Permeability of Hydrate-Bearing Sediment Formed from CO2-N2 Mixture
Abstract
1. Introduction
2. Experimental Design
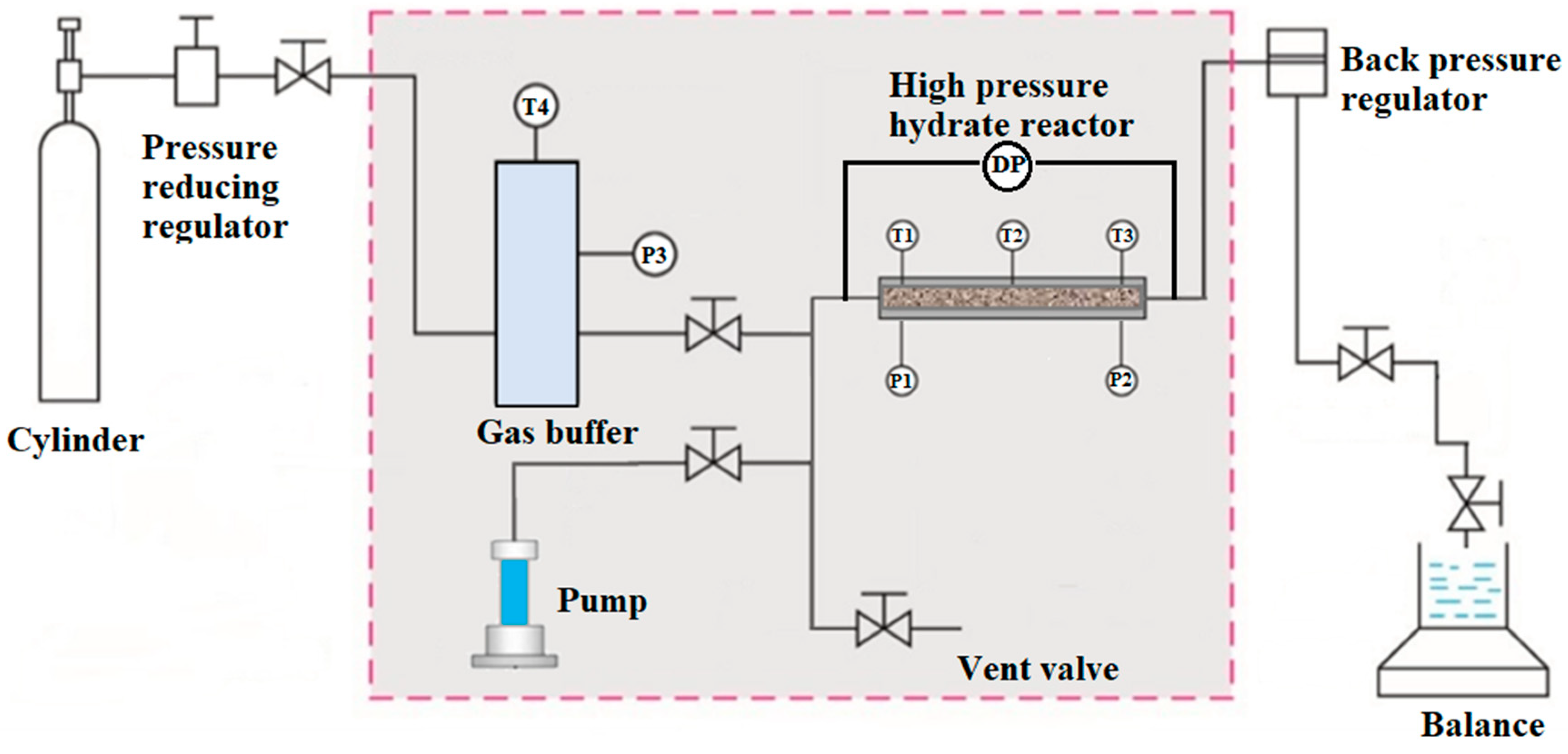
2.1. Devices and Materials
2.2. Procedures
2.3. Data Processing
3. Results and Discussions
3.1. Hydrate Saturation
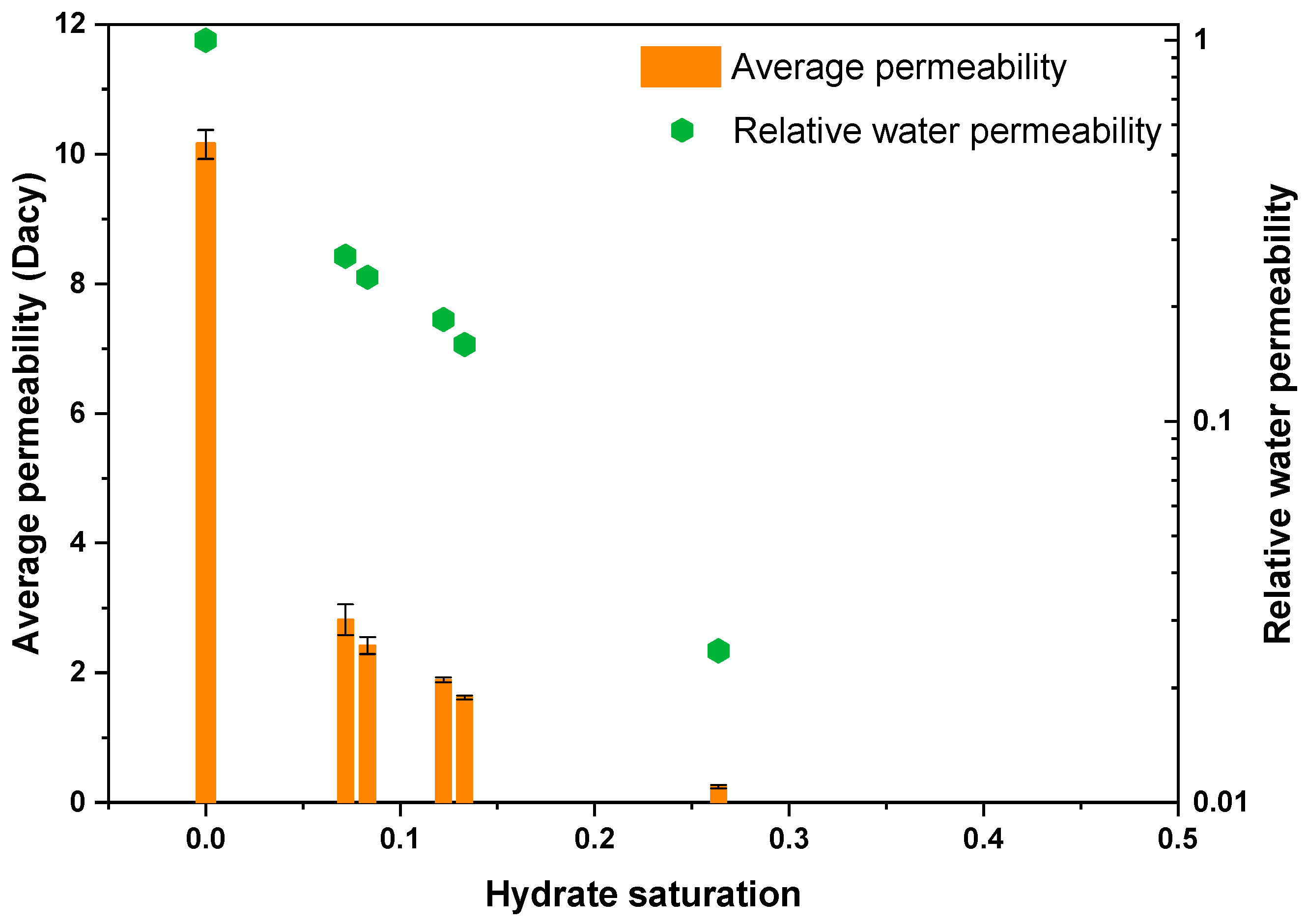
