarely = 1; and options 1–5 = 1, 6–10 = 2, 11–20 = 3, and >20 = 4) and then PSa was estimated using the following formula:
PSa = Q1 × Q2 × Q3
Lastly, PSa values were represented through a heat map for visualisation purposes and for better interpretation. A colour scale was selected as a graphical representation of PSa in a specific area (i.e., red = high probability, light blue = low probability), which is positively correlated to the number of markers placed in that area (closer markers create an increase in the probability in that specific area).
Density was estimated using the kernel density methods [45]. For visualisation purposes, PSa values used constant rendering with a radius value of 15. The heat map was constructed using a scale of 1:270,000 km.
2.3. Recreational Fishing Contests and Landings from Commercial Fishing Fleet
Capture records from fishing contests (held between 2010 and 2019) provided by the sport fishing association APDRAM (Associação de Pesca Desportiva da Região Autónoma da Madeira, Funchal, Portugal) were analysed to supply corroborative evidence of S. aurata estimated distribution based on the questionnaire surveys. This local association organises fishing contests every year, generally in multiple locations along Madeiran coasts, and provides the capture reports to the Fisheries Department of the Madeira Government. The goal of these competitions is to reach the highest total weight of catches with no catch limits and target fishing (but it forbids the capture of fishes <12 cm). For each contest, reports include the total number of individuals caught per species, the number of participants, location, and date (day, month, and year). Unfortunately, prior to 2017, specimens of S. aurata were rarely recorded in an individual species category and often classified in a joint group named “other species”, thus limiting the data available in those years. Sparus aurata landings from the commercial fleet in fishing ports from Madeira were provided by the Madeira Fisheries Directorate, covering the period from 1996 (when the first fish farm was installed) until 2021.
2.4. Risk Assessment
The risk of S. aurata becoming invasive in Madeira Island, under the current and future climatic conditions, was evaluated using the Aquatic Species Invasiveness Screening Kit (AS-ISK 2021) [12]. AS-ISK is a decision support tool freely available online (www.cefas.co.uk/nns/tools (accessed on 9 December 2022)) that consists of 55 questions [12]. The first 49 questions address biogeographical and biological aspects of the targeted species to produce a final basic risk assessment (BRA) score. The last six questions consist of a climate change assessment (CCA), which requires the assessor to evaluate how future predicted climate conditions are likely to affect the BRA concerning risk introduction, establishment, dispersal, and impact [1]. To achieve a valid screening, the assessor must provide the following for each question: a response, a justification, and a confidence ranking for that response. To classify the level of invasiveness (i.e., low, medium, or high), the assessor must inspect BRA and BRA + CCA scores. If scores are less than one, the target species is considered to be unlikely to become invasive and thus classified as “low risk”. If the scores are higher than one, the species is considered of “medium” or “high” risk to become invasive in the risk assessment area. To determine if a species possesses a “medium” or a “high” risk, the assessor refers to a “threshold” value, which varies based on the taxonomic group to which the species belongs (e.g., reptiles, amphibians, tunicates, marine fishes, and so on) and on the climate zone of the risk assessment area [1]. Located in the Temperate Northern Atlantic, the thresholds used to evaluate the invasiveness of marine fishes in the Madeira archipelago are BRA = 19.5 and BRA + CCA = 31.5 [1].
In order to reduce the chances of individual bias, a total of seven assessors were involved in the screening of S. aurata invasiveness risk in the Madeira archipelago. The assessors were selected based on their level of expertise and familiarity with this species, including senior and junior experts in aquaculture, marine ecology, and invasion biology who are familiar with Madeira marine habitats and biodiversity.
2.5. Statistical Analysis
Descriptive statistics were used to summarise the results from the survey questionnaire, from fishing contests and landings, and from AS-ISK assessments.
Potential differences among the three recreational activities (i.e., SCUBA diving, recreational angling, and spearfishing/freediving) and the potential relationship between the frequency of site visits and S. aurata sightings were tested using Chi-squared tests. These statistical analyses were conducted using IBM SPSS Statistic version 27 with a significance level of 5%.
3. Results
3.1. Survey Questionnaire and GIS Participatory Mapping
A total of 117 surveys were filled-in. From these, 64 survey questionnaires were considered valid, reporting sightings in 108 markers (28 from SCUBA diving, 33 from recreational angling, and 47 from spearfishing/freediving).
Overall, 68% of the participants remembered the first time they observed S. aurata in the wild. The oldest sighting dated back to 1997, while 2016 was the year with the most first sightings reported (Figure 2).
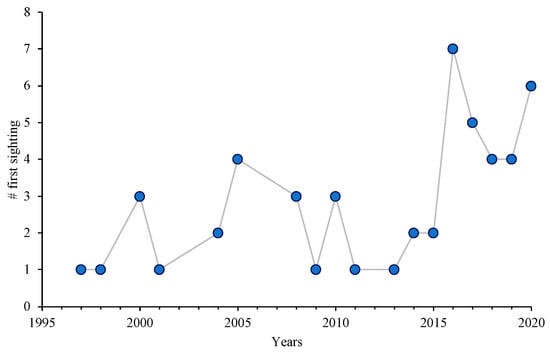
Figure 2.
