The First Record of Ocypode sinensis (Decapoda: Ocypodidae) from the Korean Peninsula: How the Complete Mitochondrial Genome Elucidates the Divergence History of Ghost Crabs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation, Morphological Characterization and Sediment Grain Analysis
2.2. Molecular Analysis for Genetically Identification
2.3. Molecular Analysis for Mitogenome
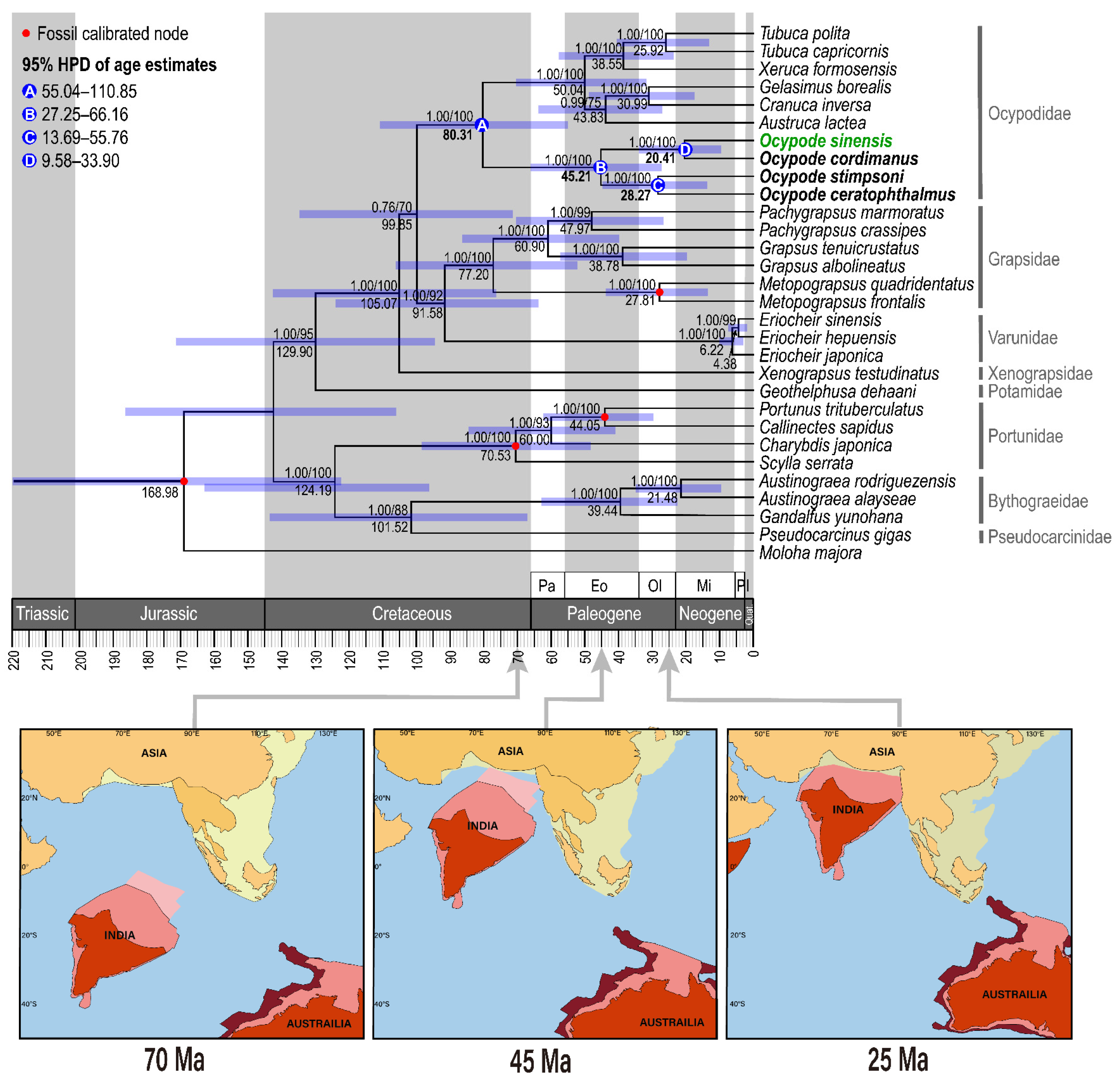
2.4. Estimation Divergence Time
| Family | Species | Size (bp) | GenBank ID | References |
|---|---|---|---|---|
| Bythograeidae | Gandalfus yunohana | 15,567 | EU647222 | [48] |
| Austinograea rodriguezensis | 15,611 | JQ035658 | [49] | |
| Austinograea alayseaea | 15,620 | JQ035660 | [49] | |
| Portunidae | Callinectes sapidus | 16,263 | AY363392 | [50] |
| Charybdis japonica | 15,738 | FJ460517 | [51] | |
| Scylla serrata | 15,721 | HM590866 | [52] | |
| Portunus trituberculatus | 16,026 | NC_005037 | [53] | |
| Potamidae | Geothelphusa dehaani | 18,197 | NC_007379 | [54] |
| Pseudocarcinidae | Pseudocarcinus gigas | 15,515 | AY562127 | [55] |
| Grapsidae | Pachygrapsus crassipes | 15,652 | KC878511 | [56] |
| Grapsus tenuicrustatus | 15,858 | KT878721 | [57] | |
| Metopograpsus frontalis | 15,587 | MH028874 | [58] | |
| Metopograpsus quadridentatus | 15,517 | MH183127 | [59] | |
| Grapsus albolineatus | 15,583 | MZ262276 | [60] | |
| Pachygrapsus marmoratus | 15,406 | MF457403.1 | [61] | |
| Ocypodidae | Tubuca capricornis | 15,629 | MF457401.1 | [61] |
| Cranuca inversa | 15,677 | MF457405.1 | [61] | |
| Austruca lactea | 15,659 | KY865330 | [62] | |
| Tubuca polita | 15,672 | MF457400 | [61] | |
| Gelasimus borealis | 15,662 | MH796170 | [63] | |
| Ocypode ceratophthalma | 15,564 | LN611669 | [24] | |
| Ocypode stimpsoni | 15,557 | MN917464 | [11] | |
| Ocypode cordimanus | 15,604 | NC_029725 | [23] | |
| Xeruca formosensis | 15,684 | OL693688 | [64] | |
| Varunidae | Eriocheir sinensis | 16,335 | FJ455505 | [65] |
| Eriocheir japonica | 16,352 | FJ455506 | [65] | |
| Eriocheir hepuensis | 16,353 | FJ455507 | [65] | |
| Xenograpsidae | Xenograpsus testudinatus | 15,796 | EU727203 | [66] |
| Homolidae | Moloha majora | 16,084 | KT182069 | [67] |
3. Results
3.1. Sediment Analysis
3.2. Morphological Characteristic
- Systematics
- The Material Examined
- Diagnosis
3.3. Genetic Distance
3.4. Mitogenome Structure
3.5. Phylogeny of the Genus Ocypode
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harley, C.D.; Randall Hughes, A.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Sydeman, W.J.; Poloczanska, E.; Reed, T.E.; Thompson, S.A. Climate change and marine vertebrates. Science 2015, 350, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Hazen, E.L.; Jorgensen, S.; Rykaczewski, R.R.; Bograd, S.J.; Foley, D.G.; Jonsen, I.D.; Shaffer, S.A.; Dunne, J.P.; Costa, D.P.; Crowder, L.B. Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Change 2013, 3, 234–238. [Google Scholar] [CrossRef]