3.2. Permeability of Hydrate-Bearing Sediments
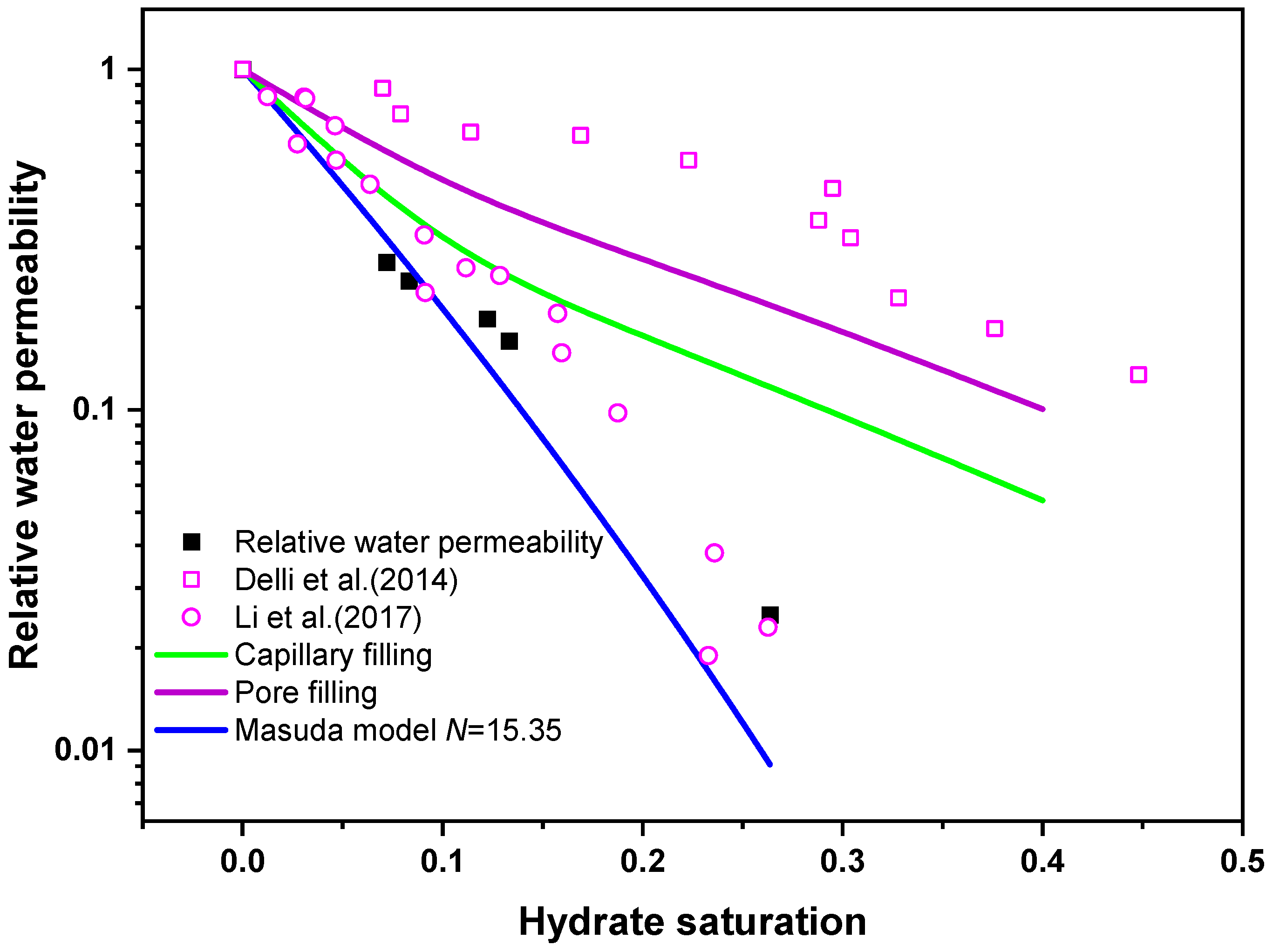
3.3. Comparison of Experimental Data and Prediction Model
4. Conclusions
- (1)
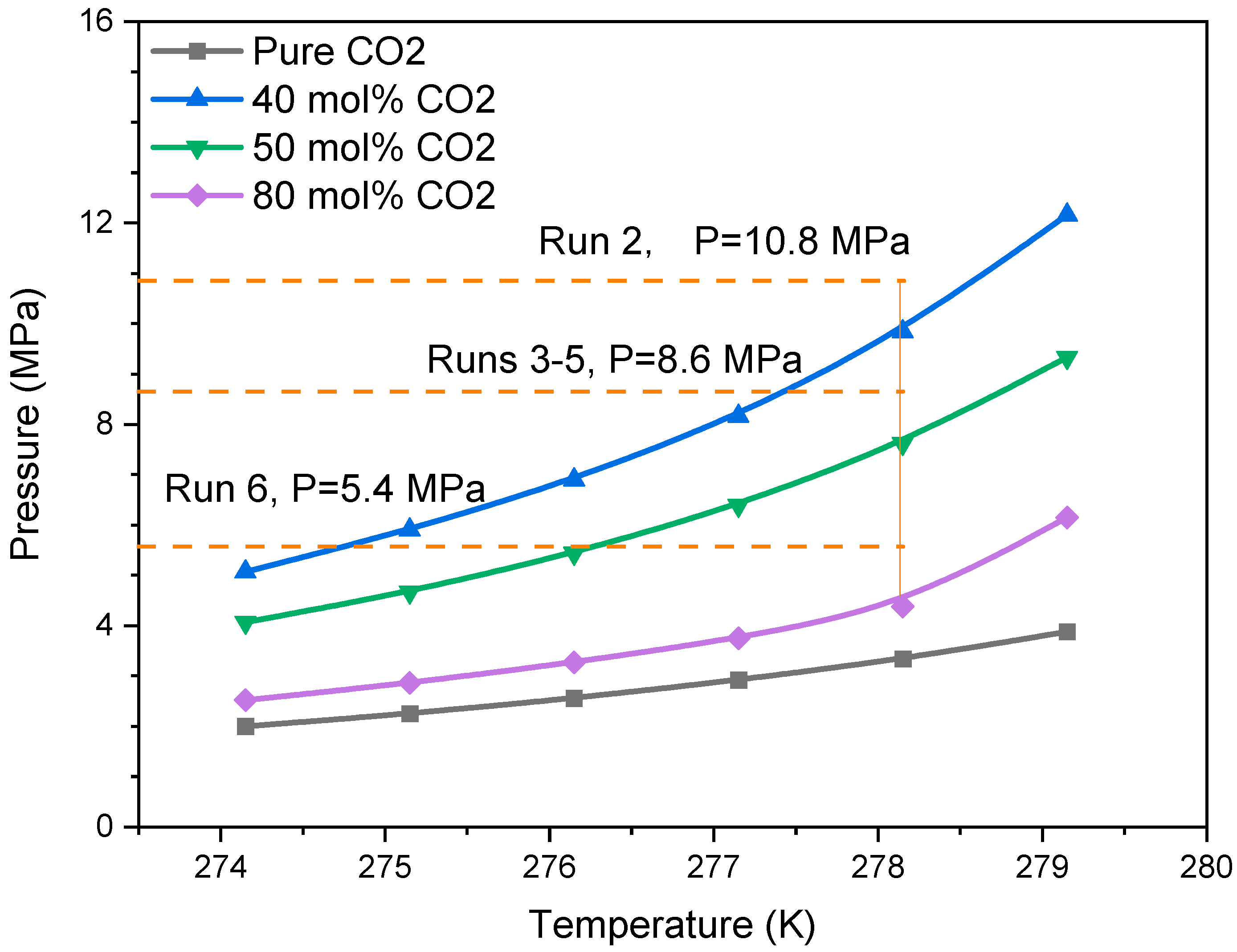
- Gas composition is the key factor affecting hydrate formation and transformation. Hydrate saturation gradually increases with increasing CO2 mole fraction in the gas mixture. However, due to the decrease in driven force during hydrate formation, the obtained hydrate saturation data with different gas composition were limited in a narrow range.
- (2)
- The presence of hydrate formed from a CO2-N2 mixture leads to a sharp reduction in sediment permeability, which shows a significant negative correlation with the CO2 mole fraction in the initial gas. With regard to NGH reservoir exploitation and reformation, high CO2 content in the CO2-N2 injection is suitable for reformation of the hydrate reservoir, and high N2 content is conductive to exploitation of hydrate by CO2 replacement.
