Predicted Changes in the Biogeographical Range of Gracilaria vermiculophylla under Present and Future Climate Scenarios
Abstract
1. Introduction
2. Methodology
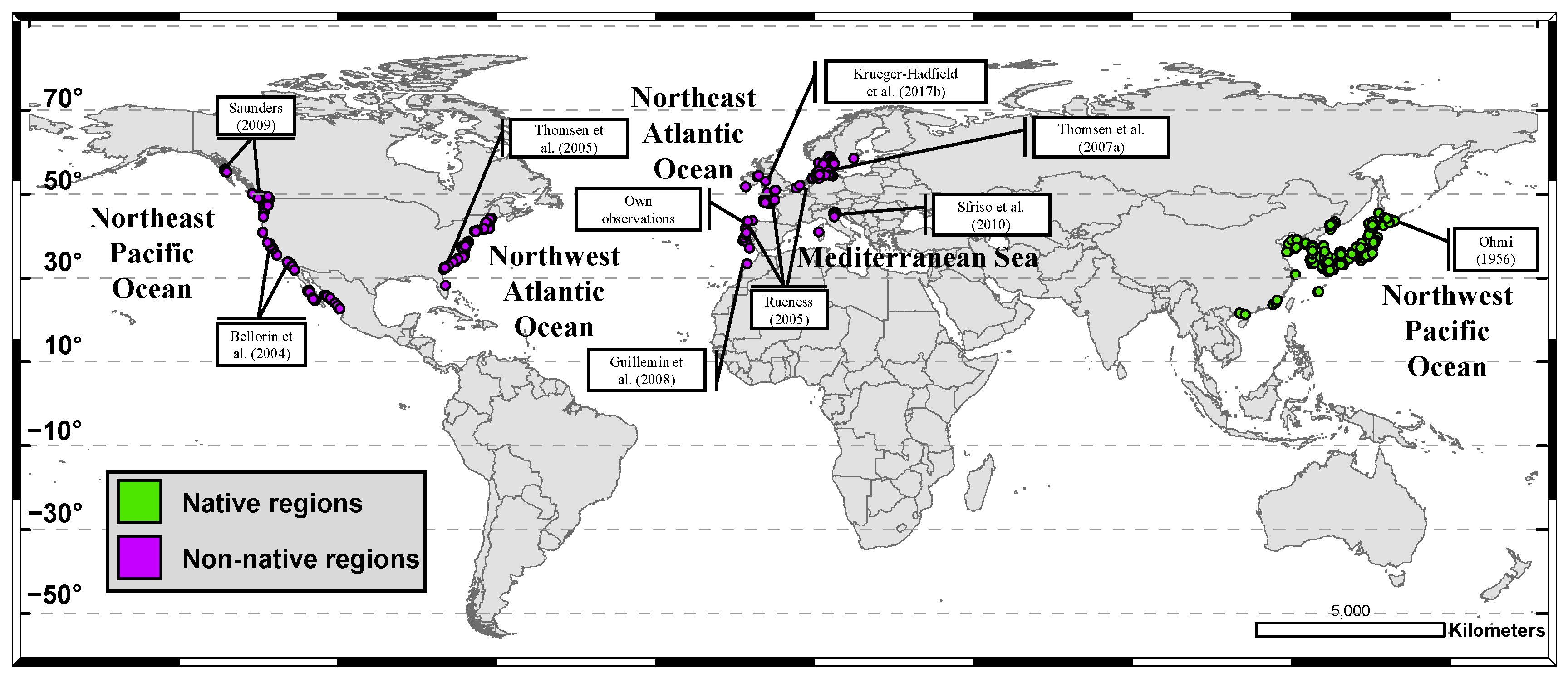
2.1. Gracilaria vermiculophylla Occurrences
2.2. MAXENT Model
2.3. Environmental Variables
2.4. Species Distribution Models (SDMs) and Spatial Analyses
3. Results
3.1. Comparison of SDMs for the Current Climate Scenario
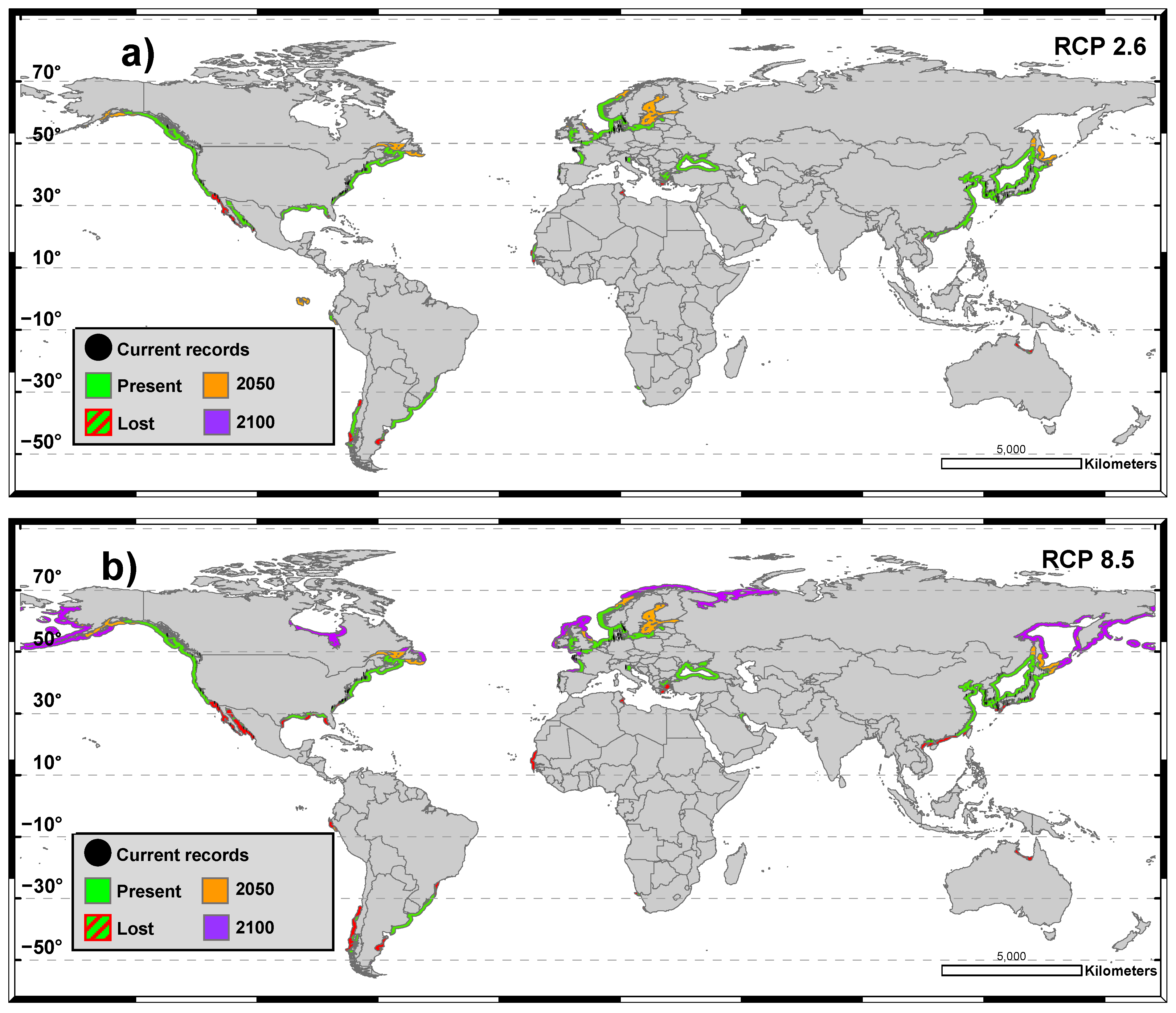
3.2. SDMs for the Future Climate Scenario
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rilov, G.; Crooks, J.A. Biological Invasions in Marine Ecosystems; Springer: Berlin/Heidelberg, Germany, 2009; Volume 204. [Google Scholar]
- Klinger, T. Optimizing Seaweed Futures under Climate Change. Bot. Mar. 2021, 64, 439–443. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Climate Change and Algal Communities. In Progress in Microalgae Research—A Path for Shaping Sustainable Futures; IntechOpen: London, UK, 2022; pp. 1–12. [Google Scholar]
- Diez, J.M.; D’Antonio, C.M.; Dukes, J.S.; Grosholz, E.D.; Olden, J.D.; Sorte, C.J.; Bluementhal, D.M.; Bradley, B.A.; Early, R.; Ibáñez, I.; et al. Will Extreme Climatic Events Facilitate Biological Invasions? Front. Ecol. Environ. 2012, 10, 249–257. [Google Scholar] [CrossRef]