- Braun, C.D.; Lezama-Ochoa, N.; Farchadi, N.; Arostegui, M.C.; Alexander, M.; Allyn, A.; Bograd, S.J.; Brodie, S.; Crear, D.P.; Curtis, T.H. Widespread habitat loss and redistribution of marine top predators in a changing ocean. Sci. Adv. 2023, 9, eadi2718. [Google Scholar] [CrossRef] [PubMed]
- Lercari, D.; Defeo, O.; Ortega, L.; Orlando, L.; Gianelli, I.; Celentano, E. Long-term structural and functional changes driven by climate variability and fishery regimes in a sandy beach ecosystem. Ecol. Model. 2018, 368, 41–51. [Google Scholar] [CrossRef]
- Scapini, F.; Degli, E.I.; Defeo, O. Behavioral adaptations of sandy beach macrofauna in face of climate change impacts: A conceptual framework. Estuar. Coast. Shelf Sci. 2019, 225, 106236. [Google Scholar] [CrossRef]
- Schoeman, D.S.; Schlacher, T.A.; Jones, A.R.; Murray, A.; Huijbers, C.M.; Olds, A.D.; Connolly, R.M. Edging along a warming coast: A range extension for a common sandy beach crab. PLoS ONE 2015, 10, e0141976. [Google Scholar] [CrossRef]
- Machado, P.M.; Tavares, D.C.; Zalmon, I.R. Synergistic effect of extreme climatic events and urbanization on population density of the ghost crab Ocypode quadrata (Fabricius, 1787). Mar. Ecol. 2019, 40, e12525. [Google Scholar] [CrossRef]
- Sakai, K.; Türkay, M. Revision of the genus Ocypode with the description of a new genus, Hoplocypode (Crustacea: Decapoda: Brachyura). Mem. Qld. Mus. 2013, 56, 665–793. [Google Scholar]
- Lee, K.-H.; Ko, H.-S. First records of three crabs (Crustacea: Decapoda) from Korea. Anim. Syst. Evol. Divers. 2008, 24, 17–24. [Google Scholar] [CrossRef]
- Kim, H.; Jung, J. Complete mitochondrial genome of the ghost crab Ocypode stimpsoni Ortmann, 1897 (Brachyura: Decapoda: Ocypodidae) and its phylogenetic relationship in Brachyura. Mitochondrial DNA Part B 2020, 5, 1699–1700. [Google Scholar] [CrossRef]
- Conservation and Management of Marine Ecosystems Act. 2022. Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=61419&type=part&key=39 (accessed on 18 October 2022).
- Chakrabarti, A. Burrow patterns of Ocypode ceratophthalma (Pallas) and their environmental significance. J. Paleontol. 1981, 55, 431–441. [Google Scholar]
- Gül, M.R.; Griffen, B.D. Burrowingz behavior and burrowing energetics of a bioindicator under human disturbance. Ecol. Evol. 2019, 9, 14205–14216. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, T.G. Ecological role of ghost crabs, Ocypode quadrata (Fabricius) on an ocean beach: Scavengers or predators? J. Exp. Mar. Biol. Ecol. 1978, 31, 67–82. [Google Scholar] [CrossRef]
- Rae, C.; Hyndes, G.A.; Schlacher, T.A. Trophic ecology of ghost crabs with diverse tastes: Unwilling vegetarians. Estuar. Coast. Shelf Sci. 2019, 224, 272–280. [Google Scholar] [CrossRef]