- (3)
- The pore-filling model underestimates the permeability of hydrate-bearing sediments formed from CO2-N2 gas. The fitted hydrate saturation index in the Masuda model is 15.35, slightly higher than the general values, which means that the formed hydrate tends to occupy the pore center, and even block the pore throat. Visual technologies are suggested to obtain the accurate morphologies of hydrate in sediments. The fitted model can be employed in numerical simulations related to hydrate exploitation by CO2-N2 injection. These results can be used as the basis for evaluation and adjustment of gas injection process in future works.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Boswell, R.; Collet, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Zhang, X.W.; Hu, T.; Pang, X.Q.; Hu, Y.; Wang, T.; Wang, E.Z.; Xu, Z.; Liu, X.H.; Wu, Z.Y. Evaluation of natural gas hydrate resources in the South China Sea by combining volumetric and trend-analysis methods. Petrol. Sci. 2022, 19, 37–47. [Google Scholar] [CrossRef]
- Yoneda, J.; Masui, A.; Konno, Y.; Jin, Y.; Egawa, K.; Kida, M.; Ito, T.; Nagao, J.; Tenma, N. Mechanical properties of hydrate bearing turbidite reservoir in the first gas production test site of the Eastern Nankai Trough. Mar. Pet. Geol. 2015, 66, 471–486. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, T.; Pang, X.Q.; Wang, E.Z.; Liu, X.H.; Wu, Z.Y.; Chen, D.; Li, C.R.; Zhang, X.W.; Wang, T. Research progress and challenges of natural gas hydrate resource evaluation in the South China Sea. Petrol. Sci. 2022, 19, 13–25. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S. Hydrate dissociation induced by depressurization in conjunction with warm brine stimulation in cubic hydrate simulator with silica sand. Appl. Energy 2016, 174, 181–191. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, C.; Zhao, J.; Zhu, Z.; Liu, W.; Yang, M.; Xue, K. Evaluation of gas production from methane hydrates using depressurization, thermal stimulation and combined methods. Appl. Energy 2015, 145, 265–277. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates—Phase equilibria for CO2-CH4 mixed hydrate system. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.Y.; Lee, J.W.; Huh, D.G.; Park, K.P.; Lee, J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. P. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef]
- Koh, D.Y.; Kang, H.; Lee, J.W.; Park, Y.; Kim, S.J.; Lee, J.; Lee, J.Y.; Lee, H. Energy-efficient natural gas hydrate production using gas exchange. Appl. Energy 2016, 162, 114–130. [Google Scholar] [CrossRef]
- Kang, H.; Koh, D.Y.; Lee, H. Nondestructive natural gas hydrate recovery driven by air and carbon dioxide. Sci. Rep. 2014, 4, 6616. [Google Scholar] [CrossRef]
- Li, B.; Xu, T.; Zhang, G.; Guo, W.; Liu, H.; Wang, Q.; Qu, L.; Sun, Y. An experimental study on gas production from fracture-filled hydrate by CO2 and CO2/N2 replacement. Energy Convers. Manag. 2018, 165, 738–747. [Google Scholar] [CrossRef]
- Kan, J.; Sun, Y.; Dong, B.; Yuan, Q.; Liu, B.; Sun, C.; Chen, G. Numerical simulation of gas production from permafrost hydrate deposits enhanced with CO2/N2 injection. Energy 2021, 221, 119919. [Google Scholar] [CrossRef]
- Sun, J.; Ning, F.; Li, S.; Zhang, K.; Liu, T.; Zhang, L.; Jiang, G.; Wu, N. Numerical simulation of gas production from hydrate-bearing sediments in the Shenhu area by depressurising: The effect of burden permeability. J. Unconv. Oil Gas Resour. 2015, 12, 23–33. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, J.; Sun, L.; Wang, J.; Hu, W.; Peng, B.; Wang, Y.; Wang, Y. Coupled simulation of hydrate-bearing and overburden sedimentary layers to study hydrate dissociation and methane Leakage. J. Mar. Sci. Eng. 2022, 10, 668. [Google Scholar] [CrossRef]