- Gorman, L.; Kraemer, G.P.; Yarish, C.; Boo, S.M.; Kim, J.K. The Effects of Temperature on the Growth Rate and Nitrogen Content of Invasive Gracilaria vermiculophylla and Native Gracilaria tikvahiae from Long Island Sound, USA. Algae 2017, 32, 57–66. [Google Scholar] [CrossRef]
- Schaffelke, B.; Smith, J.E.; Hewitt, C.L. Introduced Macroalgae—A Growing Concern. J. Appl. Phycol. 2006, 18, 529–541. [Google Scholar] [CrossRef]
- Zi Min, H.; Lopez-Bautista, J. Adaptation Mechanisms and Ecological Consequences of Seaweed Invasions: A Review Case of Gracilaria vermiculophylla. Biol. Invasions 2014, 16, 967–976. [Google Scholar] [CrossRef]
- Davidson, A.D.; Campbell, M.L.; Hewitt, C.L.; Schaffelke, B. Assessing the Impacts of Nonindigenous Marine Macroalgae: An Update of Current Knowledge. Bot. Mar. 2015, 58, 55–79. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A Global Review of the Distribution, Taxonomy, and Impacts of Introduced Seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- Fernández de la Hoz, C.; Ramos, E.; Puente, A.; Juanes, J.A. Climate Change Induced Range Shifts in Seaweeds Distributions in Europe. Mar. Environ. Res. 2019, 148, 1–11. [Google Scholar] [CrossRef]
- AlgaeBase. Available online: https://www.algaebase.org/ (accessed on 29 November 2022).
