Assessing the Fishing Impact on the Marine Ecosystem of Guishan Island in the Northeastern Waters of Taiwan Using Ecopath and Ecosim
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ecopath Model Structure
2.3. Estimation of Trophic Levels
2.4. Mixed Trophic Impact (MTI)
2.5. Keystone Species Identification
2.6. Simulations of Management Measures and Fishing Pressures Using Ecosim
2.7. Model Construction
2.7.1. Cetaceans
2.7.2. Small Skates and Rays
2.7.3. Small Sharks
2.7.4. Dolphinfish
2.7.5. Scombrids
2.7.6. Shrimps
2.7.7. Lobsters and Crabs
2.7.8. Oil Fish/Escolar
2.7.9. Mackerels
2.7.10. Carangids
2.7.11. Pomfret
2.7.12. Sciaenids
2.7.13. Cephalopods
2.7.14. Flatfishes
2.7.15. Sparids
2.7.16. Clupeids
2.7.17. Congers
2.7.18. Hairtail
2.7.19. Blackbelly Triggerfish
2.7.20. Anchovy
3. Results and Discussion
3.1. Trophic Position
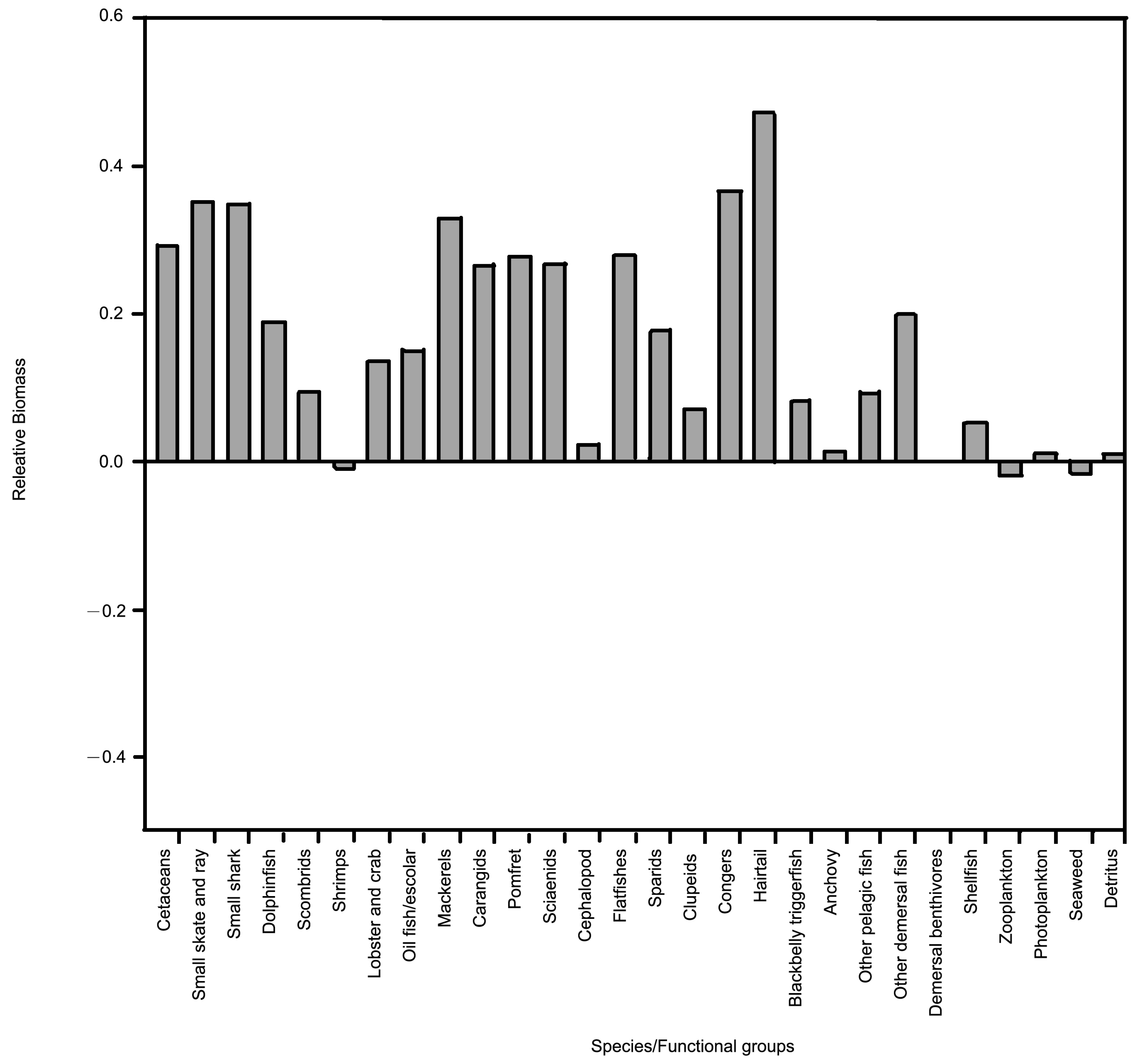
3.2. Biomass Estimate
3.3. Fishing Mortality, Exploitation Rate, and Predation Mortality
3.4. Trophic Impact and Keystone Species
3.5. Primary Production Required for Fisheries
3.6. Simulations with Reduction of Fishing Effort
3.7. Model Uncertainty
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taiwan Fisheries Year Book; Fisheries Agency, Ministry of Agriculture: Taipei, Taiwan, 2023. Available online: https://www.fa.gov.tw/list.php?theme=FS_AR&subtheme= (accessed on 10 August 2023).
