A Tidal Flat Adjacent to a Fringe Mangrove Forest Mitigates pCO2 Increases and Enhances Lateral Export of Dissolved Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Sample Collection and Fieldwork
2.3. Analytical Protocol
2.4. Data Analysis
3. Results
3.1. Physicochemical Properties of Surface Mangrove Soils
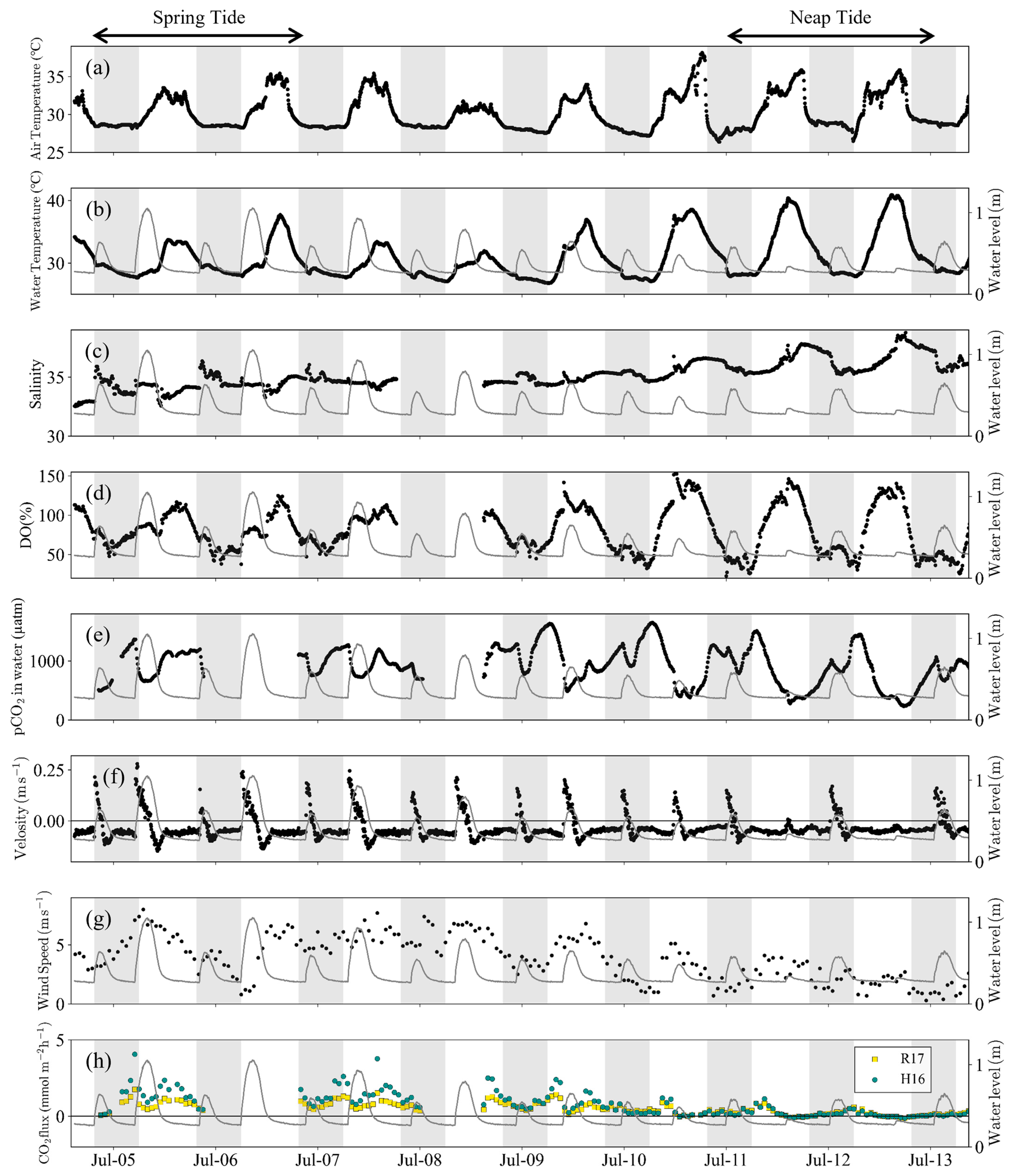
3.2. Water Quality and pCO2 Change in Front of the Mangrove Forest
3.3. Diurnal Variation of TA, DIC, and DOC
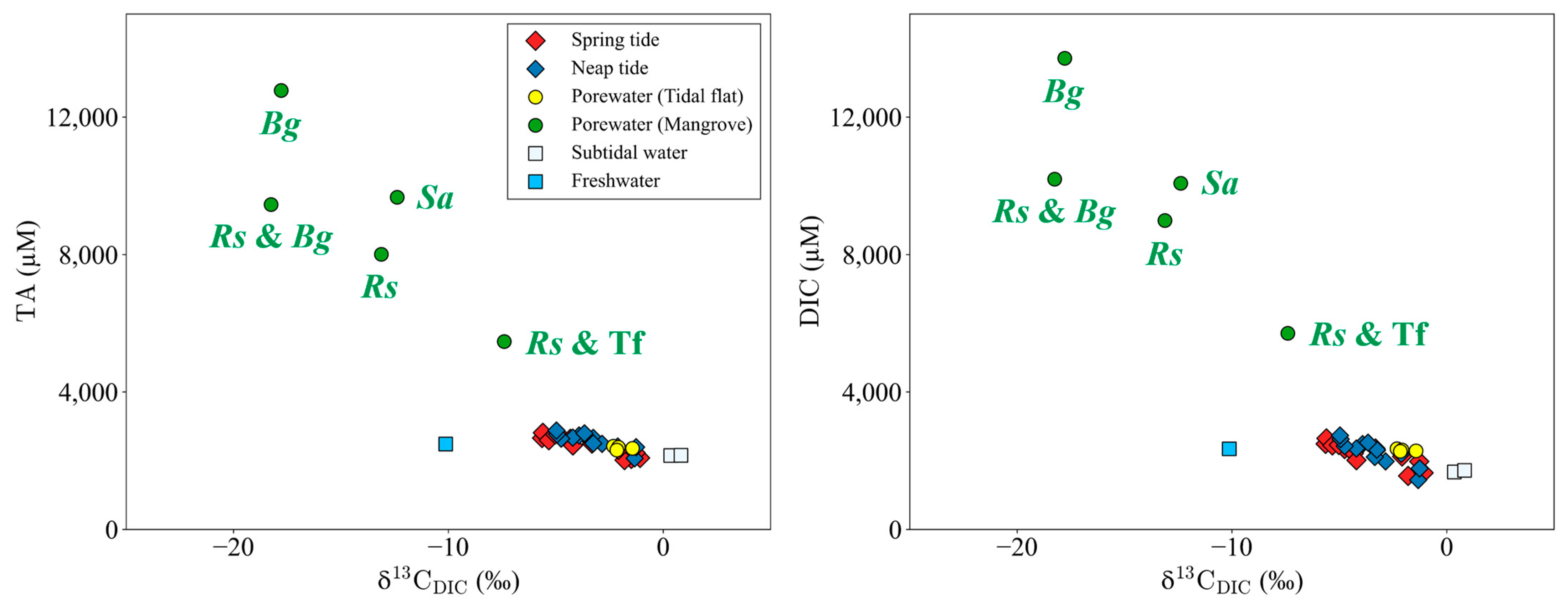
3.4. Spatial Distribution of TA, DIC, and DOC
4. Discussion
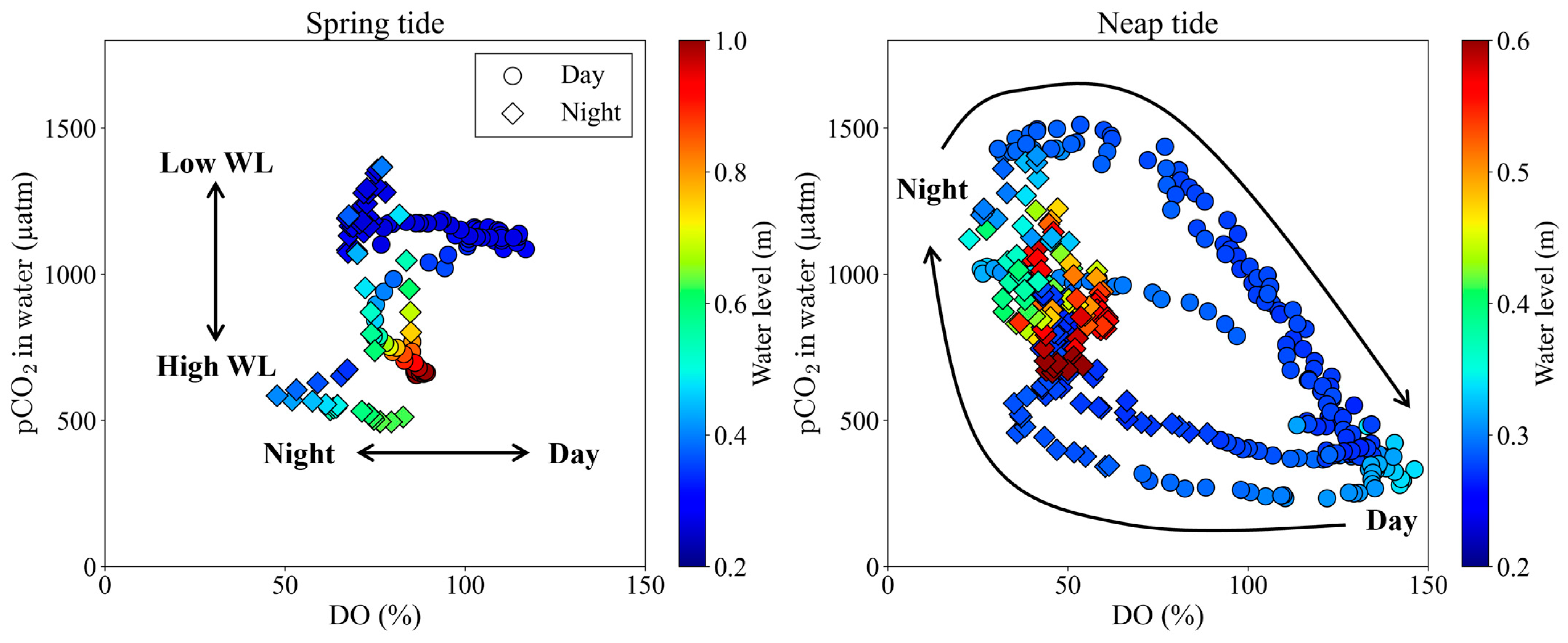
4.1. Controlling Factors of pCO2 over Spring-Neap Tidal Cycle
4.2. Low pCO2 and CO2 Efflux in Comparison to the Global Average
4.3. Lateral TA, DIC, and DOC Exports from Mangroves to the Ocean
5. Conclusions
- (1)
- Semi-monthly pCO2 variations on the tidal flat in the mangrove front were controlled by the tidal level during spring tide and by photosynthesis and respiration on the tidal flat during neap tide (Table 3 and Figure 5). This means that during neap tide, photosynthesis on the tidal flat offset the increase in pCO2 caused by the porewater export from the mangrove soil.
- (2)
- The pCO2 and CO2 efflux in front of the mangrove forest was much lower than the global average, and it may have been effectively suppressed by the primary production in the tidal flat and the limited export of porewater from the mangroves.
- (3)
- On the Yubu coast, the DIC/TA export ratio was 1.17 ± 0.08, which was lower than the global average of 1.41 ± 1.39, indicating that the DIC exported from the Yubu coast remains in the ocean compared to other mangrove sites around the world (Table 4).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdrés, L.; Young, C.D.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon; UN Environment, GRID-Arendal: Arendal, Norway, 2009. [Google Scholar]
- Howard, J.; Sutton-Grier, A.; Herr, D.; Kleypas, J.; Landis, E.; Mcleod, E.; Pidgeon, E.; Simpson, S. Clarifying the Role of Coastal and Marine Systems in Climate Mitigation. Front. Ecol. Environ. 2017, 15, 42–50. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major Role of Marine Vegetation on the Oceanic Carbon Cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Stieglitz, T.C.; Clark, J.F.; Hancock, G.J. The Mangrove Pump: The Tidal Flushing of Animal Burrows in a Tropical Mangrove Forest Determined from Radionuclide Budgets. Geochim. Cosmochim. Acta 2013, 102, 12–22. [Google Scholar] [CrossRef]