- Terada, R.; Yamamoto, H. Review of Gracilaria vermiculophylla and Other Species in Japan and Asia. In Taxonomy of Economic Seaweeds with Reference to Some Pacific Species, Vol VIII; Abbott, I.A., Ed.; California Sea Grant College Program, University of California: La Jolla, CA, USA, 2002; pp. 215–224. [Google Scholar]
- Lyra, G.d.M.; Gurgel, C.F.D.; Costa, E.d.S.; Barreto de Jesus, P.; Oliveira, M.C.; Oliveira, E.C.; Davis, C.C.; de Castro Nunes, J.M. Delimitating Cryptic Species in the Gracilaria domingensis Complex (Gracilariaceae, Rhodophyta) Using Molecular and Morphological Data. J. Phycol. 2016, 52, 997–1017. [Google Scholar] [CrossRef]
- Krueger-Hadfield, S.A.; Kollars, N.M.; Strand, A.E.; Byers, J.E.; Shainker, S.J.; Terada, R.; Greig, T.W.; Hammann, M.; Murray, D.C.; Weinberger, F.; et al. Genetic Identification of Source and Likely Vector of a Widespread Marine Invader. Ecol. Evol. 2017, 7, 4432–4447. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Kakita, H.; Obika, H.; Kitamura, T. Effects of Environmental Factors and Plant Growth Regulators on Growth of the Red Alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologia 1999, 398/399, 339–347. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Staehr, P.A.; Nyberg, C.D.; Schwaerter, S.; Krause-Jensen, D.; Silliman, B.R. Gracilaria vermiculophylla (Ohmi) Papenfuss, 1967 (Rhodophyta, Gracilariaceae) in Northern Europe, with Emphasis on Danish Conditions, and What to Expect in the Future. Aquat. Invasions 2007, 2, 83–94. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Sousa-Pinto, I.; Yarish, C. Ecophysiological Studies of the Non-Indigenous Species Gracilaria vermiculophylla (Rhodophyta) and Its Abundance Patterns in Ria de Aveiro Lagoon, Portugal. Eur. J. Phycol. 2011, 46, 453–464. [Google Scholar] [CrossRef]
- Volaric, M.P.; Berg, P.; Reidenbach, M.A. An Invasive Macroalga Alters Ecosystem Metabolism and Hydrodynamics on a Tidal Flat. Mar. Ecol. Prog. Ser. 2019, 628, 1–16. [Google Scholar] [CrossRef]
- Haram, L.E.; Sotka, E.E.; Byers, J.E. Effects of Novel, Non-Native Detritus on Decomposition and Invertebrate Community Assemblage. Mar. Ecol. Prog. Ser. 2020, 643, 49–61. [Google Scholar] [CrossRef]
- Nyberg, C.D.; Wallentinus, I. Long-Term Survival of an Introduced Red Alga in Adverse Conditions. Mar. Biol. Res. 2009, 5, 304–308. [Google Scholar] [CrossRef]
- Gulbransen, D.J. Gracilaria vermiculophylla in the Virginia Coastal Bays: Documenting the Distribution and Effects of a Non-Native Species. Ph.D. Thesis, University of Virginia, Charlottesville, VA, USA, August 2013. [Google Scholar]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and Nutrient Removal Performance of the Seaweed in a Land-Based Pilot Scale System. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Thomsen, M.S.; McGlathery, K.J.; Tyler, A.C. Macroalgal Distribution Patterns in a Shallow, Soft-Bottom Lagoon, with Emphasis on the Nonnative Gracilaria vermiculophylla and Codium fragile. Estuaries Coasts 2006, 29, 465–473. [Google Scholar] [CrossRef]
- Muangmai, N.; Vo, T.D.; Kawaguchi, S. Seasonal Fluctuation in a Marine Red Alga, Gracilaria vermiculophylla (Gracilariales, Rhodophyta), from Nokonoshima Island, Southern Japan. J. Fac. Agric. Kyushu Univ. 2014, 59, 243–248. [Google Scholar] [CrossRef]
- Raikar, S.V.; Iima, M.; Fujita, Y. Effect of Temperature, Salinity and Light Intensity on the Growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J. Mar. Sci. 2001, 30, 98–104. [Google Scholar]