- Liu, K.M.; Cheng, Y.L. Virtual population analysis of the big eye, Priacanthus macracanthus in the northeastern Taiwan waters. Fish. Res. 1999, 41, 243–254. [Google Scholar] [CrossRef]
- Schaefer, M.B. Some aspects of the dynamics of populations important to the management of the commercial marine fisheries. Inter-Am. Trop. Tuna Comm. Bull. 1954, 1, 23–56. [Google Scholar]
- Schaefer, M.B. Some considerations of population dynamics and economics in relation to the management of the commercial marine fisheries. J. Fish. Board Can. 1957, 14, 669–681. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; facsimile reprint, 1993; Chapman and Hall: London, UK, 1957; 240p. [Google Scholar]
- Latour, R.J.; Brush, M.J.; Bonzek, C.F. Toward ecosystem-based fisheries management: Strategies for multispecies modeling and associated data requirements. Fisheries 2003, 28, 10–22. [Google Scholar] [CrossRef]
- May, R.M.; Beddington, J.R.; Clark, C.W.; Holt, S.J.; Laws, R.M. Management of multispecies fisheries. Science 1979, 205, 267–277. [Google Scholar] [CrossRef]
- Sparre, P. Introduction to multispecies virtual population analysis. ICES Mar. Sci. Symp. 1991, 193, 12–21. [Google Scholar]
- Brandt, S.B.; Hartman, K.J. Innovative approaches with bioenergetics models–future application to fish ecology and management. Trans. Am. Fish. Soc. 1993, 122, 731–735. [Google Scholar] [CrossRef]
- Durbin, A.G.; Durbin, E.G. Effects of menhaden predation on plankton populations in Narragansett Bay, Rhode Island. Estuaries 1998, 21, 449–465. [Google Scholar] [CrossRef]
- Luo, J.; Hartman, K.J.; Brandt, S.B.; Cerco, F.C.; Rippetoe, T.H. A spatially-explicit approach for estimating carrying capacity: An application for the Atlantic menhaden (Brevoortia tyrannus) in Chesapeake Bay. Estuaries 2001, 24, 545–556. [Google Scholar] [CrossRef]
- Christensen, V.; Pauly, D. ECOPATH II—A software for balancing steady-state ecosystem models and calculating network characteristics. Ecol. Model. 1992, 61, 169–185. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Quantitative methods for ecological network analysis. Comput. Biol. Chem. 2004, 28, 321–339. [Google Scholar] [CrossRef]
- Dame, J.K.; Christian, R.R. Uncertainty and the use of network analysis for ecosystem-based fishery management. Fisheries 2006, 31, 331–341. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Kitchell, J.F.; Essington, T.E.; Boggs, C.H.; Schindler, D.E.; Walters, C.J. The role of sharks and longline fisheries in a pelagic ecosystem of the Central Pacific. Ecosystems 2002, 5, 202–216. [Google Scholar] [CrossRef]
- Frid, C.; Paramor, O.; Scott, C. Ecosystem-based fisheries management: Progress in the NE Atlantic. Mar. Policy 2005, 29, 461–469. [Google Scholar] [CrossRef]
- Lin, H.J.; Shao, K.T.; Hwang, J.S.; Lo, W.T.; Cheng, I.J.; Lee, L.H. A trophic model for Kuosheng Bay in northern Taiwan. J. Mar. Sci. Technol. 2004, 12, 424–432. [Google Scholar] [CrossRef]
- Lin, H.J.; Shao, K.T.; Jan, R.Q.; Chen, C.P.; Hsieh, H.L.; Hsieh, L.Y.; Hsiao, Y.T. A trophic model for the Danshuei River estuary, a hypoxic estuary in northern Taiwan. Mar. Pollut. Bull. 2007, 54, 1789–1800. [Google Scholar] [CrossRef]
- Liu, P.J.; Shao, K.T.; Jan, R.Q.; Fan, T.Y.; Wong, S.L.; Hwang, J.S.; Chen, J.P.; Chen, C.C.; Lin, H.J. A trophic model of fringing coral reefs in Nanwan Bay, southern Taiwan suggests overfishing. Mar. Environ. Res. 2009, 68, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Christensen, V.; Pauly, D. Structuring dynamic models of exploited ecosystems from trophic mass-balance assessments. Rev. Fish Biol. Fish. 1997, 7, 139–172. [Google Scholar] [CrossRef]
- Wang, S.B.; Ou, J.C.; Chang, J.J.; Liu, K.M. Characteristics of the trash fish generated by bottom trawling in surrounding waters of Guei-Shan Island, northeastern Taiwan. J. Fish. Soc. Taiwan 2007, 34, 379–395. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A Users’ Guide; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2005; 154p. [Google Scholar]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Odum, W.; Heald, E. The Detritus-Based Food Web of an Estuarine Mangrove Community, 1st ed.; Academic Press: New York, NY, USA, 1975; 265p. [Google Scholar]
- Pauly, D.; Soriano-Bartz, M.L.; Palomares, M.L.D. Improved construction, parametrization and interpretation of steady-state ecosystem models. In Trophic Models of Aquatic Ecosystems; Christensen, V., Pauly, D., Eds.; International Union for Conservation of Nature: Gland, Switzerland, 1993; Volume 26, pp. 1–13. [Google Scholar]
- Ulanowicz, R.; Puccia, C. Mixed trophic impacts in ecosystems. Coenoses 1990, 5, 7–16. [Google Scholar]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones: Identifying keystone species is difficult—But essential to understanding how loss of species will affect ecosystems. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Libralato, S.; Christensen, V.; Pauly, D. A method for identifying keystone species in food web models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Shao, K.T. The Fish Database of Taiwan. 2023. Available online: https://fishdb.sinica.edu.tw/ (accessed on 15 August 2023).