- Costa, L.L.; Madureira, J.F.; Di Beneditto, A.P.M.; Zalmon, I.R. Interaction of the Atlantic ghost crab with marine debris: Evidence from an in situ experimental approach. Mar. Pollut. Bull. 2019, 140, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Jonah, F.; Agbo, N.; Agbeti, W.; Adjei-Boateng, D.; Shimba, M. The ecological effects of beach sand mining in Ghana using ghost crabs (Ocypode species) as biological indicators. Ocean Coast. Manag. 2015, 112, 18–24. [Google Scholar] [CrossRef]
- Gül, M.R. Combined influence of human disturbance and beach geomorphology on Ghost Crab, Ocypode cursor, burrow density and size in the eastern Mediterranean. Zool. Middle East 2022, 68, 225–236. [Google Scholar] [CrossRef]
- Lucrezi, S.; Schlacher, T.A.; Walker, S. Monitoring human impacts on sandy shore ecosystems: A test of ghost crabs (Ocypode spp.) as biological indicators on an urban beach. Environ. Monit. Assess. 2009, 152, 413–424. [Google Scholar] [CrossRef]
- Barros, F. Ghost crabs as a tool for rapid assessment of human impacts on exposed sandy beaches. Biol. Conserv. 2001, 97, 399–404. [Google Scholar] [CrossRef]
- O’Brien, C.; Bracken-Grissom, H.D.; Baeza, J.A. The complete mitochondrial genome of the Atlantic ghost crab Ocypode quadrata (Fabricius, 1787) (Brachyura: Ocypodidae: Ocypodinae). J. Crustac. Biol. 2022, 42, ruac005. [Google Scholar] [CrossRef]
- Sung, J.M.; Lee, J.; Kim, S.G.; Karagozlu, M.Z.; Kim, C.B. Analysis of complete mitochondrial genome of Ocypode cordimanus (Latreille, 1818) (Decapoda, Ocypodidae). Mitochondrial DNA Part B 2016, 1, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Gan, H.M.; Lee, Y.P.; Austin, C.M. The complete mitogenome of the ghost crab Ocypode ceratophthalmus (Pallas, 1772) (Crustacea: Decapoda: Ocypodidae). Mitochondrial DNA Part A 2016, 27, 2123–2124. [Google Scholar] [CrossRef]
- Cameron, S.L.; Lambkin, C.L.; Barker, S.C.; Whiting, M.F. A mitochondrial genome phylogeny of Diptera: Whole genome sequence data accurately resolve relationships over broad timescales with high precision. Syst. Entomol. 2007, 32, 40–59. [Google Scholar] [CrossRef]
- Huang, J.F.; Yang, S.L.; Ng PK, L. Note on the taxonomy and distribution of two closely related species of ghost crab, ocypode sinensis and cordimanus. Crustaceana 1998, 77, 942–954. [Google Scholar] [CrossRef]
- Blatt, H. Origin of Sedimentary Rocks. Soil Sci. 1973, 115, 400. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, R.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 March 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Stadler, T.; Bonhoeffer, S. Uncovering epidemiological dynamics in heterogeneous host populations using phylogenetic methods. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120198. [Google Scholar] [CrossRef]
- Rinehart, L.F.; Lucas, S.G.; Heckert, A.B. An early eubrachyuran (Malacostraca: Decapoda) from the Upper Triassic Snyder Quarry, Petrified Forest Formation, north-central New Mexico. Paleontol. Geol. Up. Triassic (Rev.) Snyder Quarry New Mex. New Mex. Mus. Nat. Hist. Sci. Bull. 2003, 24, 67–70. [Google Scholar]