- Li, N.; Sun, Z.; Jia, S.; Sun, C.; Liu, B.; Yang, L.; Chen, G. A novel method to greatly increase methane hydrate exploitation efficiency via forming impermeable overlying CO2 cap. Energy Procedia 2019, 158, 5975–5981. [Google Scholar] [CrossRef]
- Sun, Z.F.; Li, N.; Jia, S.; Cui, J.; Yuan, Q.; Sun, C.; Chen, G. A novel method to enhance methane hydrate exploitation efficiency via forming impermeable overlying CO2 hydrate cap. Appl. Energy 2019, 240, 842–850. [Google Scholar] [CrossRef]
- Cui, J.L.; Sun, Z.F.; Kan, J.Y.; Jia, S.; Sun, C.; Chen, G.; Wang, X.H.; Yuan, Q.; Li, N. Study on the factors affecting the sealing performance and mechanical stability of CO2 hydrate cap during gas production from methane hydrate. J. Nat. Gas Sci. Eng. 2021, 93, 104050. [Google Scholar] [CrossRef]
- Li, N.; Jia, S.; Sun, Z.; Sun, C.; Chen, G.; Li, J. Exploitation of CH4 hydrate reservoir reformed by (CO2 + N2) gas injection. Chem. Ind. Eng. Prog. 2022, 41, 2356–2363. [Google Scholar]
- Konno, Y.; Yoneda, J.; Egawa, K.; Ito, T.; Jin, Y.; Kida, M.; Suzuki, K.; Fujii, T.; Nagao, J. Permeability of sediment cores from methane hydrate deposit in the Eastern Nankai Trough. Mar. Petrol. Geol. 2015, 66, 487–495. [Google Scholar] [CrossRef]
- Bei, K.; Xu, T.; Shang, S.; Wei, Z.; Yuan, Y.; Tian, H. Numerical modeling of gas migration and hydrate formation in heterogeneous marine sediments. J. Mar. Sci. Eng. 2019, 7, 348. [Google Scholar] [CrossRef]
- Kleinberg, R.L. Deep sea NMR: Methane hydrate growth habit in porous media and its relationship to hydraulic permeability, deposit accumulation, and submarine slope stability. J. Geophys. Res. 2003, 108, B10. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Li, C.; Zhang, Y.; Bu, Q.; Wu, N.; Liu, Y.; Chen, Q. Pore-Scale Investigation of the Electrical Property and Saturation Exponent of Archie’s Law in Hydrate-Bearing Sediments. J. Mar. Sci. Eng. 2022, 10, 111. [Google Scholar] [CrossRef]
- Bu, Q.; Xing, T.; Li, C.; Zhao, J.; Liu, C.; Wang, Z.; Zhao, W.; Kang, J.; Meng, Q.; Hu, G. Effect of hydrate microscopic distribution on acoustic characteristics during hydrate dissociation: An insight from combined acoustic-CT detection study. J. Mar. Sci. Eng. 2022, 10, 1089. [Google Scholar] [CrossRef]
- Kumar, A.; Maini, B.; Bishnoi, P.R.; Clarke, M.; Zatsepina, O.; Srinivasan, S. Experimental determination of permeability in the presence of hydrates and its effect on the dissociation characteristics of gas hydrates in porous media. J. Petrol. Sci. Eng. 2010, 70, 114–122. [Google Scholar] [CrossRef]
- Delli, M.L.; Grozic, J.L.H. Experimental determination of permeability of porous media in the presence of gas hydrates. J. Petrol. Sci. Eng. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Wu, D.; Li, X.S.; Lv, Q.N.; Li, C.; Zhang, Y. Experimental measurement and mathematical model of permeability with methane hydrate in quartz sands. Appl. Energy 2017, 202, 282–292. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhao, Q.; Xu, H.; Feng, K.; Liu, X. Relation between relative permeability and hydrate saturation in Shenhu area, South China Sea. Appl. Geophys. 2014, 11, 207–214. [Google Scholar] [CrossRef]