- Yeh, C.C. Fauna, Distribution and Habitat Features of Cetaceans in Coastal Waters of Southeastern Taiwan. Master Thesis, National Taiwan University, Taiwan, 2001; 100p. [Google Scholar]
- Benoit-Bird, K.J. Prey caloric value and predator energy needs: Foraging predictions for wild spinner dolphins. Mar. Biol. 2004, 145, 435–444. [Google Scholar] [CrossRef]
- Chou, L.S.; Bright, A.M.; Yeh, S.Y. Stomach contents of dolphins (Delphinus delphis and Lissodelphis borealis) from north Pacific Ocean. Zool. Stud. 1995, 34, 206–210. [Google Scholar]
- Pauly, D.; Trites, A.W.; Capuli, E.; Christensen, V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998, 55, 467–481. [Google Scholar] [CrossRef]
- Blanco, C.; Salomón, O.; Raga, J.A. Diet of the bottlenose dolphin (Tursiops truncatus) in the western Mediterranean Sea. J. Mar. Biol. Assoc. UK 2001, 81, 1053–1058. [Google Scholar] [CrossRef]
- Chen, K.Y. Population Fluctuation of the Spotted Mackerel (Scomber australasicus) in the Northeastern Waters off Taiwan. Master Thesis, National Taiwan Ocean University, Taiwan, 2005; 54p. [Google Scholar]
- Anon. Wildlife Conservation Act; Ministry of Agriculture: Taiwan, China, 1993. [Google Scholar]
- Paine, R.T. Food webs: Road maps of interactions or grist for theoretical development? Ecology 1988, 69, 1648–1654. [Google Scholar] [CrossRef]
- Whipple, S.J.; Link, J.S.; Garrison, L.P.; Fogarty, M.J. Models of predation and fishing mortality in aquatic ecosystems. Fish Fish. 2000, 1, 22–40. [Google Scholar] [CrossRef]
- Baird, D.; Luczkovich, J.; Christian, R.R. Assessment of spatial and temporal variability in ecosystem attributes of the St Marks National Wildlife Refuge, Apalachee Bay, Florida. Estuar. Coast. Shelf Sci. 1998, 117, 329–349. [Google Scholar] [CrossRef]
- Christian, R.R.; Luczkovich, J.J. Organizing and understanding a winter’s seagrass foodweb network through effective trophic levels. Ecol. Model. 1999, 117, 99–124. [Google Scholar] [CrossRef]
- Pinnega, J.K.; Blanchard, J.L.; Mackinson, S.; Scott, R.D.; Duplisea, D.E. Aggregation and removal of weak-links in food-web models: System stability and recovery from disturbance. Ecol. Model. 2005, 184, 229–248. [Google Scholar] [CrossRef]