- Distel, D.L.; Baco, A.R.; Chuang, E.; Morrill, W.; Cavanaugh, C.; Smith, C.R. Do mussels take wooden steps to deep-sea vents? Nature 2000, 403, 725–726. [Google Scholar] [CrossRef]
- Milne-Edwards, A.; Brocchi, P. Note sur quelques Crustacés fossiles appartenant au groupe des Macrophthalmiens. Bull. Soc. Philomath. Paris 1879, 3, 113–117. [Google Scholar]
- Karasawa, H.; Schweitzer, C.E.; Feldmann, R.M. Revision of Portunoidea (Decapoda: Brachyura) with emphasis on the fossil genera and families. J. Crustac. Biol. 2008, 28, 82–127. [Google Scholar] [CrossRef]
- Rathbun, M.J. Fossil Crustacea of the Atlantic and Gulf Coastal Plain; Geological Society of America: Boulder, CO, USA, 1935; Volume 2. [Google Scholar]
- Müller, P.M. New decapods from the Miocene of Hungary—With remarks about their environment. Földtani Közlöny 2006, 136, 37–50. [Google Scholar]
- Yang, J.S.; Nagasawa, H.; Fujiwara, Y.; Tsuchida, S.; Yang, W.J. The complete mitogenome of the hydrothermal vent crab Gandalfus yunohana (Crustacea: Decapoda: Brachyura): A link between the Bythograeoidea and Xanthoidea. Zool. Scr. 2010, 39, 621–630. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lu, B.; Chen, D.-F.; Yu, Y.-Q.; Yang, F.; Nagasawa, H.; Tsuchida, S.; Fujiwara, Y.; Yang, W.-J. When did decapods invade hydrothermal vents? Clues from the Western Pacific and Indian Oceans. Mol. Biol. Evol. 2012, 30, 305–309. [Google Scholar] [CrossRef]
- Place, A.R.; Feng, X.; Steven, C.R.; Fourcade, H.M.; Boore, J.L. Genetic markers in blue crabs (Callinectes sapidus): II. Complete mitochondrial genome sequence and characterization of genetic variation. J. Exp. Mar. Biol. Ecol. 2005, 319, 15–27. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z. Complete mitochondrial genome of the Asian paddle crab Charybdis japonica (Crustacea: Decapoda: Portunidae): Gene rearrangement of the marine brachyurans and phylogenetic considerations of the decapods. Mol. Biol. Rep. 2010, 37, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Jondeung, A.; Karinthanyakit, W.; Kaewkhumsan, J. The complete mitochondrial genome of the black mud crab, Scylla serrata (Crustacea: Brachyura: Portunidae) and its phylogenetic position among (pan) crustaceans. Mol. Biol. Rep. 2012, 39, 10921–10937. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.M.; Miya, M.U.; Nishida, M. Complete mitochondrial DNA sequence of the swimming crab, Portunus trituberculatus (Crustacea: Decapoda: Brachyura). Gene 2003, 311, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Segawa, R.D.; Aotsuka, T. The mitochondrial genome of the Japanese freshwater crab, Geothelphusa dehaani (Crustacea: Brachyura): Evidence for its evolution via gene duplication. Gene 2005, 355, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Murphy, N.P.; Burridge, C.P.; Austin, C.M. Complete mitochondrial DNA sequences of the decapod crustaceans Pseudocarcinus gigas (Menippidae) and Macrobrachium rosenbergii (Palaemonidae). Mar. Biotechnol. 