- Chen, X.; Santos, I.R.; Call, M.; Reithmaier, G.M.S.; Maher, D.; Holloway, C.; Wadnerkar, P.D.; Gómez-Álvarez, P.; Sanders, C.J.; Li, L. The Mangrove CO2 Pump: Tidally Driven Pore-water Exchange. Limnol. Oceanogr. 2021, 66, 1563–1577. [Google Scholar] [CrossRef]
- Borges, A.V.; Djenidi, S.; Lacroix, G.; Théate, J.; Delille, B.; Frankignoulle, M. Atmospheric CO2 flux from Mangrove Surrounding Waters. Geophys. Res. Lett. 2003, 30, 1558. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-Derived Dissolved Inorganic and Organic Carbon Exports from a Mangrove Tidal Creek: The Missing Mangrove Carbon Sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Call, M.; Maher, D.T.; Santos, I.R.; Ruiz-Halpern, S.; Mangion, P.; Sanders, C.J.; Erler, D.V.; Oakes, J.M.; Rosentreter, J.; Murray, R.; et al. Spatial and Temporal Variability of Carbon Dioxide and Methane Fluxes over Semi-Diurnal and Spring–Neap–Spring Timescales in a Mangrove Creek. Geochim. Cosmochim. Acta 2015, 150, 211–225. [Google Scholar] [CrossRef]
- Call, M.; Santos, I.R.; Dittmar, T.; de Rezende, C.E.; Asp, N.E.; Maher, D.T. High Pore-Water Derived CO2 and CH4 Emissions from a Macro-Tidal Mangrove Creek in the Amazon Region. Geochim. Cosmochim. Acta 2019, 247, 106–120. [Google Scholar] [CrossRef]
- Maher, D.T.; Call, M.; Santos, I.R.; Sanders, C.J. Beyond Burial: Lateral Exchange Is a Significant Atmospheric Carbon Sink in Mangrove Forests. Biol. Lett. 2018, 14, 20180200. [Google Scholar] [CrossRef]
- Santos, I.R.; Burdige, D.J.; Jennerjahn, T.C.; Bouillon, S.; Cabral, A.; Serrano, O.; Wernberg, T.; Filbee-Dexter, K.; Guimond, J.A.; Tamborski, J.J. The Renaissance of Odum’s Outwelling Hypothesis in “Blue Carbon” Science. Estuar. Coast. Shelf Sci. 2021, 255, 107361. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Tait, D.R.; Holloway, C.; Santos, I.R. Are Mangroves Drivers or Buffers of Coastal Acidification? Insights from Alkalinity and Dissolved Inorganic Carbon Export Estimates across a Latitudinal Transect. Glob. Biogeochem. Cycles 2016, 30, 753–766. [Google Scholar] [CrossRef]
- Saderne, V.; Fusi, M.; Thomson, T.; Dunne, A.; Mahmud, F.; Roth, F.; Carvalho, S.; Duarte, C.M. Total Alkalinity Production in a Mangrove Ecosystem Reveals an Overlooked Blue Carbon Component. Limnol. Oceanogr. Lett. 2021, 6, 61–67. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Anton, A.; Raven, J.A.; Beaumont, N.; Connolly, R.M.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Kuwae, T.; Lavery, P.S.; et al. The Future of Blue Carbon Science. Nat. Commun. 