- Coll, M.; Palomera, I.; Tudela, S.; Sardà, F. Trophic flows, ecosystem structure and fishing impacts in the South Catalan Sea, Northwestern Mediterranean. J. Mar. Syst. 2003, 59, 63–96. [Google Scholar] [CrossRef]
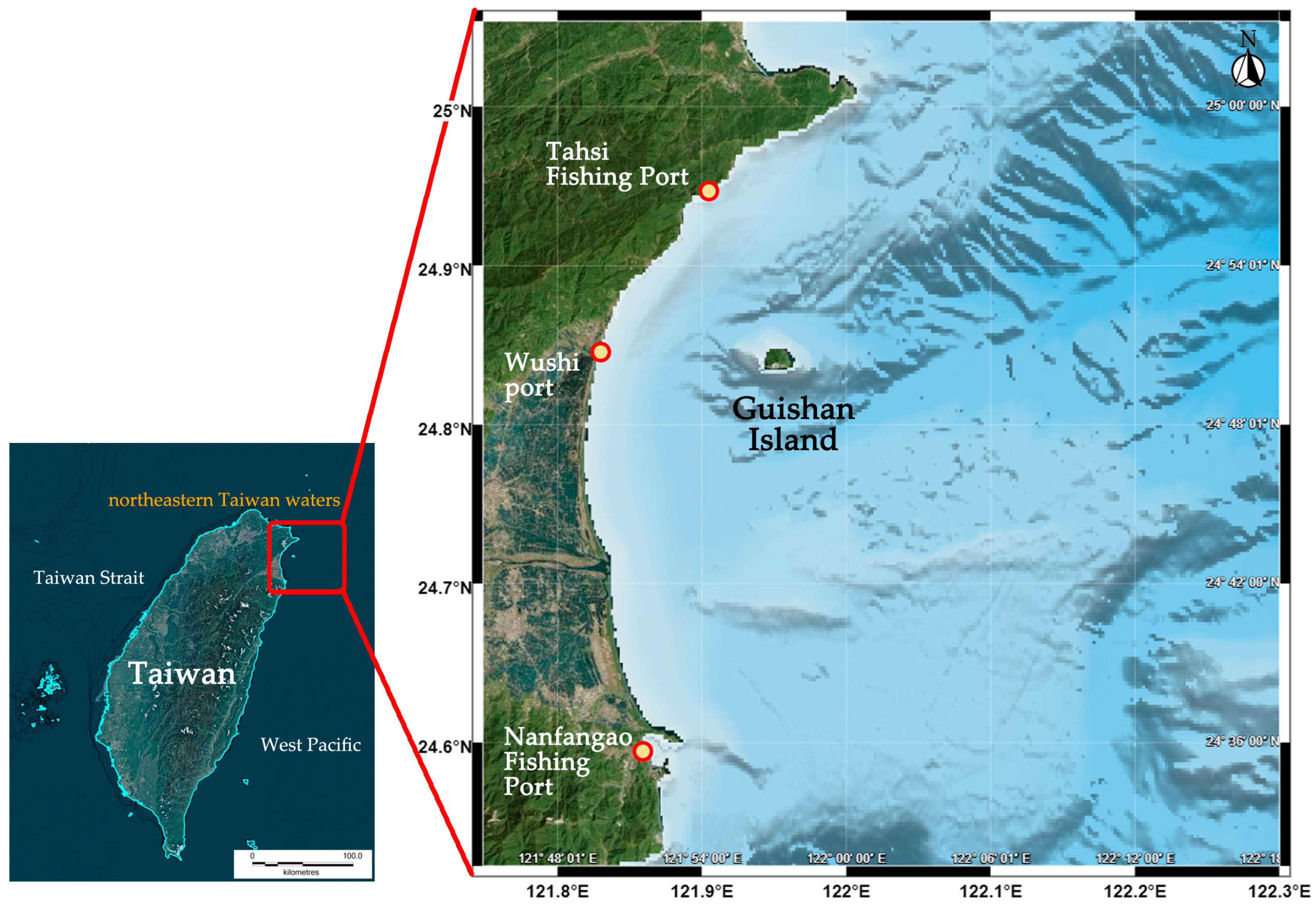
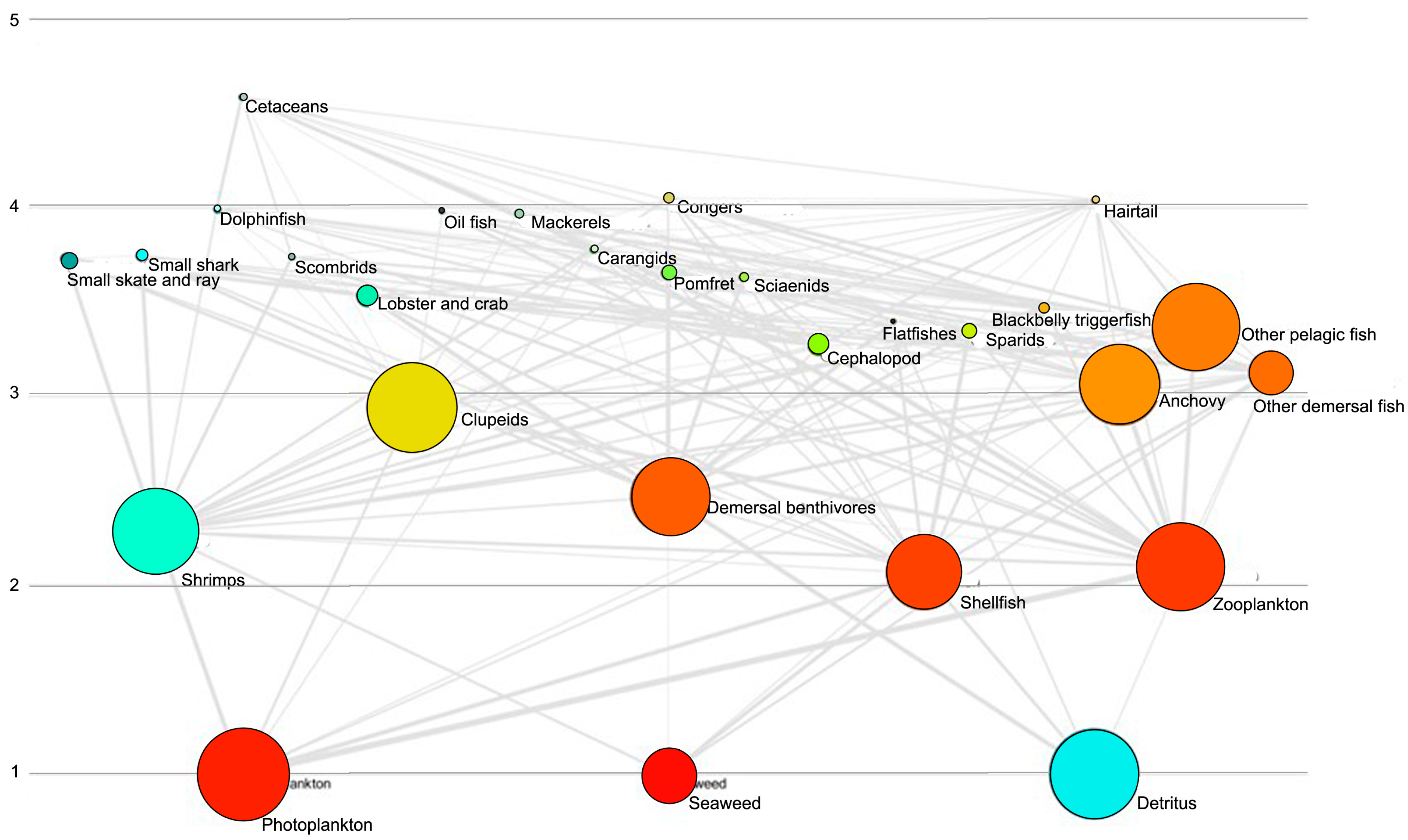
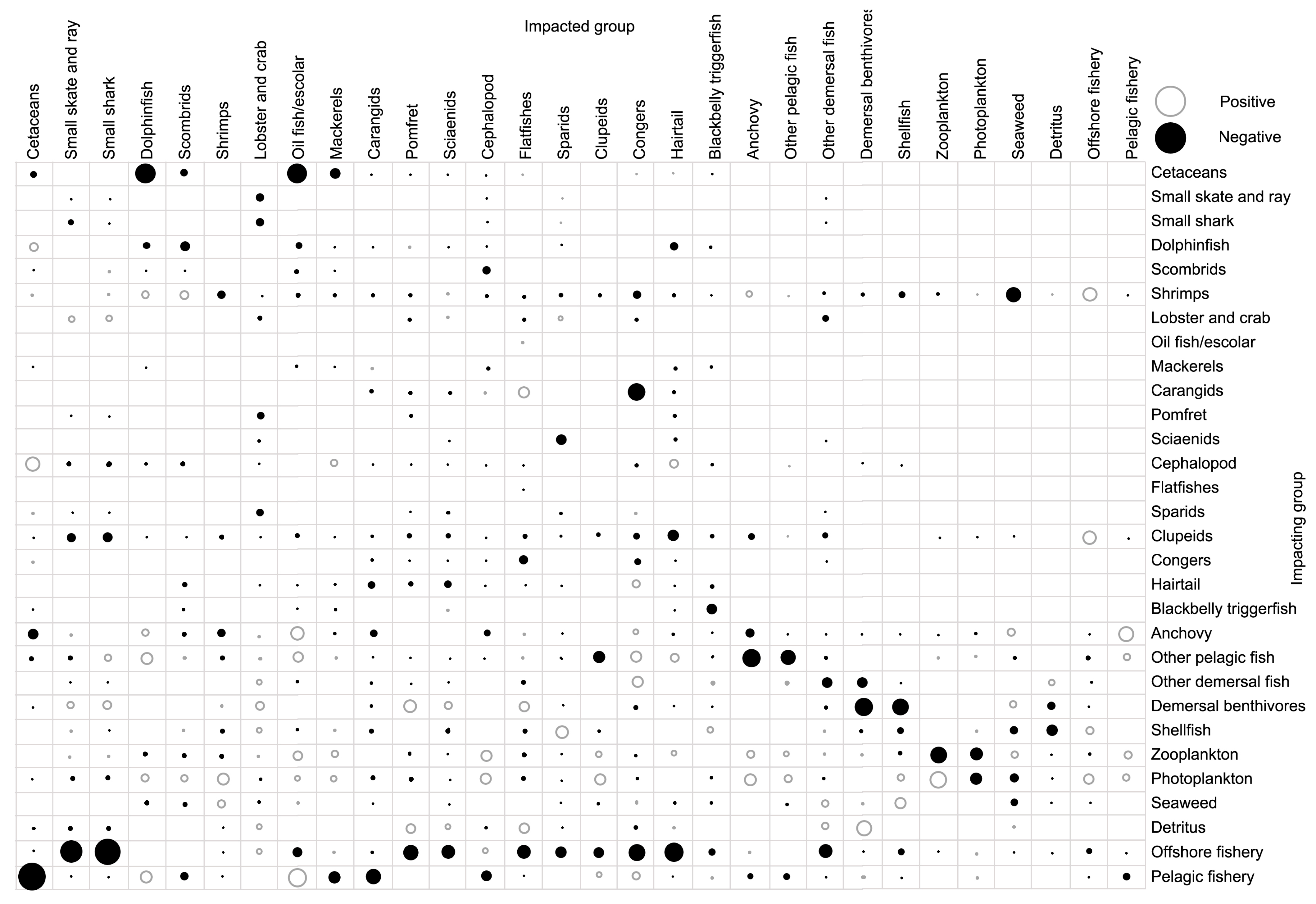
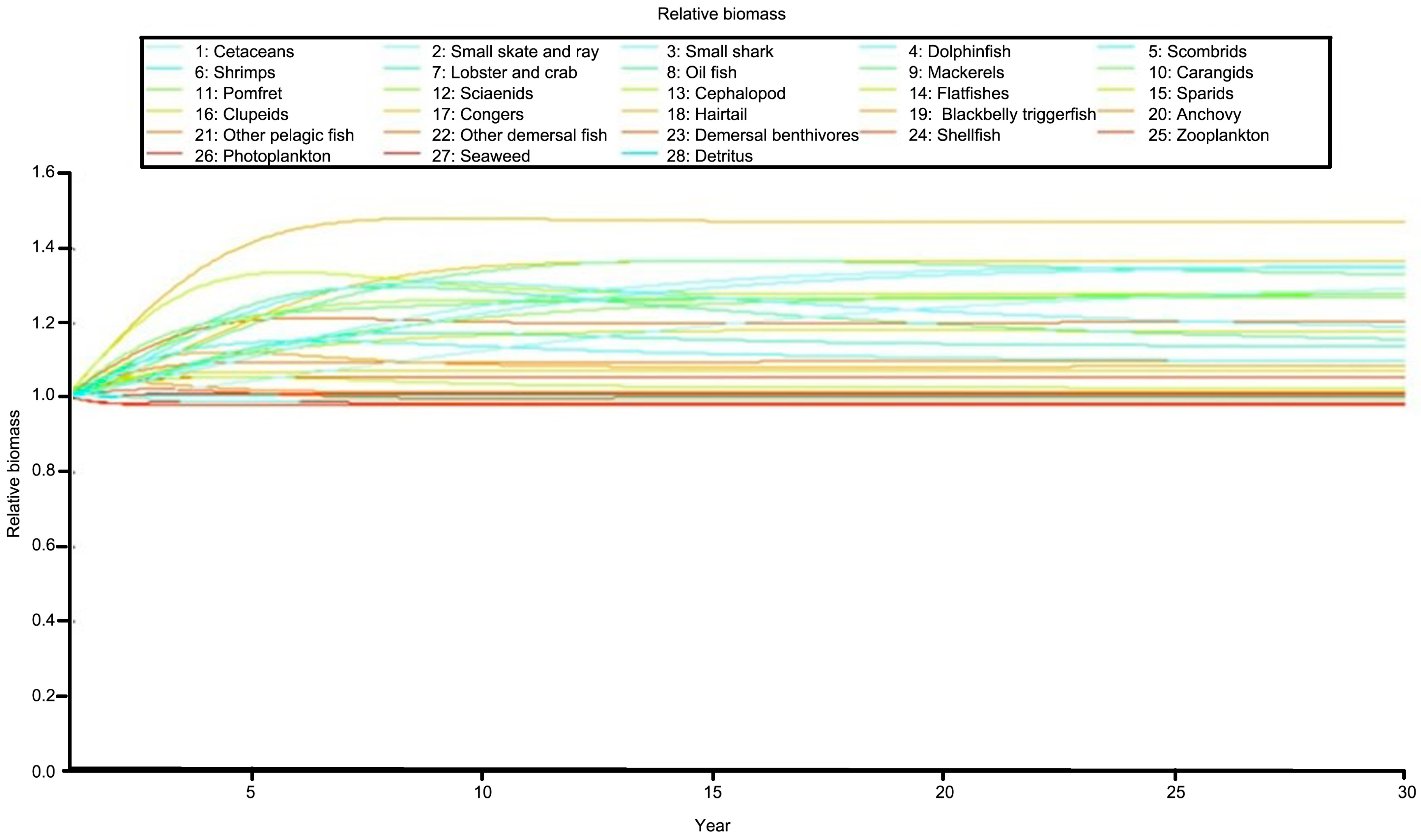
| Species/Functional Group | TP | Biomass | Production | Consumption | EE |
|---|---|---|---|---|---|
| Cetaceans | 4.59 | 0.00 | 0.32 | 18.37 | 0.03 |
| Small skate and ray | 3.73 | 0.03 | 0.50 | 2.50 | 0.67 |
| Small shark | 3.75 | 0.04 | 0.45 | 2.25 | 0.58 |
| Dolphinfish | 4.00 | 0.01 | 1.68 | 8.48 | 0.85 |
| Scombrids | 3.74 | 0.01 | 3.37 | 32.57 | 0.99 |
| Shrimps | 2.28 | 3.94 | 10.00 | 40.00 | 0.99 |
| Lobster and crab | 3.53 | 0.13 | 1.09 | 6.54 | 0.70 |
| Oil fish/escolar | 3.98 | 0.00 | 2.00 | 10.00 | 0.90 |
| Mackerels | 3.97 | 0.01 | 0.80 | 4.00 | 0.90 |
| Carangids | 3.78 | 0.02 | 1.79 | 7.14 | 0.95 |
| Pomfret | 3.66 | 0.06 | 0.57 | 2.85 | 0.95 |
| Sciaenids | 3.63 | 0.02 | 1.50 | 7.50 | 0.95 |
| Cephalopod | 3.27 | 0.11 | 2.50 | 25.00 | 0.99 |
| Flatfishes | 3.40 | 0.00 | 2.50 | 10.00 | 0.95 |
| Sparids | 3.35 | 0.06 | 0.80 | 4.00 | 0.95 |
| Clupeids | 2.94 | 5.11 | 2.00 | 10.00 | 0.99 |
| Congers | 4.05 | 0.02 | 1.00 | 5.00 | 0.85 |
| Hairtail | 4.04 | 0.01 | 2.50 | 12.00 | 0.95 |
| Blackbelly triggerfish | 3.47 | 0.02 | 2.50 | 12.00 | 0.95 |
| Anchovy | 3.06 | 3.46 | 3.00 | 15.00 | 0.99 |
| Other pelagic fish | 3.37 | 4.49 | 2.50 | 12.00 | 0.95 |
| Other demersal fish | 3.13 | 0.54 | 2.00 | 10.00 | 0.95 |
| Demersal benthivores | 2.47 | 2.64 | 2.00 | 10.00 | 0.95 |
| Shellfish | 2.06 | 2.52 | 3.00 | 15.00 | 0.95 |
| Zooplankton | 2.10 | 4.55 | 50.00 | 200.00 | 0.95 |
| Photoplankton | 1.00 | 4.74 | 400.00 | 0.00 | 0.50 |
| Seaweed | 1.00 | 0.84 | 100.00 | 0.00 | 0.50 |
| Detritus | 1.00 | 5.00 | 0.02 |
| Species/Functional Group | Mortality | Fishing Mortality | Predation Mortality | Other Mortality | Exploitation Rate |
|---|---|---|---|---|---|
| Cetaceans | 0.320 | 0.010 | 0.000 | 0.310 | 0.031 |
| Small skate and ray | 0.500 | 0.308 | 0.027 | 0.165 | 0.617 |
| Small shark | 0.450 | 0.261 | 0.000 | 0.189 | 0.580 |
| Dolphinfish | 1.681 | 0.411 | 1.018 | 0.252 | 0.245 |