- Phooprong, S.; Ogawa, H.; Hayashizaki, K. Photosynthetic and Respiratory Responses of Gracilaria vermiculophylla (Ohmi) Papenfuss Collected from Kumamoto, Shizuoka and Iwate, Japan. Ninet. Int. Seaweed Symp. 2007, 20, 293–300. [Google Scholar] [CrossRef]
- Nejrup, L.B.; Pedersen, M.F. The Effect of Temporal Variability in Salinity on the Invasive Red Alga Gracilaria vermiculophylla. Eur. J. Phycol. 2012, 47, 254–263. [Google Scholar] [CrossRef]
- Rueness, J. Life History and Molecular Sequences of Gracilaria vermiculophylla (Gracilariales, Rhodophyta), a New Introduction to European Waters. Phycologia 2005, 44, 120–128. [Google Scholar] [CrossRef]
- Kameyama, R.; Nishihara, G.N.; Kawagoe, C.; Terada, R. The Effects of Four Stressors, Irradiance, Temperature, Desiccation, and Salinity on the Photosynthesis of a Red Alga, Agarophyton vermiculophyllum (Gracilariales) from a Native Distributional Range in Japan. J. Appl. Phycol. 2021, 33, 2561–2575. [Google Scholar] [CrossRef]
- Bermejo, R.; MacMonagail, M.; Heesch, S.; Mendes, A.; Edwards, M.; Fenton, O.; Knöller, K.; Daly, E.; Morrison, L. The Arrival of a Red Invasive Seaweed to a Nutrient over-Enriched Estuary Increases the Spatial Extent of Macroalgal Blooms. Mar. Environ. Res. 2020, 158, 104944. [Google Scholar] [CrossRef]
- Samanta, P.; Jang, S.; Shin, S.; Kim, J.K. Effects of pH on Growth and Biochemical Responses in Agarophyton vermiculophyllum under Different Temperature Conditions. J. Appl. Phycol. 2020, 32, 499–509. [Google Scholar] [CrossRef]
- Nejrup, L.B.; Pedersen, M.F. Growth and Biomass Development of the Introduced Red Alga Gracilaria vermiculophylla Is Unaffected by Nutrient Limitation and Grazing. Aquat. Biol. 2010, 10, 249–259. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Johnsen, K.L. Nutrient (N and P) Dynamics of the Invasive Macroalga Gracilaria vermiculophylla: Nutrient Uptake Kinetics and Nutrient Release through Decomposition. Mar. Biol. 2017, 164, 172. [Google Scholar] [CrossRef]
- Schrofner, E.M.N.C. Nutrient Status of Major Irish Seaweed Tides. Master’s Thesis, University of the Algarve, Faro, Portugal, September 2020. [Google Scholar]
- Kim, S.Y.; Weinberger, F.; Boo, S.M. Genetic Data Hint at a Common Donor Region for Invasive Atlantic and Pacific Populations of Gracillaria vermiculophylla (Gracilariales, Rhodophyta). J. Phycol. 2010, 46, 1346–1349. [Google Scholar] [CrossRef]
- Krueger-Hadfield, S.A.; Byers, J.E.; Bonthond, G.; Terada, R.; Weinberger, F.; Sotka, E.E. Intraspecific Diversity and Genetic Structure in the Widespread Macroalga Agarophyton vermiculophyllum. J. Phycol. 2021, 57, 1403–1410. [Google Scholar] [CrossRef]
- Bellorin, A.M.; Oliveira, M.C.; Oliveira, E.C. Gracilaria vermiculophylla: A Western Pacific Species of Gracilariaceae (Rhodophyta) First Recorded from the Eastern Pacific. Phycol. Res. 2004, 52, 69–79. [Google Scholar] [CrossRef]
- Sfriso, A.; Maistro, S.; Andreoli, C.; Moro, I. First Record of Gracillaria vermiculophylla (Gracilariales, Rhodophyta) in the Po Delta Lagoons, Mediterranean Sea (Italy). J. Phycol. 2010, 46, 1024–1027. [Google Scholar] [CrossRef]
- Krueger-Hadfield, S.A.; Stephens, T.A.; Ryan, W.H.; Heiser, S. Everywhere You Look, Everywhere You Go, There’s an Estuary Invaded by the Red Seaweed Gracilaria vermiculophylla (Ohmi) Papenfuss, 1967. BioInvasions Rec. 2018, 7, 343–355. [Google Scholar] [CrossRef]