Reported first sightings of S. aurata per year in Madeira Island. Data were obtained with the survey questionnaire developed using Maptionnaire. Participants were asked to write the year in which they saw individuals of S. aurata for the first time in the coastal water of Madeira. The y-axis represents the number of answers.
Almost half of the participants (47%) perceived a yearly increase in S. aurata sightings, 17.2% believed the population remained unchanged, while 12.5% perceived a decrease in S. aurata sightings, and 23.3% were not able to point out any trend. In 43% of the cases, S. aurata was seen with other fish species, mostly accompanied by Diplodus sargus (37.5%), followed by Sarpa salpa (15.6%) and individuals of the family Mugillidae (12.5%) (for more details, see Supplementary Figure S1).
Most of the participants placed only one marker on the map (70%), and the maximum number of markers positioned by a single participant was 8 (1.6%). Most of the sightings were located on the south coast of Madeira Island, but approximately 14% of the markers (n = 15) were also placed on the north coast (Supplementary Figure S2). Overall, in 25% of the sites, reports of individuals of S. aurata larger than 30 cm were common (for more details, see Supplementary Table S1).
Chi-squared test revealed no significant relationship between the type of activity conducted by participants and the frequency of S. aurata sightings, the number of S. aurata individuals observed, or the frequency of sighting specimens larger than 30 cm (all three Chi-squared had p > 0.05).
Similar results were revealed between the reported visiting frequency of one place and the frequency or number of S. aurata sightings reported at that location (both Chi-squared had p > 0.05). However, the Chi-squared test suggests a significant relationship between the frequency of site visits and the frequency of large-sized S. aurata sightings (X2 = 28.410; df = 12; p = 0.005).
Assuming no significant relationship between the frequency of visits and reported sightings (see above), the probability of sighting S. aurata (PSa) was estimated and plotted using a heat map (Figure 2). Three areas with a high probability of sighting S. aurata can be identified on the south coast of Madeira Island: one area in the vicinity of the fish farm facilities in Calheta; one area along the shoreline of Funchal; and one more extended area in the southeast shoreline, between Caniçal harbour and the surroundings of the fish farm facilities in Baía de Abra (Figure 3).
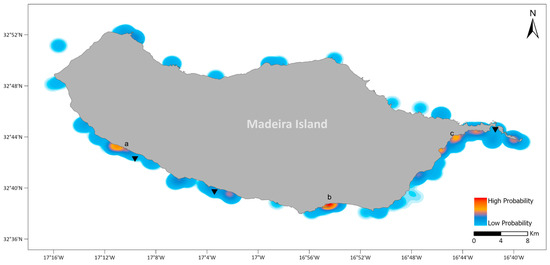
Figure 3.
Heat map representing the reported probability of S. aurata sighting (PSa) according to the questionnaire survey. In red are the areas with high probability, while light blue represents areas with low probability values. The aquaculture facilities are represented as black triangles. Letters represent the three areas with a higher probability of S. aurata sightings: (a) Calheta; (b) Funchal; (c) Caniçal.
3.2. Recreational Fishing Contests and Landings from Commercial Fishing Fleet
In the period 2010–2019, a total of 300 fishing contests were performed in 14 locations along the Madeira coasts. Captures of S. aurata were reported in 25 contests, 18 of which were in the period of 2017–2019 (Supplementary Table S2). Two years stand out when inspecting the numbers of S. aurata captures: 2010, which was the year with the highest percentage of S. aurata (90%) caught in a single contest, and 2018, in which 56% of the fishing contests had records of S. aurata (Supplementary Table S2). From 2017 to 2019, fishing contests took place in 11 locations around Madeira Island and, in 6 of these, S. aurata was caught, with a mean percentage ranging from 0.17% to 7.35% of the total catch (Figure 4; for details, see Supplementary Table S3).
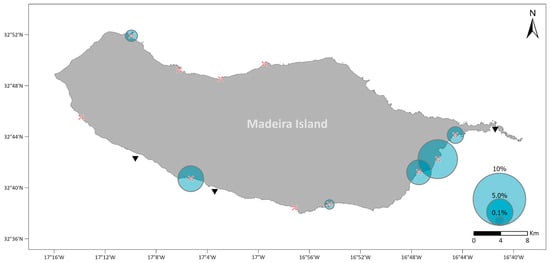
Figure 4.
Percentage of S. aurata caught from fishing contests in the period 2017–2019. The size of the circle represents the mean percentage of S. aurata recorded in each fishing contest location. The fishing contest sites are represented by red crosses and the aquaculture facilities by black triangles.
Sparus aurata was firstly reported in landings from Madeira’s commercial fishing fleet in 2008. It was included in a group of bycatch fish species, mostly unidentified and with total catch per trip by boat from 30 to 200 kg. A total of 25 landings of that group of fish was reported that year. To date, landings of bycatch group that identify S. aurata were only reported for 2009 (3.5 kg), 2011 (0.9 kg), and 2014 (6.1 kg) (Madeira Fisheries Directorates, personal communication).