| Scombrids | 3.370 | 1.347 | 1.989 | 0.034 | 0.400 |
| Shrimps | 10.000 | 1.002 | 8.898 | 0.100 | 0.100 |
| Lobster and crab | 1.090 | 0.172 | 0.591 | 0.327 | 0.158 |
| Oil fish/escolar | 2.000 | 0.535 | 1.265 | 0.200 | 0.267 |
| Mackerels | 0.800 | 0.458 | 0.262 | 0.080 | 0.573 |
| Carangids | 1.790 | 0.922 | 0.779 | 0.090 | 0.515 |
| Pomfret | 0.570 | 0.333 | 0.208 | 0.029 | 0.585 |
| Sciaenids | 1.500 | 0.796 | 0.629 | 0.075 | 0.531 |
| Cephalopod | 2.500 | 1.076 | 1.399 | 0.025 | 0.430 |
| Flatfishes | 2.500 | 1.433 | 0.942 | 0.125 | 0.573 |
| Sparids | 0.800 | 0.388 | 0.372 | 0.040 | 0.485 |
| Clupeids | 2.000 | 0.767 | 1.213 | 0.020 | 0.384 |
| Congers | 1.000 | 0.304 | 0.546 | 0.150 | 0.304 |
| Hairtail | 2.500 | 1.640 | 0.735 | 0.125 | 0.656 |
| Blackbelly triggerfish | 2.500 | 0.722 | 1.653 | 0.125 | 0.289 |
| Anchovy | 3.000 | 0.886 | 2.084 | 0.030 | 0.295 |
| Other pelagic fish | 2.500 | 0.732 | 1.643 | 0.125 | 0.293 |
| Other demersal fish | 2.000 | 1.073 | 0.827 | 0.100 | 0.537 |
| Demersal benthivores | 2.000 | 0.189 | 1.711 | 0.100 | 0.095 |
| Shellfish | 3.000 | 0.703 | 2.147 | 0.150 | 0.234 |
| Impacting/Impacted | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Small skate and ray | 0.03 | ||||||||||||
| 2 | Dolphinfish | 1.02 | ||||||||||||
| 3 | Scombrids | 1.03 | 0.52 | |||||||||||
| 4 | Shrimps | 0.01 | 0.01 | 0 | 0.01 | 0.92 | 0 | 0 | 0.01 | 0.07 | ||||
| 5 | Lobster and crab | 0.13 | 0.14 | 0.13 | 0.01 | |||||||||
| 6 | Oil fish/escolar | 1.27 | ||||||||||||
| 7 | Mackerels | 0.26 | ||||||||||||
| 8 | Carangids | 0.04 | 0.11 | 0 | 0.03 | 0.08 | ||||||||
| 9 | Pomfret | 0.01 | 0.03 | 0 | 0.01 | 0.02 | ||||||||
| 10 | Sciaenids | 0.03 | 0.09 | 0 | 0.03 | 0.06 | ||||||||
| 11 | Cephalopod | 0.16 | 0.07 | 0.08 | 0.17 | 0.37 | 0.01 | 0.1 | 0.1 | 0.01 | 0.01 | |||
| 12 | Flatfishes | 0.04 | ||||||||||||
| 13 | Sparids | 0.01 | 0.03 | 0 | 0.01 | 0.02 | 0.26 | |||||||
| 14 | Clupeids | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | ||||||
| 15 | Congers | 0.55 | ||||||||||||
| 16 | Hairtail | 0.29 | 0.29 | 0 | 0.05 | |||||||||
| 17 | Blackbelly triggerfish | 0.15 | 0.23 | 0.03 | 0.25 | |||||||||
| 18 | Anchovy | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0.02 | |||||