- Krueger-Hadfield, S.A.; Kollars, N.M.; Byers, J.E.; Greig, T.W.; Hammann, M.; Murray, D.C.; Murren, C.J.; Strand, A.E.; Terada, R.; Weinberger, F.; et al. Invasion of Novel Habitats Uncouples Haplo-Diplontic Life Cycles. Mol. Ecol. 2016, 25, 3801–3816. [Google Scholar] [CrossRef] [PubMed]
- Nejrup, L.B.; Pedersen, M.F.; Vinzent, J. Grazer Avoidance May Explain the Invasiveness of the Red Alga Gracilaria vermiculophylla in Scandinavian Waters. Mar. Biol. 2012, 159, 1703–1712. [Google Scholar] [CrossRef]
- Hammann, M.; Wang, G.; Boo, S.M.; Aguilar-Rosas, L.E.; Weinberger, F. Selection of Heat-Shock Resistance Traits during the Invasion of the Seaweed Gracilaria vermiculophylla. Mar. Biol. 2016, 163, 104. [Google Scholar] [CrossRef]
- Surget, G.; Le Lann, K.; Delebecq, G.; Kervarec, N.; Donval, A.; Poullaouec, M.A.; Bihannic, I.; Poupart, N.; Stiger-Pouvreau, V. Seasonal Phenology and Metabolomics of the Introduced Red Macroalga Gracilaria vermiculophylla, Monitored in the Bay of Brest (France). J. Appl. Phycol. 2017, 29, 2651–2666. [Google Scholar] [CrossRef]
- Thomsen, M.S.; McGlathery, K.J.; Schwarzschild, A.; Silliman, B.R. Distribution and Ecological Role of the Non-Native Macroalga Gracilaria vermiculophylla in Virginia Salt Marshes. Biol. Invasions 2009, 11, 2303–2316. [Google Scholar] [CrossRef]
- Besterman, A.F.; Karpanty, S.M.; Pace, M.L. Impact of Exotic Macroalga on Shorebirds Varies with Foraging Specialization and Spatial Scale. PLoS ONE 2020, 15, e0231337. [Google Scholar] [CrossRef]
- Sfriso, A.; Mistri, M.; Munari, C.; Buosi, A.; Sfriso, A.A. Management and Exploitation of Macroalgal Biomass as a Tool for the Recovery of Transitional Water Systems. Front. Ecol. Evol. 2020, 8, 20. [Google Scholar] [CrossRef]
- Sotka, E.E.; Baumgardner, A.W.; Bippus, P.M.; Destombe, C.; Duermit, E.A.; Endo, H.; Flanagan, B.A.; Kamiya, M.; Lees, L.E.; Murren, C.J.; et al. Combining Niche Shift and Population Genetic Analyses Predicts Rapid Phenotypic Evolution during Invasion. Evol. Appl. 2018, 11, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhong, K.-L.; Jueterbock, A.; Satoshi, S.; Choi, H.-G.; Weinberger, F.; Assis, J.; Hu, Z.-M. The Invasive Alga Gracilaria vermiculophylla in the Native Northwest Pacific under Ocean Warming: Southern Genetic Consequence and Northern Range Expansion. Front. Mar. Sci. 2022, 9, 983685. [Google Scholar] [CrossRef]
- Besterman, A.F.; McGlathery, K.J.; Reidenbach, M.A.; Wiberg, P.L.; Pace, M.L. Predicting Benthic Macroalgal Abundance in Shallow Coastal Lagoons from Geomorphology and Hydrologic Flow Patterns. Limnol. Oceanogr. 2020, 66, 123–140. [Google Scholar] [CrossRef]
- Laeseke, P.; Martínez, B.; Mansilla, A.; Bischof, K. Future Range Dynamics of the Red Alga Capreolia Implexa in Native and Invaded Regions: Contrasting Predictions from Species Distribution Models versus Physiological Knowledge. Biol. Invasions 2020, 22, 1339–1352. [Google Scholar] [CrossRef]
- Verbruggen, H.; Tyberghein, L.; Belton, G.S.; Mineur, F.; Jueterbock, A.; Hoarau, G.; Gurgel, C.F.D.; De Clerck, O. Improving Transferability of Introduced Species’ Distribution Models: New Tools to Forecast the Spread of a Highly Invasive Seaweed. PLoS ONE 2013, 8, e68337. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Varela, S.; Stengel, D.B. A Novel Method Combining Species Distribution Models, Remote Sensing, and Field Surveys for Detecting and Mapping Subtidal Seagrass Meadows. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1098–1110. [Google Scholar] [CrossRef]
- GBIF | Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 17 November 2022).