3.3. AS-ISK Invasiveness Risk
Sparus aurata average BRA score for the Madeira archipelago was estimated at 22.16 ± 6.77 (average ± SD). Considering that the threshold for marine fishes in Madeira’s ecoregion is 19.5 [1] and the average confidence value for BRA was 0.72 ± 0.06, S. aurata is considered as carrying a high risk of being invasive in Madeira (Figure 4). Concerning the level of invasiveness in future projected climate conditions (BRA + CCA), the output of the risk assessment corresponds to a value of 25.83 ± 8.91 with a confidence of 0.69 ± 0.07 (average ± SD). As the BRA + CCA threshold value for marine fishes in this ecoregion is 31.5, S. aurata represents a medium risk of becoming invasive under future climatic conditions predicted for the Madeira archipelago (Figure 5).
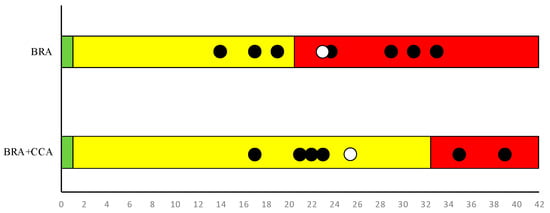
Figure 5.
Results of AS-ISK for BRA and BRA + CCA conducted by seven assessors. The black dots are the single assessments and the white dots are the average values for all assessors. In BRA + CCA, two dots present the same value (21). The colour inside each column represents the invasiveness risk: green (value less than 1) is low risk; yellow corresponds to medium risk, and red corresponds to high risk. The threshold values between medium and high risk are in accordance with the species (marine fish) and the ecoregion (temperate marine) analysed [1].
Individual values of BRA and CCA for each assessor and overall results from the AS-ISK are shown in the Supplementary Materials (Table S4).
4. Discussion
The combined methodologies applied in this study are revealed to be suitable and with great potential to make important contributions to local management and conservation practices as well as in broader scientific studies of NIS, dispersal rates, and distribution assessments.
For Madeira Island, this approach has been demonstrated to be suitable to create an updated map of S. aurata distribution and provide insight into future potential invasive consequences.
The participatory mapping showcased locations with a higher estimated probability of S. aurata sighting, with both estimates and reported sightings suggesting that this species can be found in most locations around Madeira. However, the interpretation of the produced heat map needs some careful considerations. Firstly, the zero probability of S. aurata sightings (white colour in Figure 2) represents missing information and not S. aurata absence. Secondly, the estimated probability is influenced by the frequency of visits to different areas. Despite no significant relationship between reported visit frequency and reported S. aurata sightings, it is, in fact, possible that the island’s south coast is associated with a higher probability than the north because of urban development and higher population density (i.e., cities and marinas). This pattern is likely to be partly driven by convenience and ease of access, such as the presence of infrastructure that facilitates marine recreational activities. Considering all of this, it is plausible that the distribution of S. aurata is underrepresented on the north coast of the island and overestimated in the south. However, such errors in the estimation do not challenge the presence of this species around the entire island, nor the possibility of an established population.
Additional information that supports this hypothesis is the reported sighting of large specimens of S. aurata (>30 cm), which are larger than the average length at which fish farmed in Madeira enter the commercial circuit (approx. 28.5 cm). Thus, the presence of large fish in the wild suggests that S. aurata can survive for extended periods outside of the cages and is likely capable of breeding in the wild. In fact, a local breeding programme using natural spawning and reproduction of the species—without environmental control or the use of hormones—assessed that the average size of broodstock is 35 cm [46].
However, with these results, it is difficult to be certain if the present distribution of S. aurata in Madeira Island is due to a well-established population of this species or continuous input from open-ocean fish farming. According to respondents of the GIS participatory mapping survey, the first S. aurata sighting goes back to 1997, just a year after the installation of the first fish farming sea-cages in Madeira Island. Although it was not possible to verify the authenticity of this record, it suggests that the first fish farm breakaway may have occurred soon after the beginning of the aquaculture activities on this island. Official reports from Madeiran farmers refer only to large escape events, which is common for other species (i.e., Atlantic Salmon, rainbow trout, and Atlantic cod) and other countries (i.e., Norway, Italy, or Spain) [47,48,49]. Indeed, given the positive correlation between fisheries and available biomass [50], it is possible that the high percentage of S. aurata caught in 2018 fishing contests (90%) was related to a massive escape event that occurred previously in the same year [51]. Considering that many smaller escape incidents are either not detected or not reported [52], the true level of escape incidents could be underestimated by 2–4 times [53].
The lack of standardisation in S. aurata detection and quantification efforts also contributes to the current level of uncertainty to support the hypothesis of a continuous S. aurata spillover from aquaculture. Fishing contest reports show higher percentages of S. aurata in areas closer to aquaculture facilities (i.e., the one located on the South East coast of Madeira Island) compared with the ones furthest away (i.e., areas in the North coast) (Figure 3). However, this result must be interpreted with caution because the fishing effort was not identical and angling is commonly influenced by multiple attributes of anglers, fishes, and the environment [54].