| 19 | Other pelagic fish | 0 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0.04 | |||||
| 20 | Other demersal fish | 0.01 | 0.02 | 0 | 0.38 | 0 | 0.06 | 0.05 | ||||||
| 21 | Demersal benthivores | 0.01 | 0.01 | 0 | 0.16 | 0 | 0.03 | 0.02 | 0.11 | |||||
| 22 | Shellfish | 0 | 0 | 0.08 | 0 | 0.01 | 0.01 | |||||||
| 23 | Zooplankton | 0 | 0.01 | 7.96 | 0 | 0 | 0 | 0 | 0.44 | |||||
| 24 | Photoplankton | 19.08 | 0 | |||||||||||
| 25 | Seaweed | 32.21 | ||||||||||||
| Impacting/Impacted | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||
| 1 | Small skate and ray | |||||||||||||
| 2 | Dolphinfish | |||||||||||||
| 3 | Scombrids | 0.44 | ||||||||||||
| 4 | Shrimps | 0 | 0.01 | 1.69 | 0 | 0 | 0.01 | 2.96 | 1.56 | 0.17 | 0.99 | 0.48 | ||
| 5 | Lobster and crab | 0.01 | 0.17 | 0.01 | ||||||||||
| 6 | Oil fish/escolar | |||||||||||||
| 7 | Mackerels | |||||||||||||
| 8 | Carangids | 0.07 | 0.45 | |||||||||||
| 9 | Pomfret | 0.02 | 0.12 | |||||||||||
| 10 | Sciaenids | 0.06 | 0.36 | |||||||||||
| 11 | Cephalopod | 0.12 | 0.19 | |||||||||||
| 12 | Flatfishes | 0.1 | 0.8 | |||||||||||
| 13 | Sparids | 0.02 | 0.02 | |||||||||||
| 14 | Clupeids | 0 | 1.2 | |||||||||||
| 15 | Congers | |||||||||||||
| 16 | Hairtail | 0.1 | ||||||||||||
| 17 | Blackbelly triggerfish | |||||||||||||
| 18 | Anchovy | 0.19 | 0 | 0 | 0.01 | 1.77 | 0.08 | |||||||
| 19 | Other pelagic fish | 0.15 | 0 | 0 | 0.01 | 1.36 | 0.06 | |||||||
| 20 | Other demersal fish | 0 | 0 | 0.07 | 0.01 | 0.01 | 0.2 | |||||||
| 21 | Demersal benthivores | 0 | 0.02 | 0 | 0 | 0.01 | 0.62 | 0.74 | ||||||
| 22 | Shellfish | 0 | 0.04 | 0 | 0 | 0.03 | 0.43 | 1.54 | ||||||
| 23 | Zooplankton | 0.01 | 7.31 | 0 | 0.01 | 8.04 | 5.39 | 0.06 | 0.09 | 18.18 | ||||
| 24 | Photoplankton | 2.1 | 0.78 | 0.52 | 3.19 | 174 | ||||||||
| 25 | Seaweed | 2.91 | 0.97 | 0.46 | 13.45 |
| Species/Functional Group | Keystone Index | Keystone Index #2 | Relative Total Impact |
|---|---|---|---|
| Cetaceans | −0.0464 | 3.982 | 1.000 |
| Small skate and ray | −0.811 | 2.202 | 0.172 |
| Small shark | −0.737 | 2.204 | 0.204 |
| Dolphinfish | −0.515 | 2.919 | 0.340 |
| Scombrids | −0.751 | 2.979 | 0.197 |
| Shrimps | −0.252 | 0.731 | 0.706 |
| Lobster and crab | −0.523 | 1.899 | 0.335 |
| Oil fish/escolar | −1.662 | 3.156 | 0.0242 |