2005, 7, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Q.; Ma, W.-M.; Yang, W.-J.; Yang, J.-S. The complete mitogenome of the lined shore crab Pachygrapsus crassipes Randall 1840 (Crustacea: Decapoda: Grapsidae). Mitochondrial DNA 2014, 25, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.-M.; Lee, J.; Kim, S.-K.; Karagozlu, M.Z.; Kim, C.-B. The complete mitochondrial genome of Grapsus tenuicrustatus (Herbst, 1783) (Decapoda, Grapsidae). Mitochondrial DNA Part B 2016, 1, 441–442. [Google Scholar] [CrossRef]
- Guan, M.; Liu, X.; Lin, F.; Xie, Z.; Fazhan, H.; Ikhwanuddin, M.; Tan, H.; Ma, H. The whole mitochondrial genome of the mangrove crab, Metopograpsus frontalis (Miers, 1880) (Decapoda, Grapsidae) and its phylogenetic relationship. Mitochondrial DNA Part B 2018, 3, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xing, Y.; Yao, W.; Zhang, C.; Zhang, Z.; Jiang, G.; Ding, Z. Characterization of four new mitogenomes from Ocypodoidea & Grapsoidea, and phylomitogenomic insights into thoracotreme evolution. Gene 2018, 675, 27–35. [Google Scholar] [PubMed]
- Lü, J.; Xia, L.; Liu, X.; Ma, Y.; Li, J.; Ye, Y.; Guo, B. The mitochondrial genome of Grapsus albolineatus (Decapoda: Brachyura: Grapsidae) and phylogenetic associations in Brachyura. Sci. Rep. 2022, 12, 2104. [Google Scholar] [CrossRef]
- Tan, M.H.; Gan, H.M.; Lee, Y.P.; Linton, S.; Grandjean, F.; Bartholomei-Santos, M.L.; Miller, A.D.; Austin, C.M. ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Mol. Phylogenetics Evol. 2018, 127, 320–331. [Google Scholar] [CrossRef]
- Yang, T.-T.; Liu, Y.; Xin, Z.-Z.; Liu, Q.-N.; Zhang, D.-Z.; Tang, B.-P. The complete mitochondrial genome of Uca lactea (Ocypodidae, Brachyura) and phylogenetic relationship in Brachyura. Mitochondrial DNA Part B 2019, 4, 1319–1320. [Google Scholar] [CrossRef]
- Guo, H.; Tang, D.; Wang, Z.; Shi, X.; Shen, C.; Cheng, X.; Ji, C.; Wang, Z. Complete mitochondrial genome and phylogenetic analysis of Uca Borealis. Mitochondrial DNA Part B 2019, 4, 89–90. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Shih, H.-T. The complete mitogenome of Xeruca formosensis (Rathbun, 1921) (Crustacea: Brachyura: Ocypodidae), a fiddler crab endemic to Taiwan, with its phylogenetic position in the family. Zool. Stud. 2022, 61, e69. [Google Scholar]
- Wang, J.; Huang, L.; Cheng, Q.; Lu, G.; Wang, C. Complete mitochondrial genomes of three mitten crabs, Eriocheir sinensis, E. hepuensis, and E. japonica. Mitochondrial DNA Part A 2016, 27, 1175–1176. [Google Scholar] [CrossRef]
- Ki, J.-S.; Dahms, H.-U.; Hwang, J.-S.; Lee, J.-S. The complete mitogenome of the hydrothermal vent crab Xenograpsus testudinatus (Decapoda, Brachyura) and comparison with brachyuran crabs. Comp. Biochem. Physiol. Part D Genom. Proteom. 2009, 4, 290–299. [Google Scholar] [CrossRef]
- Shi, G.; Cui, Z.; Hui, M.; Liu, Y.; Chan, T.-Y.; Song, C. Unusual sequence features and gene rearrangements of primitive crabs revealed by three complete mitochondrial genomes of Dromiacea. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 20, 65–73. [Google Scholar] [CrossRef]