Regardless of which theory justifies the present distribution of S. aurata in the coastal water of Madeira Island, understanding the risks associated with these fish farm escapes is particularly relevant for management and policymakers, especially in the case of expanding mariculture activities [55]. In Madeira Island, S. aurata farming is a growing economic sector; in the first semester of 2022, 1.0229 tons were produced, 51.2% more than the same period of the previous year [35]. The risks that escaped farmed fish pose are a function of the probability of escape; the magnitude of each escape event; and the impact of escaped fish on wild populations, ecosystems, and society [56]. Considering that S. aurata is classified as an NIS in Madeira [37,57], it reinforces the need to assess the relevance of its impacts and to more effectively preserve and protect the marine ecosystem, thus promoting their sustainable use. The results from the AS-ISK showed that S. aurata is currently a species with a high risk of invasiveness in Madeira Island. Sparus aurata is known to quickly adapt to local native food sources such as molluscs, crustaceans, and macrophytes, potentially changing the trophic web structure or habitat composition [42]. The establishment of this species may enhance trophic competition with native species like the red porgy (Pagrus pagrus, [37]) and the white seabream (Diplodus sargus, [58]). The latter species presents an important diet overlap and was the one that was more commonly seen with S. aurata (37.5% see Supplementary Figure S1). Moreover, although potential predators of S. aurata (i.e., dolphins, seabirds, monk seals, and local fishermen) are present at Madeira throughout the year, their abundance may not be enough to control a new growing population [59].
Another issue related to NIS, including S. aurata, is the possibility to host or act as a vector of recognized pests and infectious agents [60]. However, knowledge about the nature and mechanisms of infectious pathogens between farmed stocks and wild populations is still scarce [42].
To date, S. aurata in Madeira seems not to affect any ecosystem services directly nor have negative socio-economic impacts. In fact, S. aurata is still not commonly reported from fishery landings of the local commercial fleet. However, ecological functions can change dramatically over time, or changes can become evident only after long periods of the innocuous presence of the NIS [14]. The accuracy of the AS-ISK responses evidenced a lower confidence ranking from the CCA (0.43) compared with the BRA (0.72), highlighting the intrinsically high level of uncertainty in future climate change projections. Indeed, it is possible that, in future climate change scenarios for Madeira Island, S. aurata could potentially reduce natural breeding and recruitment success in the wild and at local hatcheries. In fact, an increase in minimum sea temperatures, presently at a monthly average of 18 °C [61], would dramatically reduce the spawning window for this species, which reproduces under 19 °C [62]. Moreover, several studies have indicated that S. aurata is particularly sensitive to prolonged exposure to elevated water temperature (i.e., >28–30 °C) and unable to acclimate to temperatures beyond 26 °C [63,64]. Moreover, thermal sensitivity of S. aurata differs between life stages [62,65], making it even more challenging to predict the impacts of this species in the ecosystem.
Given the high risk of S. aurata becoming invasive in Madeira and the suggested establishment of this species, the findings of this study highlight the need for implementing a regional monitoring program that consistently and periodically evaluates present and future impacts of S. aurata on coastal ecosystems. Such a program should include commercial fisheries’ independent data, as well as data from recreational fisheries and the regular participation and contributions of relevant stakeholders and citizens. As seen in this study and other research targeting marine NIS [66,67], citizen science is a valuable low-cost tool to monitor species distribution. Moreover, encouraging constant citizen participation through GIS mapping tools will help to understand species’ temporal and spatial distribution patterns. However, the use of citizen science is not flawless and requires data interpretation and integration by scientists [26,27,28,29]. To overcome issues related to spatial data collected with citizen participation and validate local distribution, the suggested monitoring program should also collect in situ data periodically and follow a distance gradient from both aquaculture facilities and areas with urban development. In addition, to confirm the presence of a self-sustained S. aurata population, this program should also inspect individuals captured in the wild to assess if both sexually mature males and females are already present and breeding in coastal waters around Madeira. Moreover, genetic monitoring programs of reared stocks and of their conspecifics already in the wild should also be established [68].
Lastly, this study also highlights the need for regulations that require aquaculture farms to report escape events independently of their sizes, which has recently been adopted under new regulations (Dec.L.R. 5/2023/M de 9 de Janeiro de 2023). We also recommend the requirement for recreational fishing contests to report captures and the addition of S. aurata as a separate category in the commercial landings annual report of the Madeira Fisheries Directorate. All of these recommendations will help comply with EU regulations under the Marine Strategy Framework Directive for NIS [16].
Based on local results, this approach, leveraging citizen participation and experts’ knowledge to assess the distribution and invasion risk of S. aurata, has the potential to be applied in other locations (or other target species) where time and funding are limiting factors. In fact, up-to-date data on S. aurata distribution were collected in a short period of time, covering almost the whole perimeter of the island and with virtually no costs except for those associated with launching and maintaining the questionnaire online. Moreover, the risk assessments were conducted with a freely available toolkit (AS-ISK, [12]) and relied on volunteer and collaborative work from experts, showcasing that species invasive risk can be assessed with almost no associated costs based on expert opinion and knowledge. Finally, the visual and numeric output of this approach is straightforward to interpret for managers and policymakers as well, making it an easy-to-use early warning system for potentially invasive NIS and allowing the prompt development of management measures.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jmse11020438/s1. Figure S1: Fish species seen together with S. aurata expressed in percentage; Figure S2: Sighting of S. aurata for each marine activity; Figure S3: Sighting of S. aurata larger than 30 cm; Table S1: Frequency of S. aurata individuals bigger than 30 cm; Table S2: Fishing contests performed along Madeira coasts in the period between 2010 and 2019; Table S3: Fishing contests performed along Madeira coasts in the period between 2017 and 2019; Table S4: AS-ISK results for S. aurata in Madeira Archipelago.