2019, 10, 3998. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, H.P.; Ganguly, D.; Purvaja, R.; Singh, G.; Das, S.; Rao, M.N.; Kazip Ys, A.; Arumugam, K.; Ramesh, R. Interspecific Variations in Leaf Litter Decomposition and Nutrient Release from Tropical Mangroves. J. Environ. Manag. 2023, 328, 116902. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; Lao, Y.; Wang, X.; Du, J.; Santos, I.R. Submarine Groundwater Discharge-Derived Carbon Fluxes in Mangroves: An Important Component of Blue Carbon Budgets? J. Geophys. Res. C Oceans 2018, 123, 6962–6979. [Google Scholar] [CrossRef]
- Alongi, D.M. Lateral Export and Sources of Subsurface Dissolved Carbon and Alkalinity in Mangroves: Revising the Blue Carbon Budget. J. Mar. Sci. Eng. 2022, 10, 1916. [Google Scholar] [CrossRef]
- Shoji, J.; Tomiyama, T. Influence of Vegetation Coverage on Dissolved Oxygen Concentration in Seagrass Bed in the Seto Inland Sea: Possible Effects on Fish Nursery Function. Estuaries Coasts 2023, 46, 1098–1109. [Google Scholar] [CrossRef]
- Smith, K.J.; Able, K.W. Dissolved Oxygen Dynamics in Salt Marsh Pools and Its Potential Impacts on Fish Assemblages. Mar. Ecol. Prog. Ser. 2003, 258, 223–232. [Google Scholar] [CrossRef]
- Dubuc, A.; Waltham, N.; Malerba, M.; Sheaves, M. Extreme Dissolved Oxygen Variability in Urbanised Tropical Wetlands: The Need for Detailed Monitoring to Protect Nursery Ground Values. Estuar. Coast. Shelf Sci. 2017, 198, 163–171. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Ho, D.T.; Johnston, S.G.; Maher, D.T. Mangroves as a Source of Greenhouse Gases to the Atmosphere and Alkalinity and Dissolved Carbon to the Coastal Ocean: A Case Study from the Everglades National Park, Florida. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005812. [Google Scholar] [CrossRef]
- Taillardat, P.; Ziegler, A.D.; Friess, D.A.; Widory, D.; Truong Van, V.; David, F.; Thành-Nho, N.; Marchand, C. Carbon Dynamics and Inconstant Porewater Input in a Mangrove Tidal Creek over Contrasting Seasons and Tidal Amplitudes. Geochim. Cosmochim. Acta 2018, 237, 32–48. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrón, S.; Engel, V.C.; Anderson, W.T.; Swart, P.K.; Price, R.M.; Barbero, L. Dissolved Carbon Biogeochemistry and Export in Mangrove-Dominated Rivers of the Florida Everglades. Biogeosciences 2017, 14, 2543–2559. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.; Eyre, B.D. Seasonal and Temporal CO2 Dynamics in Three Tropical Mangrove Creeks—A Revision of Global Mangrove CO2 Emissions. Geochim. Cosmochim. Acta 2018, 222, 729–745. [Google Scholar] [CrossRef]