- Elith, J.H.; Graham, C.P.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Liang, W.; Papeş, M.; Tran, L.; Grant, J.; Washington-Allen, R.; Stewart, S.; Wiggins, G. The Effect of Pseudo-Absence Selection Method on Transferability of Species Distribution Models in the Context of Non-Adaptive Niche Shift. Ecol. Model. 2018, 388, 1–9. [Google Scholar] [CrossRef]
- Franklin, J. Species Distribution Models in Conservation Biogeography: Developments and Challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field Validation of an Invasive Species Maxent Model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Teichberg, M.; Winters, G.; Procaccini, G.; Reuter, H. Projected Rapid Habitat Expansion of Tropical Seagrass Species in the Mediterranean Sea as Climate Change Progresses. Front. Plant Sci. 2020, 11, 555376. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Steinitz, O.; Rotem, D.; Rosenfeld, A.; Kadmon, R. Incorporating Distance Constraints into Species Distribution Models. J. Appl. Ecol. 2008, 45, 599–609. [Google Scholar] [CrossRef]
- Manel, S.; Ceri Williams, H.; Ormerod, S.J. Evaluating Presence-Absence Models in Ecology: The Need to Account for Prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Bio-ORACLE Marine Data Layers for Ecological Modelling. Available online: www.bio-oracle.org (accessed on 17 November 2022).
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A Global Environmental Dataset for Marine Species Distribution Modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Meyer, L.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Jiang, K.; Jiménez Cisneros México, B.; Kattsov, V.; Lee, H.; et al. Cambio Climático 2014. Informe de Síntesis. Contribución de Los Grupos de Trabajo I, II Y III Al Quinto Informe de Evaluación Del Grupo Intergubernamental de Expertos Sobre El Cambio Climático; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Brown, J.H. On the Relationship between Abundance and Distribution of Species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Saunders, G.W. Routine DNA Barcoding of Canadian Gracilariales (Rhodophyta) Reveals the Invasive Species Gracilaria Vermiculophylla in British Columbia. Mol. Ecol. Resour. 2009, 9, 140–150. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Gurgel, C.F.D.; Fredericq, S.; McGlathery, K.J. Gracilaria vermiculophylla (Rhodophyta, Gracilariales) in Hog Island Bay, Virginia: A Cryptic Alien and Invasive Macroalga and Taxonomic Correction. J. Phycol. 2005, 42, 139–141. [Google Scholar] [CrossRef]
- Guillemin, M.L.; Akki, S.A.; Givernaud, T.; Mouradi, A.; Valero, M.; Destombe, C. Molecular Characterisation and Development of Rapid Molecular Methods to Identify Species of Gracilariaceae from the Atlantic Coast of Morocco. Aquat. Bot. 2008, 89, 324–330. [Google Scholar] [CrossRef]
- Krueger-Hadfield, S.A.; Magill, C.L.; Bunker, F.S.P.D.; Mieszkowska, N.; Sotka, E.E.; Maggs, C.A. When Invaders Go Unnoticed: The Case of Gracilaria Vermiculophylla in the British Isles. Cryp. Alg. 2017, 38, 379–400. [Google Scholar] [CrossRef]
- Ohmi, H. Contributions to the Knowledge of Gracilariaceae from Japan: Ⅱ. On a New Species of the Genus Gracilariopsis, with Some Considerations on Its Ecology. Hokkaido Univ. Collect. Sch. Acad. Pap. 1956, 6, 271–279. [Google Scholar]
- Wesselmann, M.; Anton, A.; Duarte, C.M.; Hendriks, I.E.; Agustí, S.; Savva, I.; Apostolaki, E.T.; Marbà, N. Tropical Seagrass Halophila stipulacea Shifts Thermal Tolerance during Mediterranean Invasion. Proc. R. Soc. B Biol. Sci. 2020, 287, 20193001. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Pereira, R.; Buschmann, A.H.; Sousa-Pinto, I.; Yarish, C. Nitrogen Uptake Responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under Combined and Single Addition of Nitrate and Ammonium. J. Exp. Mar. Bio. Ecol. 2011, 407, 190–199. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Moustakas, A. Uncertainty in Marine Invasion Science. Front. Mar. Sci. 2018, 5, 38. [Google Scholar] [CrossRef]