| Mackerels | −0.957 | 2.479 | 0.123 |
| Carangids | −0.217 | 3.125 | 0.675 |
| Pomfret | −0.783 | 1.987 | 0.184 |
| Sciaenids | −0.490 | 2.760 | 0.361 |
| Cephalopod | −0.408 | 2.059 | 0.436 |
| Flatfishes | −1.821 | 2.578 | 0.0168 |
| Sparids | −0.642 | 2.140 | 0.254 |
| Clupeids | −0.292 | 0.595 | 0.671 |
| Congers | −0.488 | 2.711 | 0.362 |
| Hairtail | −0.339 | 3.144 | 0.510 |
| Blackbelly triggerfish | −0.971 | 2.235 | 0.119 |
| Anchovy | −0.243 | 0.789 | 0.709 |
| Other pelagic fish | −0.263 | 0.671 | 0.702 |
| Other demersal fish | −0.457 | 1.339 | 0.395 |
| Demersal benthivores | −0.261 | 0.876 | 0.663 |
| Shellfish | −0.413 | 0.743 | 0.465 |
| Zooplankton | −0.247 | 0.682 | 0.729 |
| Photoplankton | −0.195 | 0.720 | 0.828 |
| Seaweed | −0.533 | 1.076 | 0.335 |
| Species/Functional Group | No. of Paths | TP | PPR | PPR/Catch | PPR/TotPP (%) |
|---|---|---|---|---|---|
| Cetaceans | 29,520 | 4.59 | 0.33 | 10,604.19 | 0.01 |
| Small skate and ray | 202 | 3.73 | 2.73 | 273.23 | 0.08 |
| Small shark | 404 | 3.75 | 2.93 | 293.28 | 0.09 |
| Dolphinfish | 7451 | 4.00 | 1.53 | 302.23 | 0.05 |
| Scombrids | 114 | 3.74 | 2.27 | 271.76 | 0.07 |
| Shrimps | 3 | 2.28 | 25.65 | 6.51 | 0.79 |
| Lobster and crab | 68 | 3.53 | 2.78 | 127.26 | 0.09 |
| Oil fish/escolar | 7412 | 3.98 | 0.06 | 236.00 | 0.00 |
| Mackerels | 7272 | 3.97 | 1.31 | 233.37 | 0.04 |
| Carangids | 3176 | 3.78 | 3.08 | 219.76 | 0.09 |
| Pomfret | 202 | 3.66 | 3.52 | 185.80 | 0.11 |
| Sciaenids | 369 | 3.63 | 2.52 | 168.17 | 0.08 |
| Cephalopod | 63 | 3.27 | 9.53 | 77.51 | 0.29 |
| Flatfishes | 140 | 3.40 | 0.17 | 91.73 | 0.01 |
| Sparids | 140 | 3.35 | 2.79 | 130.15 | 0.09 |
| Clupeids | 21 | 2.94 | 81.27 | 20.71 | 2.51 |
| Congers | 4070 | 4.05 | 1.63 | 254.05 | 0.05 |
| Hairtail | 3176 | 4.04 | 5.71 | 317.35 | 0.18 |
| Blackbelly triggerfish | 98 | 3.47 | 0.84 | 55.75 | 0.03 |
| Anchovy | 5 | 3.06 | 65.01 | 21.21 | 2.00 |
| Other pelagic fish | 21 | 3.37 | 113.52 | 34.56 | 3.50 |
| Other demersal fish | 50 | 3.13 | 27.88 | 47.90 | 0.86 |
| Demersal benthivores | 12 | 2.47 | 6.58 | 13.15 | 0.20 |
| Shellfish | 6 | 2.06 | 11.31 | 6.39 | 0.35 |
| Group/Functional Group | Biomass | Production | Predation | Consumption | Catch | |
|---|---|---|---|---|---|---|