- Akhand, A.; Watanabe, K.; Chanda, A.; Tokoro, T.; Chakraborty, K.; Moki, H.; Tanaya, T.; Ghosh, J.; Kuwae, T. Lateral Carbon Fluxes and CO2 Evasion from a Subtropical Mangrove-Seagrass-Coral Continuum. Sci. Total Environ. 2021, 752, 142190. [Google Scholar] [CrossRef] [PubMed]
- Ewel, K.; Twilley, R.; Ong, J. Different Kinds of Mangrove Forests Provide Different Goods and Services. Glob. Ecol. Biogeogr. Lett. 1998, 7, 83–94. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Rogers, K.; McKee, K.L.; Lovelock, C.E.; Mendelssohn, I.A.; Saintilan, N. Mangrove Sedimentation and Response to Relative Sea-Level Rise. Ann. Rev. Mar. Sci. 2016, 8, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Hasada, K.; Taniguchi, S.; Furukawa, K.; Ono, K.; Watanabe, S. Progressing influences of sea-level rise to mangrove forests on Iriomote Island, southwestern Japan. In Proceedings of the Annual Meeting of the Association of Japanese Geographers; The Association of Japanese Geographers: Tokyo, Japan, 2018. (In Japanese) [Google Scholar] [CrossRef]
- Nakamura, W.; Nakamura, Y.; Fujimoto, K.; Suzuki, T.; Higa, H. Analysis on Mangroves Distribution Change over the last 40 Years by using RGB Images of Aira River Mouth in Iriomote Island, Japan and Effect by Sea Level Rise. J. Japan Soc. Civil Eng. Ser. B2 (Coastal Eng.) 2021, 77, I_925–I_930. (In Japanese) [Google Scholar] [CrossRef]
- Miyagi, T.; Miyagi, F.; Baba, S. The idea and significance of non-disturbed corer“Geoslicer NM series”for mangrove forest-floor investigation. Mangrove Sci. 2023, 14, 15–20. (In Japanese) [Google Scholar]
- Endo, T.; Tanaka, T.; Otani, S.; Fujita, T.; Yamoch, S. Validity Verification of Measurement Method of Carbon Dioxide Concentration in Water using Water-resistant Breathable Tube. J. Jpn. Soc. Civ. Eng. Ser. B2 2013, 69, I_1251–I_1255. (In Japanese) [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007; 191p. [CrossRef]
- Torres, M.E.; Mix, A.C.; Rugh, W.D. Precise Δ13C Analysis of Dissolved Inorganic Carbon in Natural Waters Using Automated Headspace Sampling and Continuous-flow Mass Spectrometry. Limnol. Oceanogr. Methods 2005, 3, 349–360. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon Dioxide in Water and Seawater: The Solubility of a Non-Ideal Gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Ho, D.T.; Call, M.; Barr, J.G.; Eyre, B.D. Spatial and Temporal Variability of CO2 and CH4 Gas Transfer Velocities and Quantification of the CH4 Microbubble Flux in Mangrove Dominated Estuaries. Limnol. Oceanogr. 2017, 62, 561–578. [Google Scholar] [CrossRef]