- Sainz-Villegas, S.; Fernández de la Hoz, C.; Juanes, J.A.; Puente, A. Predicting Non-Native Seaweeds Global Distributions: The Importance of Tuning Individual Algorithms in Ensembles to Obtain Biologically Meaningful Results. Front. Mar. Sci. 2022, 9, 1009808. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the Global Threat of Invasive Species to Marine Biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Staehr, P.A.; Nejrup, L.; Schiel, D.R. Effects of the Invasive Macroalgae Gracilaria vermiculophylla on Two Co-Occurring Foundation Species and Associated Invertebrates. Aquat. Invasions 2013, 8, 133–145. [Google Scholar] [CrossRef]
- Keller, E.L.; Berke, S.K.; Needham, C.N.; Salerno, C.R. A Double-Edged Sword: Infaunal Responses to Agarophyton vermiculophyllum in the Mid-Atlantic United States. Estuaries Coasts 2019, 42, 1924–1937. [Google Scholar] [CrossRef]
- Ramus, A.P.; Silliman, B.R.; Thomsen, M.S.; Long, Z.T. An Invasive Foundation Species Enhances Multifunctionality in a Coastal Ecosystem. Proc. Natl. Acad. Sci. USA 2017, 114, 8580–8585. [Google Scholar] [CrossRef]
- Pacheco, D.; Araújo, G.S.; Cotas, J.; Gaspar, R.; Neto, J.M.; Pereira, L. Invasive Seaweeds in the Iberian Peninsula: A Contribution for Food Supply. Mar. Drugs 2020, 18, 560. [Google Scholar] [CrossRef]
- Wood, M.A.; Lipcius, R.N. Non-Native Red Alga Gracilaria vermiculophylla Compensates for Seagrass Loss as Blue Crab Nursery Habitat in the Emerging Chesapeake Bay Ecosystem. PLoS ONE 2022, 17, e0267880. [Google Scholar] [CrossRef]
- Wernberg, T.; Coleman, M.A.; Bennett, S.; Thomsen, M.S.; Tuya, F.; Kelaher, B.P. Genetic Diversity and Kelp Forest Vulnerability to Climatic Stress. Sci. Rep. 2018, 8, 1851. [Google Scholar] [CrossRef] [PubMed]
- Krueger-Hadfield, S.A. What’s Ploidy Got to Do with It? Understanding the Evolutionary Ecology of Macroalgal Invasions Necessitates Incorporating Life Cycle Complexity. Evol. Appl. 2019, 13, 486–499. [Google Scholar] [CrossRef]
- Jousson, O.; Pawlowski, J.; Zaninetti, L.; Zechman, F.W.; Dini, F.; Di Guiseppe, G.; Woodfield, R.; Millar, A.; Meinesz, A. Invasive Alga Reaches California. Nature 2000, 408, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Benedetti-Cecchi, L.; Pannacciulli, F.; Bulleri, F.; Moschella, P.S.; Airoldi, L.; Relini, G.; Cinelli, F. Predicting the Consequences of Anthropogenic Disturbance: Large-Scale Effects of Loss of Canopy Algae on Rocky Shores. Mar. Ecol. Prog. Ser. 2001, 214, 137–150. [Google Scholar] [CrossRef]
- Fletcher, R.; Fortin, M. Spatial Ecology and Conservation Modeling; Springer International Publishing: Cham, Switzerland, 2018; p. 523. [Google Scholar]
- Afonso, C.; Correia, A.; Freitas, M.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bippus, P.M.; Krueger-Hadfield, S.A.; Sotka, E.E. Palatability of an introduced seaweed does not differ between native and non-native populations. Mar. Biol. 2018, 165, 39. [Google Scholar] [CrossRef]
- Burdick, D.M.; Moore, G.; Mathieson, A.C.; Payne, A.; Martin, L.; Peter, C. Seaweed Monitoring in the Great Bay Estuary: 2019 Annual Report. PREP Reports & Publications. 442. 2020. Available online: https://scholars.unh.edu/prep/442 (accessed on 17 November 2022).
- Cacabelos, E.; Engelen, A.H.; Mejia, A.; Arenas, F. Comparison of the assemblage functioning of estuary systems dominated by the seagrass Nanozostera noltii versus the invasive drift seaweed Gracilaria vermiculophylla. J. Sea Res. 2012, 72, 99–105. [Google Scholar] [CrossRef]
- García-Rodríguez, L.D.; Riosmena-Rodríguez, R.; Kim, S.Y.; López-Meyer, M.; Orduña-Rojas, J.; López-Vivas, J.M.; Boo, S.M. Recent introduction of Gracilaria parvispora (Gracilariales, Rhodophyta) in Baja California, Mexico. Bot. Mar. 2013, 56, 143–150. [Google Scholar] [CrossRef]
- Guidone, M.; Newton, C.; Thornber, C.S. Utilization of the invasive alga Gracilaria vermiculophylla (Ohmi) Papenfuss by the native mud snail Ilyanassa obsoleta (Say). J. Exp. Mar. Biol. Ecol. 2014, 452, 119–124. [Google Scholar] [CrossRef]
- Myung, K.; Mi Yeon, Y.; Ga Youn, C. Applying DNA barcoding to Korean Gracilariaceae (Rhodophyta). Cryptogam. Algol. 2010, 31, 387–401. [Google Scholar]
- Nehls, G. Gracilaria vermiculophylla (Gracilariales, Rhodophyta)—A Pacific algae spreading on tidal flats of the Wadden Sea. 2006. Available online: http://www.bioconsult-sh.de/pdf/PosterGracilaria.pdf (accessed on 20 August 2009).