| 1 | Cetacean | 4 | 6 | 7 | 6 | 4 |
| 2 | Small skate and ray | 1 | 3 | 4 | 5 | 4 |
| 3 | Small shark | 1 | 3 | 4 | 5 | 4 |
| 4 | Dolphin fish | 1 | 7 | 4 | 6 | 6 |
| 5 | Scombrids | 1 | 3 | 4 | 4 | 4 |
| 6 | Shrimps | 1 | 3 | 3 | 3 | 4 |
| 7 | Lobster and crab | 1 | 3 | 3 | 3 | 4 |
| 8 | Oil fish/escolar | 1 | 3 | 4 | 3 | 4 |
| 9 | Mackerels | 1 | 3 | 4 | 3 | 4 |
| 10 | Carangids | 1 | 3 | 4 | 3 | 4 |
| 11 | Pomfret | 1 | 3 | 4 | 3 | 4 |
| 12 | Sciaenids | 1 | 3 | 4 | 3 | 4 |
| 13 | Cephalopod | 1 | 3 | 4 | 3 | 4 |
| 14 | Flatfishes | 1 | 3 | 4 | 3 | 4 |
| 15 | Sparids | 1 | 3 | 4 | 3 | 4 |
| 16 | Clupeids | 1 | 3 | 4 | 3 | 4 |
| 17 | Congers | 1 | 3 | 4 | 3 | 4 |
| 18 | Hairtail | 1 | 3 | 4 | 3 | 4 |
| 19 | Blackbelly triggerfish | 1 | 3 | 4 | 3 | 4 |
| 20 | Anchovy | 1 | 3 | 4 | 3 | 4 |
| 21 | Other pelagic fish | 1 | 3 | 4 | 3 | 4 |
| 22 | Other demersal fish | 1 | 3 | 4 | 3 | 4 |
| 23 | Demersal benthivores | 1 | 3 | 4 | 3 | 4 |
| 24 | Shellfish | 3 | 3 | 3 | 3 | 4 |
| 25 | Zooplankton | 1 | 3 | 2 | 3 | |
| 26 | Photoplankton | 1 | 3 | |||
| 27 | Seaweed | 1 | 3 | |||
| 28 | Detritus | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, C.-P.; Su, K.-Y.; Liu, K.-M. Assessing the Fishing Impact on the Marine Ecosystem of Guishan Island in the Northeastern Waters of Taiwan Using Ecopath and Ecosim. J. Mar. Sci. Eng. 2023, 11, 2368. https://doi.org/10.3390/jmse11122368
Chin C-P, Su K-Y, Liu K-M. Assessing the Fishing Impact on the Marine Ecosystem of Guishan Island in the Northeastern Waters of Taiwan Using Ecopath and Ecosim. Journal of Marine Science and Engineering. 2023; 11(12):2368. https://doi.org/10.3390/jmse11122368
Chicago/Turabian StyleChin, Chien-Pang, Kuan-Yu Su, and Kwang-Ming Liu. 2023. "Assessing the Fishing Impact on the Marine Ecosystem of Guishan Island in the Northeastern Waters of Taiwan Using Ecopath and Ecosim" Journal of Marine Science and Engineering 11, no. 12: 2368. https://doi.org/10.3390/jmse11122368
APA StyleChin, C.-P., Su, K.-Y., & Liu, K.-M. (2023). Assessing the Fishing Impact on the Marine Ecosystem of Guishan Island in the Northeastern Waters of Taiwan Using Ecopath and Ecosim. Journal of Marine Science and Engineering, 11(12), 2368. https://doi.org/10.3390/jmse11122368