- Ho, D.T.; Coffineau, N.; Hickman, B.; Chow, N.; Koffman, T.; Schlosser, P. Influence of Current Velocity and Wind Speed on Air-water Gas Exchange in a Mangrove Estuary. Geophys. Res. Lett. 2016, 43, 3813–3821. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between Wind Speed and Gas Exchange over the Ocean Revisited. Limnol. Oceanogr. Methods 2014, 12, 351–362. [Google Scholar] [CrossRef]
- Ray, R.; Baum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of Dissolved (Inorganic and Organic) and Particulate Carbon from Mangroves and Its Implication to the Carbon Budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef]
- Alling, V.; Porcelli, D.; Mörth, C.-M.; Anderson, L.G.; Sanchez-Garcia, L.; Gustafsson, Ö.; Andersson, P.S.; Humborg, C. Degradation of Terrestrial Organic Carbon, Primary Production and out-Gassing of CO2 in the Laptev and East Siberian Seas as Inferred from Δ13C Values of DIC. Geochim. Cosmochim. Acta 2012, 95, 143–159. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of Intertidal Intertidal Sediment Processes and Porewater Exchange on the Water Column Biogeochemistry in a Pristine Mangrove Creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Manna, S.; Das, S.; Hazra, S.; Roy, R.; Choudhury, S.B.; Rao, K.H.; Dadhwal, V.K.; Chakraborty, K.; et al. A Comparison of CO2 Dynamics and Air-Water Fluxes in a River-Dominated Estuary and a Mangrove-Dominated Marine Estuary. Geophys. Res. Lett. 2016, 43, 11726–11735. [Google Scholar] [CrossRef]
- Taillardat, P.; Willemsen, P.; Marchand, C.; Friess, D.A.; Widory, D.; Baudron, P.; Truong, V.V.; Nguyễn, T.-N.; Ziegler, A.D. Assessing the Contribution of Porewater Discharge in Carbon Export and CO2 Evasion in a Mangrove Tidal Creek (Can Gio, Vietnam). J. Hydrol. 2018, 563, 303–318. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Liu, C.T.; Pai, S.C. Variations in Oxygen, Nutrient and Carbonate Fluxes of the Kuroshio Current. La Mer 1995, 33, 161–176. [Google Scholar]
- Chou, W.-C.; Sheu, D.D.; Chen, C.T.A.; Wen, L.-S.; Yang, Y.; Wei, C.-L. Transport of the South China Sea Subsurface Water Outflow and Its Influence on Carbon Chemistry of Kuroshio Waters off Southeastern Taiwan. J. Geophys. Res. 2007, 112, C12008. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Watanabe, K.; Das, S.; Tokoro, T.; Chakraborty, K.; Hazra, S.; Kuwae, T. Low CO2 Evasion Rate from the Mangrove-Surrounding Waters of the Sundarbans. Biogeochemistry 2021, 153, 95–114. [Google Scholar] [CrossRef]