Author Contributions
Conceptualization, P.P., J.G.M., F.G., R.M.-E. and J.C.-C.; methodology, P.P., J.G.M., F.G., R.M.-E., F.A. (Filipe Alves), S.C., S.A., M.P.P., F.A. (Frederico Almada), M.F., N.N., C.A. and J.C.-C.; software, S.A. and M.F.; validation, P.P., J.G.M., F.G., R.M.-E. and J.C.-C.; formal analysis, P.P., J.G.M., F.G., R.M.-E. and J.C.-C.; investigation, P.P., J.G.M., F.G., R.M.-E. and J.C.-C.; resources, P.P. and R.M.-E.; data curation, P.P., J.G.M., F.G., R.M.-E. and M.P.P.; writing—original draft preparation, P.P., J.G.M., F.G., R.M.-E. and M.P.P.; writing—review and editing, P.P., J.G.M., F.G., R.M.-E., F.A. (Filipe Alves), S.C., S.A., M.P.P., F.A. (Frederico Almada), M.F., N.N., C.A. and J.C.-C.; visualization, P.P. and S.A.; supervision, J.C.-C.; project administration, J.C.-C.; funding acquisition, J.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
P.P. was funded by a PhD grant ref. M3.1.a/F/065/2015 by Fundo Regional de Ciência e Tecnologia (FRCT) and the program AÇORES 2020; a research scholarship granted by Oceanic Observatory of Madeira (OOM) ref 1797; and by a post-doctoral research fellowship within the CLEANATLANTIC project (ARDITI-CLEAN-ATLANTIC-2022-01). J.G.M. was supported by post-doctoral research fellowship by ARDITI (ARDITI–M1420-09-5369-FSE-000002) and by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P., under the Scientific Employment Stimulus—Institutional Calls—(CEEC-INST/00037/2021). F.G. was supported by a post-doctoral research fellowship granted by ARDITI in the framework of the projects RAGES (ARDITI-RAGES-2019-001), IMPLAMAC (MAC2/1.1a/265), MIMAR+ (MAC2/4.6d/249), and OCEANLIT (MAC2/4.6d/302). R.M.-E. was financially supported by the Oceanic Observatory of Madeira Project (M1420-01-0145-FEDER-000001-Observatório Oceânico da Madeira-OOM). F.A. (Filipe Alves) had the support of the FCT project through UIDB/04292/2020 and UIDP/04292/2020 granted to MARE. S.C. was financially supported by doctoral fellowships by Agência Regional para o Desenvolvimento da Investigação, Tecnologia e Inovação (ARDITI—M1420-09-5369-FSE-000002). S.A. was financially supported by doctoral fellowship by MARE ref UI/BD/151020/2021. M.P.P. is funded by FCT–Fundação para a Ciência e a Tecnologia, through researcher contract DL57/2016/CP1479/CT0020. F.A. (Frederico Almada) had the support through researcher contract DL57/2016/CP1339/CT0003 and the FCT through the strategic project UIDB/04292/2020 awarded to MARE and through the project LA/P/0069/2020 granted to the Associate Laboratory ARNET. J.C.-C. is funded by national funds through FCT—Fundação para a Ciência e Tecnologia, I.P., under the Scientific Employment Stimulus—Institutional Call—[CEECINST/00098/2018]. This work was partially supported by the following projects: PLASMAR+ (MAC2/1.1a/347) in the framework of the INTERREG MAC 2014-2020 Programme. This study also had the support of FCT through the strategic project UIDB/04292/2020 awarded to MARE and through project LA/P/0069/2020 granted to the Associate Laboratory ARNET.
Institutional Review Board Statement
Ethical review and approval were waived for this study since no personal data have been collected. Definition of “personal data” is according to the Portuguese National General Data Protection Regulation (Capítulo i, Artigo 4.o).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.