- Tsang, L.M.; Schubart, C.D.; Ahyong, S.T.; Lai, J.C.; Au, E.Y.; Chan, T.-Y.; Ng, P.K.; Chu, K.H. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol. Biol. Evol. 2014, 31, 1173–1187. [Google Scholar] [CrossRef]
- Artal, P. “Uca miocenica” (Crustacea, Decapoda), Nueva Especie del Mioceno de la Prov. de Barcelona (Cataluña, España). Scr. Mussei Geol. Semin. Barc. Ser. Palaeontol. 2008, 6, 3–16. [Google Scholar]
- Casadío, S.; Feldmann, R.M.; Parras, A.; Schweitzer, C.E. Miocene fossil Decapoda (Crustacea: Brachyura) from Patagonia, Argentina, and their paleoecological setting. Ann. Carnegie Mus. 2005, 74, 151–188. [Google Scholar] [CrossRef]
- Hall, R. Late Jurassic–Cenozoic reconstructions of the Indonesian region and the Indian Ocean. Tectonophysics 2012, 570, 1–41. [Google Scholar] [CrossRef]
- Ocaña, F.A.; de Jesús-Navarrete, A.; Hernández-Arana, H.A. Co-occurring factors affecting ghost crab density at four sandy beaches in the Mexican Caribbean. Reg. Stud. Mar. Sci. 2020, 36, 101310. [Google Scholar] [CrossRef]
- Watson, G.S.; Gregory, E.A.; Johnstone, C.; Berlino, M.; Green, D.W.; Peterson, N.R.; Schoeman, D.S.; Watson, J.A. Like night and day: Reversals of thermal gradients across ghost crab burrows and their implications for thermal ecology. Estuar. Coast. Shelf Sci. 2018, 203, 127–136. [Google Scholar] [CrossRef]
- Yong, A.Y.P.; Lim, S.S.L. Coexistence of Juvenile with Adult Ocypode gaudichaudii at Culebra Beach, Panama: A Temporal-spatial Partitioning Compromise. Zool. Stud. 2022, 60, e8. [Google Scholar] [CrossRef]
- Wong, K.J.; Shih, H.-T.; Chan, B.K. The ghost crab Ocypode mortoni George, 1982 (Crustacea: Decapoda: Ocypodidae): Redescription, distribution at its type locality, and the phylogeny of East Asian Ocypode species. Zootaxa 2012, 3550, 71–87. [Google Scholar] [CrossRef]
- Naderi, M.; Zare, P.; Lastra, M.; Pishehvarzad, F. First record of ghost crab Ocypode sinensis (Dai, Song & Yang, 1985) (Decapoda: Brachyura: Ocypodidae) from Qeshm Island, Persian Gulf, Iran. Cah. Biol. Mar. 2018, 59, 527–531. [Google Scholar] [CrossRef]
- Ackiss, A.S.; Bird, C.E.; Akita, Y.; Santos, M.D.; Tachihara, K.; Carpenter, K.E. Genetic patterns in peripheral marine populations of the fusilier fish Caesio cuning within the Kuroshio Current. Ecol. Evol. 2018, 8, 11875–11886. [Google Scholar] [CrossRef] [PubMed]
- Lie, H.J.; Cho, C.H.; Lee, J.H.; Niiler, P.; Hu, J.H. Separation of the Kuroshio water and its penetration onto the continental shelf west of Kyushu. J. Geophys. Res. Ocean. 1998, 103, 2963–2976. [Google Scholar] [CrossRef]
- Wada, T.; Wada, K. First record of the tropical ghost crab Ocypode sinensis Dai, Song & Yang, 1985 (Ocypodidae) from the coast of Sea of Japan. Cancer 2015, 24, 15–19. [Google Scholar]