- Ray, R.; Thouzeau, G.; Walcker, R.; Vantrepotte, V.; Gleixner, G.; Morvan, S.; Devesa, J.; Michaud, E. Mangrove-Derived Organic and Inorganic Carbon Exchanges Between the Sinnamary Estuarine System (French Guiana, South America) and Atlantic Ocean. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005739. [Google Scholar] [CrossRef]
- Ray, R.; Miyajima, T.; Watanabe, A.; Yoshikai, M.; Ferrera, C.M.; Orizar, I.; Nakamura, T.; San Diego-McGlone, M.L.; Herrera, E.C.; Nadaoka, K. Dissolved and Particulate Carbon Export from a Tropical Mangrove-Dominated Riverine System. Limnol. Oceanogr. 2021, 66, 3944–3962. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Onishi, T.; Yoshitake, S.; Tomotsune, M.; Kida, M.; Iimura, Y.; Kondo, M.; Suchewaboripont, V.; Cao, R.; Kinjo, K.; et al. Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation. Forests 2020, 11, 1041. [Google Scholar] [CrossRef]
- Call, M.; Sanders, C.J.; Macklin, P.A.; Santos, I.R.; Maher, D.T. Carbon Outwelling and Emissions from Two Contrasting Mangrove Creeks during the Monsoon Storm Season in Palau, Micronesia. Estuar. Coast. Shelf Sci. 2019, 218, 340–348. [Google Scholar] [CrossRef]
- Faber, P.A.; Evrard, V.; Woodland, R.J.; Cartwright, I.C.; Cook, P.L.M. Pore-Water Exchange Driven by Tidal Pumping Causes Alkalinity Export in Two Intertidal Inlets. Limnol. Oceanogr. 2014, 59, 1749–1763. [Google Scholar] [CrossRef]
- Sadat-Noori, M.; Maher, D.T.; Santos, I.R. Groundwater Discharge as a Source of Dissolved Carbon and Greenhouse Gases in a Subtropical Estuary. Estuaries Coasts 2016, 39, 639–656. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Schulz, K.G.; Sanders, C.J.; McMahon, A.; Tucker, J.; Santos, I.R. Carbon Outwelling across the Shelf Following a Massive Mangrove Dieback in Australia: Insights from Radium Isotopes. Geochim. Cosmochim. Acta 2019, 253, 142–158. [Google Scholar] [CrossRef]
- Santos, I.R.; Maher, D.T.; Larkin, R.; Webb, J.R.; Sanders, C.J. Carbon Outwelling and Outgassing vs. Burial in an Estuarine Tidal Creek Surrounded by Mangrove and Saltmarsh Wetlands. Limnol. Oceanogr. 2019, 64, 996–1013. [Google Scholar] [CrossRef]
- Volta, C.; Ho, D.T.; Maher, D.T.; Wanninkhof, R.; Friederich, G.; Del Castillo, C.; Dulai, H. Seasonal Variations in Dissolved Carbon Inventory and Fluxes in a Mangrove-dominated Estuary. Glob. Biogeochem. Cycles 2020, 34, e2019GB006515. [Google Scholar] [CrossRef]