Acknowledgments
The authors would like to acknowledge Festa Isufi for her kindness in providing support for the use of Maptionnaire. We would also like to thank COM/EMGFA—Madeira Command Operations/General Staff of The Armed Forces for sharing aerial images included in Figure 1 within the scope of a collaboration with ARDITI, MARE-Madeira, and the Oceanic Observatory of Madra (OOM). We would like to thank all the participants in the on-line survey. The authors also acknowledge four anonymous reviewers for their helpful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F. Biological Invaders in Inland Waters: Profiles, Distribution and Threats; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2007; Volume 2, p. 733. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.G.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish. Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Lodge, D.M. Biological invasions: Lessons for ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Manchester, S.J.; Bullock, J.M. The impacts of non native species on UK biodiversity and the effectiveness of control. J. Appl. Ecol. 2000, 37, 845–864. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Biological invasions of fresh water: Empirical rules and assembly theory. Bio. Cons. 1996, 78, 149–161. [Google Scholar] [CrossRef]
- Roy, H.E.; Rabitsch, W.; Scalera, R.; Stewart, A.; Gallardo, B.; Genovesi, P.; Essl, F.; Adriaens, T.; Bacher, S.; Booy, O.; et al. Developing a framework of minimum standards for the risk assessment of alien species. J. Appl. Ecol. 2018, 55, 526–538. [Google Scholar] [CrossRef]
- Verbrugge, L.N.H.; Leuven, R.S.E.; Van der Velde, G. Evaluation of International Risk Assessment Protocols for Exotic Species; Department of Environmental Science, Radboud University Nijmegen: Nijmegen, The Netherlands, 2010; Reports Environmental Science, 366. [Google Scholar]
- González-Moreno, P.; Lazzaro, L.; Vilà, M.; Preda, C.; Adriaens, T.; Bacher, S.; Brundu, G.; Copp, G.H.; Essl, F.; García-Berthou, E.; et al. Consistency of impact assessment protocols for non-native species. Neobiota 2019, 44, 1–25. [Google Scholar] [CrossRef]
- Panov, V.E.; Alexandrov, B.; Arbačiauskas, K.; Binimelis, R.; Copp, G.H.; Grabowski, M.; Lucy, F.; Leuven, R.S.; Nehring, S.; Paunović, M.; et al. Assessing the risks of aquatic species invasions via European inland waterways: From concepts to environmental indicators. Integr. Environ. Assess. Manag. 2009, 5, 110–126. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti-Ambrogi, A. Biopollution by Invasive Marine Non-Indigenous Species: A Review of Potential Adverse Ecological Effects in a Changing Climate. Int. J. Environ. Res. Public Health 2021, 18, 4268. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Miller, D.L.; Holman, L.E.; Gittenberger, A.; Ardura, A.; Rius, M.; Mirimin, L. Environmental DNA sampling protocols for the surveillance of marine non-indigenous species in Irish coastal waters. Mar. Pollut. Bull. 2021, 172, 112893. [Google Scholar] [CrossRef] [PubMed]
- MSFD [Marine Strategy Framework Directive]. European Commission Directive 2008/56/EC of the European parliament and of the council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy. Off. J. Eur. Union L 2008, 164, 19–40. [Google Scholar]
- Feldman, M.J.; Imbeau, L.; Marchand, P.; Mazerolle, M.J.; Darveau, M.; Fenton, N.J. Trends and gaps in the use of citizen science derived data as input for species distribution models: A quantitative review. PLoS ONE 2021, 16, e0234587. [Google Scholar] [CrossRef]
- Azzurro, E.; Cerri, J. Participatory mapping of invasive species: A demonstration in a coastal lagoon. Mar. Policy 2021, 126, 104412. [Google Scholar] [CrossRef]
- Garcia-Soto, C.; Seys, J.J.C.; Zielinski, O.; Busch, J.A.; Luna, S.I.; Baez, J.C.; Domegan, C.; Dubsky, K.; Kotynska-Zielinska, I.; Loubat, P.; et al. Marine Citizen Science: Current State in Europe and New Technological Developments. Front. Mar. Sci. 2021, 8, 621472. [Google Scholar] [CrossRef]
- Kohl, H.A.; Nelson, P.V.; Pring, J.; Weaver, K.L.; Wiley, D.M.; Danielson, A.B.; Cooper, R.M.; Mortimer, H.; Overoye, D.; Burdick, A.; et al. GLOBE Observer and the GO on a Trail Data Challenge: A Citizen Science Approach to Generating a Global Land Cover Land Use Reference Dataset. Front. Clim. 2021, 3, 620497. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Bio. Cons. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Asaad, I.; Lundquist, C.J.; Erdmann, M.V.; Costello, M.J. An interactive atlas for marine biodiversity conservation in the Coral Triangle. Earth Syst. Sci. Data. 2018, 11, 163–174. [Google Scholar] [CrossRef]
- Kaschner, K.; Rius-Barile, J.; Kesner-Reyes, K.; Garilao, C.; Kullander, S.O.; Rees, T.; Froese, R. AquaMaps: Predicted Range Maps for Aquatic Species. World Wide Web Electronic Publication. 2010. Version 08/2010. Available online: www.aquamaps.org (accessed on 9 December 2022).
- Pauly, D.; Zeller, D.; Palomares, M.L.D. (Eds.) Sea Around Us Concepts, Design and Data. 2020. Available online: seaaroundus.org (accessed on 9 December 2022).