- Kocsis, Á.T.; Scotese, C.R. Mapping paleocoastlines and continental flooding during the Phanerozoic. Earth-Sci. Rev. 2021, 213, 103463. [Google Scholar] [CrossRef]
- Zahirovic, S.; Seton, M.; Müller, R. The cretaceous and cenozoic tectonic evolution of Southeast Asia. Solid Earth 2014, 5, 227–273. [Google Scholar] [CrossRef]
- Keep, M.; Holbourn, A.; Kunht, W.; Gallagher, S.J. Progressive Western Australian collision with Asia: Implications for regional orography, oceanography, climate and marine biota. J. R. Soc. West. Aust. 2018, 101, 1–17. [Google Scholar]



| Jeju | Busan | |
|---|---|---|
| Abbreviation | JJ | BS |
| Latitude | 33°12′39.16″ N | 35°15′43.26″ N |
| Longitude | 126°15′38.41″ E | 129°14′1.44″ E |
| Collection date | 29–30 August 2022 | 14 September 2021 |
| Number of specimens | 2 | 1 |
| Sediment | ||
| 0.5–1 mm (%) | 3.16 | 13.61 |
| 0.25–0.5 mm (%) | 47.45 | 63.19 |
| 0.12–0.25 mm (%) | 49.25 | 22.66 |
| 0.06–0.12 mm (%) | 0.05 | 0.18 |
| 0.03–0.06 mm (%) | 0.02 | 0.11 |
| <0.03 mm (%) | 0.07 | 0.24 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||||||||||
| 2 | 0.36 | |||||||||||||||
| 3 | 0.36 | 0.00 | ||||||||||||||
| 4 | 0.91 | 0.54 | 0.54 | |||||||||||||
| 5 | 13.48 | 13.48 | 13.48 | 14.17 | ||||||||||||
| 6 | 18.22 | 17.74 | 17.74 | 17.98 | 18.53 | |||||||||||
| 7 | 16.41 | 15.94 | 15.94 | 16.17 | 18.86 | 12.66 | ||||||||||
| 8 | 17.24 | 17.00 | 17.00 | 17.00 | 17.98 | 6.67 | 13.73 | |||||||||
| 9 | 18.03 | 18.03 | 18.03 | 18.27 | 20.55 | 14.96 | 13.55 | 15.12 | ||||||||
| 10 | 15.72 | 15.26 | 15.26 | 15.72 | 21.38 | 14.83 | 14.14 | 16.42 | 14.38 | |||||||
| 11 | 14.83 | 14.37 | 14.37 | 14.37 | 17.41 | 16.19 | 16.26 | 16.63 | 17.64 | 6.49 | ||||||
| 12 | 17.48 | 17.01 | 17.01 | 17.25 | 19.17 | 14.00 | 14.03 | 14.66 | 16.08 | 15.50 | 15.30 | |||||
| 13 | 16.14 | 15.67 | 15.67 | 15.43 | 18.24 | 17.43 | 15.20 | 18.85 | 20.38 | 16.97 | 17.24 | 18.14 | ||||
| 14 | 17.17 | 16.93 | 16.93 | 17.17 | 19.94 | 21.58 | 17.14 | 19.84 | 17.52 | 16.71 | 16.51 | 16.92 | 19.97 | |||
| 15 | 17.75 | 17.51 | 17.51 | 18.23 | 19.91 | 20.09 | 18.41 | 19.84 | 18.74 | 17.45 | 17.92 | 20.29 | 21.62 | 20.27 | ||
| 16 | 15.92 | 15.46 | 15.46 | 15.46 | 18.03 | 19.14 | 19.50 | 17.25 | 20.23 | 17.95 | 15.63 | 18.40 | 20.13 | 19.72 | 15.88 | |
| 17 | 17.68 | 17.68 | 17.68 | 18.16 | 19.12 | 17.77 | 17.92 | 19.48 | 18.44 | 14.94 | 17.04 | 20.30 | 21.97 | 20.92 | 19.00 | 17.82 |
| Gene | Type | Position | Strand | Length (bp) | Intergenic Space | Start Codon | Stop Codon |
|---|---|---|---|---|---|---|---|
| cox1 | CDS | 1–1534 | H | 1534 | 0 | ATG | TAA |
| trnL2-taa | tRNA | 1535–1599 | H | 65 | 10 | TCT | GAA |
| cox2 | CDS | 1610–2299 | H | 690 | 0 | ATG | TAG |
| trnK-ttt | tRNA | 2298–2366 | H | 69 | 0 | AGT | ACT |
| trnD-gtc | tRNA | 2367–2430 | H | 64 | 0 | AAG | TTA |
| atp8 | CDS | 2431–2589 | H | 159 | −5 | ATG | TAA |
| atp6 | CDS | 2583–3257 | H | 675 | −1 | ATT | TAA |
| cox3 | CDS | 3257–4048 | H | 792 | −1 | ATG | TAA |