- Cabral, A.; Dittmar, T.; Call, M.; Scholten, J.; de Rezende, C.E.; Asp, N.; Gledhill, M.; Seidel, M.; Santos, I.R. Carbon and Alkalinity Outwelling across the Groundwater-Creek-Shelf Continuum off Amazonian Mangroves. Limnol. Oceanogr. Lett. 2021, 6, 369–378. [Google Scholar] [CrossRef]


| Sample | Sand (%) | Silt (%) | Clay (%) | Texture | Color (dry) | SOC (%) | TN (%) | C/N | Ignition Loss (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tidal flat | 93.9 | 3.9 | 2.2 | S | 5 Y 6/2 | 1.1 | 0.04 | 21.5 | 4.1 |
| S. alba | 86.9 | 8.6 | 4.5 | LS | 5 Y 5/2 | 1.7 | 0.1 | 21.0 | 7.1 |
| R. stylosa | 71.4 | 19.3 | 9.3 | SL | 2.5 YR 3/1 | 4.8 | 0.1 | 41.7 | 25.5 |
| B. gymnorrhiza | 89.1 | 8.0 | 2.9 | LS | 10 YR 4/1 | 2.7 | 0.1 | 40.0 | 8.0 |
| Spring Tide | Neap Tide | Average | |
|---|---|---|---|
| Q (m3 d−1) | 12,906 | 13,665 | 13,286 |
| ΔTA (μM) | 280 ± 250 | 332 ± 193 | 306 ± 224 |
| TA flux (mol d−1) | 3614 ± 3221 | 4534 ± 2634 | 4064 ± 2982 |
| TA export (mmol m−2 d−1) | 32 ± 29 | 40 ± 23 | 36 ± 26 |
| ΔDIC (μM) | 306 ± 342 | 413 ± 316 | 359 ± 333 |
| DIC flux (mol d−1) | 3947 ± 4415 | 5639 ± 4315 | 4772 ± 4430 |
| DIC export (mmol m−2 d−1) | 35 ± 39 | 50 ± 38 | 42 ± 39 |
| ΔDOC (μM) | 88 ± 103 | 82 ± 47 | 85 ± 80 |
| DOC flux (mol d−1) | 1135 ± 1325 | 1120 ± 643 | 1129 ± 1062 |
| DOC export (mmol m−2 d−1) | 10 ± 12 | 10 ± 6 | 10 ± 9 |
| Adj. R2 | F Statistics p Value | Depth(m) | DO (%) | Day-Night | ||||
|---|---|---|---|---|---|---|---|---|
| Coef. | p Value | Coef. | p Value | Coef. | p Value | |||
| Spring tide | 0.625 | <0.001 | −0.72 | <0.001 | 0.27 | <0.01 | 0.01 | >0.05 |
| Middle tide | 0.651 | <0.001 | −0.41 | <0.001 | −0.91 | <0.001 | 0.43 | <0.001 |
| Neap tide | 0.561 | <0.001 | 0.07 | >0.05 | −1.05 | <0.001 | −0.63 | <0.001 |
| Location | Target | TA | DIC | DOC | Reference |
|---|---|---|---|---|---|
| Yubu Coast, Japan | Spring tide | 32 ± 29 | 35 ± 39 | 10 ± 12 | This study |
| Yubu Coast, Japan | Neap tide | 40 ± 23 | 50 ± 38 | 10 ± 6 | This study |
| Fukido River, Japan | Winter | NA | 113 | 17 | [49] |
| Fukido River, Japan | Summer | NA | 279 | 72 | [49] |
| Yutsun River, Japan | River mouth | 363 | 179 | 66 | [26] |
| Katunggan It Ibajay Ecopark, Philippines | Estuary flux | NA | 361 ± 185 | 16.5 ± 1.7 | [48] |
| Palau, Micronesia | Creek1 | 48 ± 17 | 79 ± 28 | 35 ± 12 | [50] |
| Palau, Micronesia | Creek2 | 6 ± 5 | 10 ± 4 | 8 ± 3 | [50] |
| Can Gio, Vietnam | Symmetric tidal cycle | NA | 352 ± 34 | 21 ± 2 | [43] |
| Can Gio, Vietnam | Asymmetric tidal cycle | NA | 678 ± 79 | 68 ± 8 | [43] |