- Ernoul, L.; Wardell-Johnson, A.; Willm, L.; Béchet, A.; Boutron, O.; Mathevet, R.; Arnassant, S.; Sandoz, A. Participatory mapping: Exploring landscape values associated with an iconic species. Appl. Geogr. 2018, 95, 71–78. [Google Scholar] [CrossRef]
- Aceves-Bueno, E.; Adeleye, A.S.; Feraud, M.; Huang, Y.; Tao, M.; Yang, Y.; Anderson, S.E. The Accuracy of Citizen Science Data: A Quantitative Review. Bull. Ecol. Soc. Am. 2017, 98, 278–290. [Google Scholar] [CrossRef]
- Adriaens, T.; Tricarico, E.; Reyserhove, L.; Cardoso, A.C.; Gervasini, E.; Lopez Canizares, C.; Mitton, I.; Schade, S.; Spinelli, F.A.; Tsiamis, K. Data-Validation Solutions for Citizen Science Data on Invasive Alien Species, Tailoring Validation Tools for the JRC App “Invasive Alien Species in Europe”, EUR 30857 EN; Publications Office of the European: Luxembourg, 2021; ISBN 978-92-76-42055-2. [Google Scholar] [CrossRef]
- Delaney, D.G.; Sperling, C.D.; Adams, C.S.; Leung, B. Marine invasive species: Validation of citizen science and implications for national monitoring networks. Biol. Invasions 2008, 10, 117–128. [Google Scholar] [CrossRef]
- Wiggins, A.; Crowston, K. From conservation to crowdsourcing: A typology of citizen science. In Proceedings of the 2011 44th Hawaii International Conference on System Sciences, Kauai, HI, USA, 4–7 January 2011; pp. 1–10. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Sfakianakis, D.G.; Somarakis, S. Morphological differences between wild and farmed Mediterranean fish. Hydrobiologia 2012, 679, 217–231. [Google Scholar] [CrossRef]
- Caldeira, R.M.A.; Groom, S.; Miller, P.; Pilgrim, D.; Nezlin, N.P. Sea-surface signatures of the island mass effect phenomena around Madeira Island, Northeast Atlantic. Remote Sens. Environ. 2002, 80, 336–360. [Google Scholar] [CrossRef]
- Andrade, C.A.P. A fish farm pilot-project in Madeira Archipelago, Northeastern Atlantic-I. In The offshore option. In Proceedings of the International Conference, Portland, ME, USA, 8–10 May 1996; pp. 371–376. [Google Scholar]
- Andrade, C.A.P.; Gouveia, N.M.A. Ten years of marine aquaculture in Madeira archipelago. In Proceedings of the International Workshop: Developing a Sustainable Aquaculture Industry in the Azores, Horta, Portugal, 2–5 June 2008; pp. 3–32. [Google Scholar]
- Stirling Aquatic Resources. A Study of the Market for Fish Cultured in Madeira; The Department of Marketing/Institute of Aquaculture, University of Stirling: Stirling, UK, 1996. [Google Scholar]
- DREM [Direção Regional de Estatística da Madeira]. Available online: https://perma.cc/DF2M-GCS3 (accessed on 2 February 2023).
- Jardas, I. The Adriatic Ichthyofauna; Skolska Knjiga: Zagreb, Croatia, 1996; Croatian with English Abstract. [Google Scholar]
- Alves, F.M.A.; Alves, C.M.A. Two new records of seabreams (Pisces: Sparidae) from the Madeira Archipelago. Arquipélago Life Mar. Sci. 2002, 19A, 107–111. Available online: http://hdl.handle.net/10400.3/172 (accessed on 1 January 2023).
- González-Lorenzo, G.; Brito, A.; Barquin, J. Impacts of the escapees from mariculture cage in Canary Islands. Vieraea 2005, 33, 449–454. [Google Scholar]
- Wirtz, P.; Fricke, R.; Biscoito, M.J. The coastal fishes of Madeira Island–new records and an annotated check-list. Zootaxa 2008, 1715, 1–26. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Hermida, M.; Villasante, S.; Gouveia, L.; Gouveia, N.; Pita, P. Importance of recreational shore angling in the archipelago of Madeira, Portugal (northeast Atlantic). Sci. Mar. 2020, 84, 331–341. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Vieira, C.; Gouveia, L.; Gouveia, N.; Hermida, M. Characterization and evolution of spearfishing in Madeira archipelago, Eastern Atlantic. Aquat. Living Resour. 2020, 33, 15. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Toledo-Guedes, K.; Izquierdo-Gomez, D.; Šegvić-Bubicć, T.; Sanchez-Jerez, P. Implications of Sea Bream and Sea Bass Escapes for Sustainable Aquaculture Management: A Review of Interactions, Risks and Consequences. Rev. Fish. Sci. Aquac. 2018, 26, 214–234. [Google Scholar] [CrossRef]
- Harris, P.T.; Whiteway, T. High seas marine protected areas: Benthic environmental conservation priorities from a GIS analysis of global ocean biophysical data. Ocean. Coast. Manag. 2009, 52, 22–38. [Google Scholar] [CrossRef]
- Redlands, C.A. ArcGIS desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2010. [Google Scholar]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Chapman and Hall: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Andrade, C.A.P.; Nogueira, N.; Silva, P.; Dinis, M.T.; Narciso, L. Mesocosm hatcheries using semi-intensive methodologies and species diversification in aquaculture. J. Agric. Sci. Technol. 2012, 2, 428–437. [Google Scholar]
- Jackson, D.; Drumm, A.; McEvoy, S.; Jensen, O.; Mendiola, D.; Gabiña, G.; Borg, J.A.; Papageorgiou, N.; Karakassis, Y.; Black, K.D. A pan-European valuation of the extent, causes and cost of escape events from sea cage fish farming. Aquaculture 2015, 436, 21–26. [Google Scholar] [CrossRef]