- Dai, S.; Seol, Y. Water permeability in hydrate-bearing sediments: A pore-scale study. Geophy. Res. Lett. 2014, 41, 4176–4184. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Zhang, Y.; Wang, D.; Li, Y.; Song, Y. Analysis of the influence of wettability on permeability in hydrate-bearing porous media using pore network models combined with computed tomography. J. Nat. Gas Sci. Eng. 2015, 26, 1372–1379. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Zhang, Y.; Wang, D.; Li, Y.; Song, Y. Analysis of the effect of particle size on permeability in hydrate-bearing porous media using pore network models combined with CT. Fuel 2016, 163, 34–40. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yang, M.; Li, Y.; Liu, W.; Song, Y. Permeability of laboratory-formed porous media containing methane hydrate: Observations using X-ray computed tomography and simulations with pore network models. Fuel 2015, 145, 170–179. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. CO2 capture by injection of flue Gas or CO2−u2 mixtures into hydrate reservoirs: Dependence of CO2 capture efficiency on gas hydrate reservoir conditions. Environ. Sci. Technol. 2018, 52, 4324–4330. [Google Scholar] [CrossRef]
- Pandey, J.S.; Khan, S.; Karcz, A.P.; Solms, N. Chemically modifed hydrate swapping and hydrate stability during multistage CO2-N2 injection schemes. Fuel 2021, 299, 120711. [Google Scholar] [CrossRef]
- Niu, M.; Wu, G.; Yin, Z.; Sun, Y.; Liu, K.; Chen, D. Effectiveness of CO2-N2 injection for synergistic CH4 recovery and CO2 sequestration at marine gas hydrates condition. Chem. Eng. J. 2021, 420, 129615. [Google Scholar] [CrossRef]
- Niu, M.; Yin, Z.; Sun, Y.; Fang, W.; Chen, G.; Chen, D. CH4 hydrate production coupled with CO2 sequestration and hydrate restoration employing depressurization assisted by CO2-N2 injection at marine conditions. Chem. Eng. J. 2023, 456, 140981. [Google Scholar] [CrossRef]
- Chen, G.J.; Guo, T.M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar]
- Starling, K.E.; Han, M.S. Thermo data refined for LPG-14. mixtures. Hydrocarb. Process. 1972, 51, 129–132. [Google Scholar]
- Masuda, Y.; Naganawa, S.; Ando, S.; Sato, K. Numerical calculation of gas production performance from reservoirs containing natural gas hydrates. SPE J. 1997, 29, 201–210. [Google Scholar]
- Gong, G.J.; Zhao, G.J.; Pang, W.X.; Yang, M.J.; Chen, B.B.; Zheng, J.N. Review of hydrate-bearing sediment permeability for natural gas hydrate exploitation: Measurement and application development. J. Petrol. Sci. Eng. 2023, 220, 111217. [Google Scholar] [CrossRef]


| Run | Initial Water Saturation (SW) | CO2 Mole Fraction | Initial Pressure (MPa) | Hydrate Saturation | Average Permeability (Darcy) | Relative Water Permeability |
|---|---|---|---|---|---|---|
| 1 | / | / | / | 0 | 10.15 | 1.000 |
| 2 | 0.3 | 0.4 | 10.8 | 0.072 | 2.82 | 0.278 |
| 3 | 0.2 | 0.5 | 8.6 | 0.083 | 2.42 | 0.238 |
| 4 | 0.3 | 0.5 | 8.6 | 0.122 | 1.89 | 0.186 |
| 5 | 0.4 | 0.5 | 8.6 | 0.133 | 1.62 | 0.160 |
| 6 | 0.3 | 0.8 | 5.4 | 0.264 | 0.243 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Fan, Z.; Ma, H.; Jia, S.; Kan, J.; Sun, C.; Liu, S. Permeability of Hydrate-Bearing Sediment Formed from CO2-N2 Mixture. J. Mar. Sci. Eng. 2023, 11, 376. https://doi.org/10.3390/jmse11020376
Li N, Fan Z, Ma H, Jia S, Kan J, Sun C, Liu S. Permeability of Hydrate-Bearing Sediment Formed from CO2-N2 Mixture. Journal of Marine Science and Engineering. 2023; 11(2):376. https://doi.org/10.3390/jmse11020376
Chicago/Turabian StyleLi, Nan, Ziyang Fan, Haoran Ma, Shuai Jia, Jingyu Kan, Changyu Sun, and Shun Liu. 2023. "Permeability of Hydrate-Bearing Sediment Formed from CO2-N2 Mixture" Journal of Marine Science and Engineering 11, no. 2: 376. https://doi.org/10.3390/jmse11020376
APA StyleLi, N., Fan, Z., Ma, H., Jia, S., Kan, J., Sun, C., & Liu, S. (2023). Permeability of Hydrate-Bearing Sediment Formed from CO2-N2 Mixture. Journal of Marine Science and Engineering, 11(2), 376. https://doi.org/10.3390/jmse11020376