| Darwin, Australia | Creek | 116 | 85 | NA | [13] |
| Hinchinbrook Island, Australia | Creek | 21 | 22 | NA | [13] |
| Seventeen Seventy, Australia | Creek | 81 | −97 | NA | [13] |
| Jacobs Well, Australia | Creek | 12 | 83 | NA | [13] |
| Newcastle, Australia | Creek | 116 | 77 | NA | [13] |
| Barwon Heads, Australia | Creek | −1 | −3 | NA | [13] |
| Watson Inlet, Australia | Creek | 310 | 440 | 25 | [51] |
| Chinaman Inlet, Australia | Creek | 46 | 120 | 0 | [51] |
| Had Head, Australia | Wet Season | 27 ± 5 | 24 ± 5 | 14 ± 3 | [52] |
| Had Head, Australia | Dry Season | 19 ± 4 | 16 ± 3 | 3 ± 1 | [52] |
| Gulf of Carpentaria, Australia | Alive mangrove | 519–950 | 584–1050 | 17 | [53] |
| Gulf of Carpentaria, Australia | Dead mangrove | 254–599 | 291–605 | 20 | [53] |
| Coffs Creek, Australia | Creek | 2.2 ± 1.9 | 2.2 ± 1.5 | −0.3± 1.0 | [54] |
| Evans Head, Australia | Creek | 12 ± 6 | 12 ± 6 | 2 ± 2 | [54] |
| Everglades, USA | SharkTREx 3 | NA | 269 ± 19 | 68 ± 4 | [55] |
| Everglades, USA | SharkTREx 5 | NA | 253 ± 8 | 39 ± 1 | [55] |
| Everglades, USA | Shark River | 97 | 142 | 39 | [22] |
| Amazon, Brazil | Estuary | 15 ± 8 | 20 ± 14 | 0.3 ± 10 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, W.; Wang, K.; Ono, K.; Endo, T.; Watanabe, S.; Mori, T.; Furukawa, K.; Fujimoto, K.; Sasaki, J. A Tidal Flat Adjacent to a Fringe Mangrove Forest Mitigates pCO2 Increases and Enhances Lateral Export of Dissolved Carbon. J. Mar. Sci. Eng. 2023, 11, 2356. https://doi.org/10.3390/jmse11122356
Nakamura W, Wang K, Ono K, Endo T, Watanabe S, Mori T, Furukawa K, Fujimoto K, Sasaki J. A Tidal Flat Adjacent to a Fringe Mangrove Forest Mitigates pCO2 Increases and Enhances Lateral Export of Dissolved Carbon. Journal of Marine Science and Engineering. 2023; 11(12):2356. https://doi.org/10.3390/jmse11122356
Chicago/Turabian StyleNakamura, Wataru, Kangnian Wang, Kenji Ono, Toru Endo, Shin Watanabe, Taiki Mori, Keita Furukawa, Kiyoshi Fujimoto, and Jun Sasaki. 2023. "A Tidal Flat Adjacent to a Fringe Mangrove Forest Mitigates pCO2 Increases and Enhances Lateral Export of Dissolved Carbon" Journal of Marine Science and Engineering 11, no. 12: 2356. https://doi.org/10.3390/jmse11122356
APA StyleNakamura, W., Wang, K., Ono, K., Endo, T., Watanabe, S., Mori, T., Furukawa, K., Fujimoto, K., & Sasaki, J. (2023). A Tidal Flat Adjacent to a Fringe Mangrove Forest Mitigates pCO2 Increases and Enhances Lateral Export of Dissolved Carbon. Journal of Marine Science and Engineering, 11(12), 2356. https://doi.org/10.3390/jmse11122356