- Sicuro, B.; Tarantola, M.; Valle, E. Italian aquaculture and the diffusion of alien species: Costs and benefits. Aquac. Res. 2016, 47, 3718–3728. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Fleming, I.A.; McGinnity, P.; Soto, D.; Wennevik, V.; Whoriskey, F. Incidence and Impacts of Escaped Farmed Atlantic salmon Salmo Salar in Nature; NINA Special Report 36; FAO: Rome, Italy, 2008; 110p, ISSN 0804-421X. ISBN 978-82-426-1966-2. [Google Scholar]
- Toledo-Guedes, K.; Sanchez-Jerez, P.; Brito, A. Influence of a massive aquaculture escape event on artisanal fisheries. Fish. Manag. Ecol. 2014, 21, 113–121. [Google Scholar] [CrossRef]
- Müller, J. Sparus aurata Escapes from Offshore Fish Farms around MADEIRA. Master’s Thesis, Universidade do Algarve, Faro, Portugal, 2019. [Google Scholar]
- Dempster, T.; Arechavala-Lopez, P.; Barrett, L.T.; Fleming, I.A.; Sanchez-Jerez, P.; Uglem, I. Recapturing escaped fish from marine aquaculture is largely unsuccessful: Alternatives to reduce the number of escapees in the wild. Rev. Aquac. 2018, 10, 153–167. [Google Scholar] [CrossRef]
- Skilbrei, O.T.; Heino, M.; Svåsand, T. Using simulated escape events to assess the annual numbers and destinies of escaped farmed Atlantic salmon of different life stages from farm sites in Norway. ICES J. Mar. Sci. 2015, 72, 670–685. [Google Scholar] [CrossRef]
- Shaw, S.L.; Renik, K.M.; Sass, G.G. Angler and environmental influences on walleye Sander vitreus and muskellunge Esox masquinongy angler catch in Escanaba Lake, Wisconsin 2003–2015. PLoS ONE 2021, 16, e0257882. [Google Scholar] [CrossRef]
- Clavelle, T.; Lester, S.E.; Gentry, R.; Froehlich, H.E. Interactions and management for the future of marine aquaculture and capture fisheries. Fish Fish 2019, 20, 368–388. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hindar, K.; Fleming, I.A.; Goldburg, R.J.; E I Williams, S.; Volpe, J.P.; Whoriskey, F.G.; Eagle, J.; Kelso, D.J.; Mangel, M. Fugitive Salmon: Assessing the Risks of Escaped Fish from Net-Pen Aquaculture. BioScience 2005, 55, 427–437. [Google Scholar] [CrossRef]
- Castro, N.; Carlton, J.T.; Costa, A.C.; Marques, C.S.; Hewitt, C.L.; Cacabelos, E.; Lopes, E.; Gizzi, F.; Gestoso, I.; Monteiro, J.G.; et al. Diversity and patterns of marine non-native species in the archipelagos of Macaronesia. Divers. Distrib. 2022, 28, 667–684. [Google Scholar] [CrossRef]
- Figueiredo, M.; Morato, T.; Barreiros, J.P.; Afonso, P.; Santos, R.S. Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores. Fish. Res. 2005, 75, 107–119. [Google Scholar] [CrossRef]
- Bax, N.J. The significance and prediction of predation in marine fisheries. ICES J. Mar. Sci. 1998, 55, 997–1030. [Google Scholar] [CrossRef]
- Balart, E.F.; Pérez-Urbiola, J.C.; Campos-Dávila, L.; Monteforte, M.; Ortega-Rubio, A. On the first record of a potentially harmful fish, Sparus aurata in the Gulf of California. Biol. Invasions 2009, 11, 547–550. [Google Scholar] [CrossRef]
- Schäfer, S.; Monteiro, J.; Castro, N.; Rilov, G.; Canning-Clode, J. Cronius ruber (Lamarck, 1818) arrives to Madeira Island: A new indication of the ongoing tropicalization of the northeastern Atlantic. Mar. Biodivers. 2021, 49, 2699–2707. [Google Scholar] [CrossRef]
- Polo, A.; Yúfera, M.; Pascual, E. Effects of temperature on egg and larval development of Sparus aurata L. Aquaculture 1991, 92, 367–375. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Costa, P.M.; Diniz, M.S. Histopathological alterations, physiological limits, and molecular changes of juvenile Sparus aurata in response to thermal stress. Mar. Ecol. Prog. 2014, 505, 253–266. [Google Scholar] [CrossRef]
- Feidantsis, K.; Pörtner, H.O.; Lazou, A.; Kostoglou, B.; Michaelidis, B. Metabolic and molecular stress responses of the gilthead seabream Sparus aurata during long-term expo-sure to increasing temperatures. Mar. Biol. 2009, 156, 797–809. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of temperature on the development of skeletal deformities in gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Encarnação, J.; Baptista, V.; Teodósio, M.A.; Morais, P. Low-Cost Citizen Science Effectively Monitors the Rapid Expansion of a Marine Invasive Species. Front. Environ. Sci. 2021, 9, 752705. [Google Scholar] [CrossRef]
- Kousteni, V.; Tsiamis, K.; Gervasini, E.; Zenetos, A.; Karachle, P.K.; Cardoso, A.C. Citizen scientists contributing to alien species detection: The case of fishes and mollusks in European marine waters. Ecosphere 2022, 13, e03875. [Google Scholar] [CrossRef]
- Cossu, P.; Scarpa, F.; Sanna, D.; Lai, T.; Dedola, G.L.; Curini-Galletti, M.; Mura, L.; Fois, N.; Casu, M. Influence of genetic drift on patterns of genetic variation: The footprint of aquaculture practices in Sparus aurata (Teleostei: Sparidae). Mol. Ecol. 2019, 28, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).