- Nettleton, J.C.; Mathieson, A.C.; Thornber, C.; Neefus, C.D.; Yarish, C. Introduction of Gracilaria vermiculophylla (Rhodophyta, Gracilariales) to New England, USA: Estimated Arrival Times and Current Distribution. Rhodora 2013, 115, 28–41. [Google Scholar] [CrossRef]
- Schories, D.; Selig., U.; Schubert, H. Species and synonym list of the German marine macroalgae based on historical and recent records. Rostocker Meeresbiologische Beiträge 2009, 21, 7–135. [Google Scholar]
- Skriptsova, A.V.; Choi, H.-G. Taxonomic revision of Gracilaria “verrucosa” from the Russian Far East based on morphological and molecular data. Bot. Mar. 2009, 52, 331–340. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Sousa, A.M.M.; Gonçalves, M.P.; Nilsson, M.; Hilliou, L. Production and properties of agar from the invasive marine alga, Gracilaria vermiculophylla (Gracilariales, Rhodophyta). J. Appl. Phycol. 2010, 22, 211–220. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Weinberger, F.; Bian, D.; Nakaoka, M.; Lenz, M. Anti-epiphyte defences in the red seaweed Gracilaria vermiculophylla: Non-native algae are better defended than their native conspecifics. J. Ecol. 2016, 105, 445–457. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Montgomery, F.; Greene, J.K.; Hamner, R.M.; Williams, M.; Whitfield, P.E. Distribution and Identification of an Invasive Gracilaria Species that is Hampering Commercial Fishing Operations in Southeastern North Carolina, USA. Biol. Invasions 2006, 8, 631–637. [Google Scholar] [CrossRef]
- Wright, J.T.; Byers, J.E.; DeVore, J.L.; Sotka, E.E. Engineering or food? mechanisms of facilitation by a habitat-forming invasive seaweed. Ecology 2014, 95, 2699–2706. [Google Scholar] [CrossRef]


| Mean Temp. | Max. Temp. | Min. Temp. | Max. Salinity | Min. Salinity | |
|---|---|---|---|---|---|
| Northwest Pacific Ocean | 11.6 ± 2.1 | 19.3 ± 1.1 | 3.0 ± 4.6 | 30.4 ± 5.3 | 21.3 ± 10.7 |
| Northeast Pacific Ocean | 16.3 ± 3.9 | 25.6 ± 2.9 | 5.8 ± 4.7 | 33.0 ± 2.4 | 28.5 ± 4.8 |
| Northwest Atlantic Ocean | 15.0 ± 5.6 | 20.2 ± 5.9 | 10.8 ± 4.6 | 32.9 ± 1.6 | 27.3 ± 5.0 |
| Northeast Atlantic Ocean | 17.4 ± 3.5 | 26.6 ± 2.2 | 9.3 ± 5.1 | 33.7 ± 1.5 | 30.2 ± 2.8 |
| Mediterranean Sea | 17.6 ± 0.3 | 26.9 ± 0.1 | 7.4 ± 1.1 | 33.8 ± 1.0 | 27.2 ± 2.4 |
| Average values | 15.6 ± 2.2 | 23.7 ± 3.3 | 7.2 ± 2.7 | 32.8 ± 1.2 | 26.9 ± 3.0 |
| Level | Type | Description | References |
|---|---|---|---|
| Economic | Industry |
| [17,31,46,79] |
| Ecological | Ecosystem services |
| [5,17,19,43,45,77,78,80] |
| Interactions with organisms |
| [5,17,45,77] | |
| Environmental Risks |
| [5,45,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Segura, C.; Fernández, E.; Beca-Carretero, P. Predicted Changes in the Biogeographical Range of Gracilaria vermiculophylla under Present and Future Climate Scenarios. J. Mar. Sci. Eng. 2023, 11, 367. https://doi.org/10.3390/jmse11020367
Mendoza-Segura C, Fernández E, Beca-Carretero P. Predicted Changes in the Biogeographical Range of Gracilaria vermiculophylla under Present and Future Climate Scenarios. Journal of Marine Science and Engineering. 2023; 11(2):367. https://doi.org/10.3390/jmse11020367
Chicago/Turabian StyleMendoza-Segura, Clara, Emilio Fernández, and Pedro Beca-Carretero. 2023. "Predicted Changes in the Biogeographical Range of Gracilaria vermiculophylla under Present and Future Climate Scenarios" Journal of Marine Science and Engineering 11, no. 2: 367. https://doi.org/10.3390/jmse11020367
APA StyleMendoza-Segura, C., Fernández, E., & Beca-Carretero, P. (2023). Predicted Changes in the Biogeographical Range of Gracilaria vermiculophylla under Present and Future Climate Scenarios. Journal of Marine Science and Engineering, 11(2), 367. https://doi.org/10.3390/jmse11020367