| trnG-tcc | tRNA | 4048–4110 | H | 63 | −1 | ATT | ATA |
| nd3 | CDS | 4111–4461 | H | 351 | −2 | ATT | TAA |
| trnA-tgc | tRNA | 4460–4524 | H | 65 | 6 | AAG | TTA |
| trnR-tcg | tRNA | 4531–4593 | H | 63 | 0 | TAT | AAT |
| trnN-gtt | tRNA | 4594–4658 | H | 65 | 0 | TTG | AAG |
| trnS1-tct | tRNA | 4659–4724 | H | 66 | 1 | GAA | TCT |
| trnE-ttc | tRNA | 4726–4792 | H | 67 | −1 | GTT | ACT |
| trnH-gtg | tRNA | 4792–4854 | L | 63 | 0 | TAT | AAT |
| trnF-gaa | tRNA | 4855–4918 | L | 64 | −1 | TAT | AAT |
| nd5 | CDS | 4918–6651 | L | 1734 | 46 | TTA | CAT |
| nd4 | CDS | 6698–8035 | L | 1338 | −7 | TTA | CAT |
| nd4l | CDS | 8029–8328 | L | 300 | 8 | TTA | CAT |
| trnT-tgt | tRNA | 8337–8402 | H | 66 | 0 | GTT | ACT |
| trnP-tgg | tRNA | 8403–8467 | L | 65 | −16 | TCA | CTG |
| nd6 | CDS | 8452–8967 | H | 516 | 6 | CTT | TAA |
| cytB | CDS | 8974–10,107 | H | 1134 | 1 | ATG | AAA |
| trnS-tga | tRNA | 10,109–10,175 | H | 67 | 16 | GAT | TCG |
| nd1 | CDS | 10,192–11,130 | L | 939 | 31 | TTA | CAT |
| trnL-tag | tRNA | 11,162–11,228 | L | 67 | −58 | TAC | AGT |
| rrn-16S | rRNA | 11,171–12,541 | L | 1371 | 2 | TAG | ATA |
| trnV-tac | tRNA | 12,544–12,615 | L | 72 | 1 | TCA | TTG |
| rrn-12S | rRNA | 12,617–13,449 | L | 833 | 721 | TTA | TCT |
| trnl-gat | tRNA | 14,171–14,236 | H | 66 | −3 | AAT | TTA |
| trnQ-ttg | tRNA | 14,234–14,302 | L | 69 | 5 | TTA | ATA |
| trnM-cat | tRNA | 14,308–14,377 | H | 70 | 0 | TAA | TTA |
| nd2 | CDS | 14,378–15,388 | H | 1011 | −2 | ATG | TAG |
| trnW-tca | tRNA | 15,387–15,455 | H | 69 | 1 | AGA | CTA |
| trnC-gca | tRNA | 15,457–15,522 | L | 66 | 2 | AAA | GTT |
| trnY-gta | tRNA | 15,525–15,589 | L | 65 | 0 | TAA | ATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-I.; Jang, S.-J.; Kim, T. The First Record of Ocypode sinensis (Decapoda: Ocypodidae) from the Korean Peninsula: How the Complete Mitochondrial Genome Elucidates the Divergence History of Ghost Crabs. J. Mar. Sci. Eng. 2023, 11, 2348. https://doi.org/10.3390/jmse11122348
Kim D-I, Jang S-J, Kim T. The First Record of Ocypode sinensis (Decapoda: Ocypodidae) from the Korean Peninsula: How the Complete Mitochondrial Genome Elucidates the Divergence History of Ghost Crabs. Journal of Marine Science and Engineering. 2023; 11(12):2348. https://doi.org/10.3390/jmse11122348
Chicago/Turabian StyleKim, Da-In, Sook-Jin Jang, and Taewon Kim. 2023. "The First Record of Ocypode sinensis (Decapoda: Ocypodidae) from the Korean Peninsula: How the Complete Mitochondrial Genome Elucidates the Divergence History of Ghost Crabs" Journal of Marine Science and Engineering 11, no. 12: 2348. https://doi.org/10.3390/jmse11122348
APA StyleKim, D.-I., Jang, S.-J., & Kim, T. (2023). The First Record of Ocypode sinensis (Decapoda: Ocypodidae) from the Korean Peninsula: How the Complete Mitochondrial Genome Elucidates the Divergence History of Ghost Crabs. Journal of Marine Science and Engineering, 11(12), 2348. https://doi.org/10.3390/jmse11122348







