Diversity of CO2 Concentrating Mechanisms in Macroalgae Photosynthesis: A Case Study of Ulva sp.
Abstract
1. Introduction
2. Composition of CCMs
2.1. Inorganic Carbon Transporters
2.2. Carbonic Anhydrase
2.3. Rubisco and Pyrenoid
3. Indicators to Measure CCMs in Macroalgae
3.1. CO2 Affinity of Photosynthesis versus Rubisco
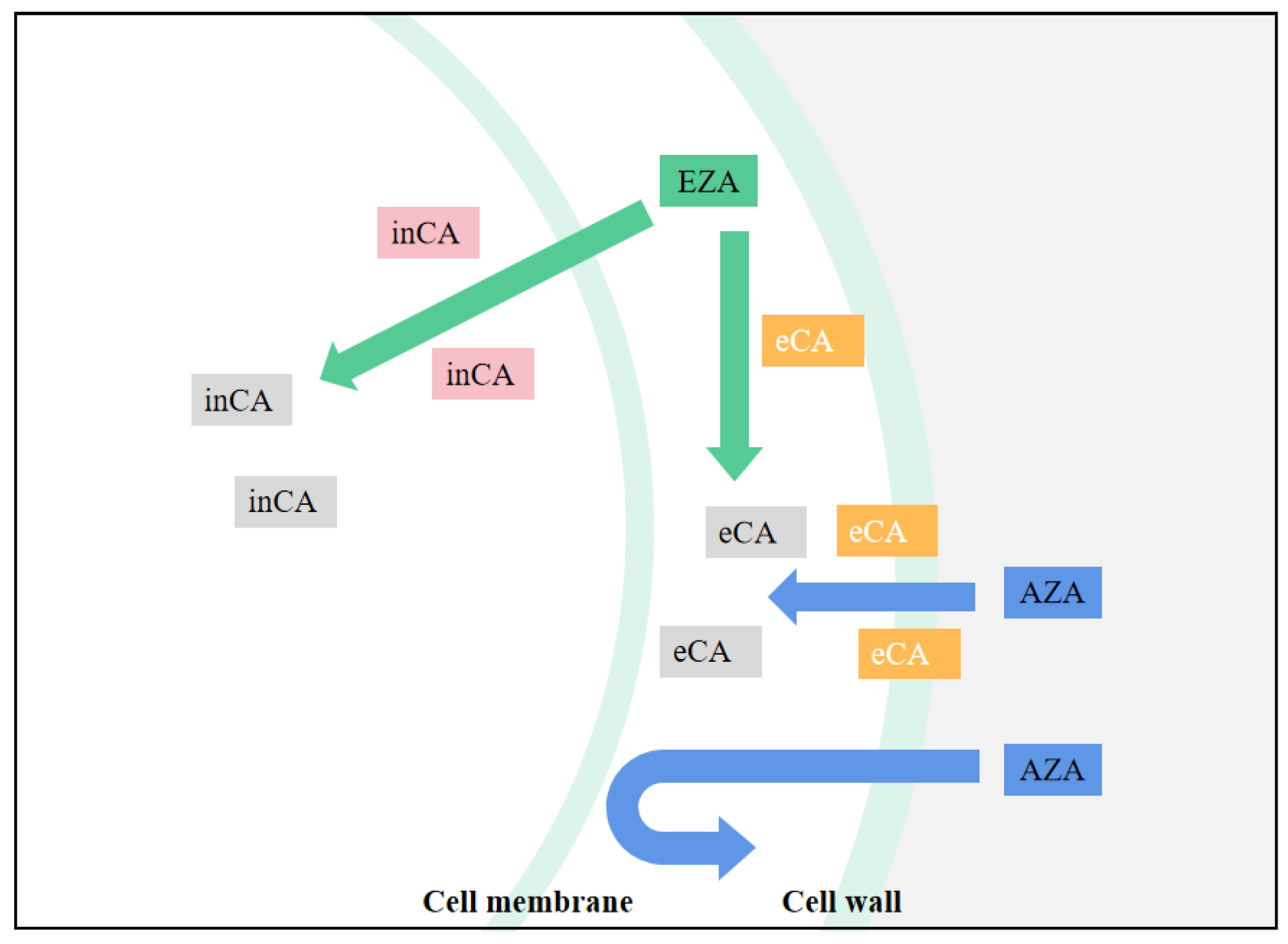
3.2. Effects of CA Inhibitors
3.3. Using HCO3− as Photosynthetic Substrate
3.4. Changes in Affinity to External Ci Depends on Growth Ci Conditions
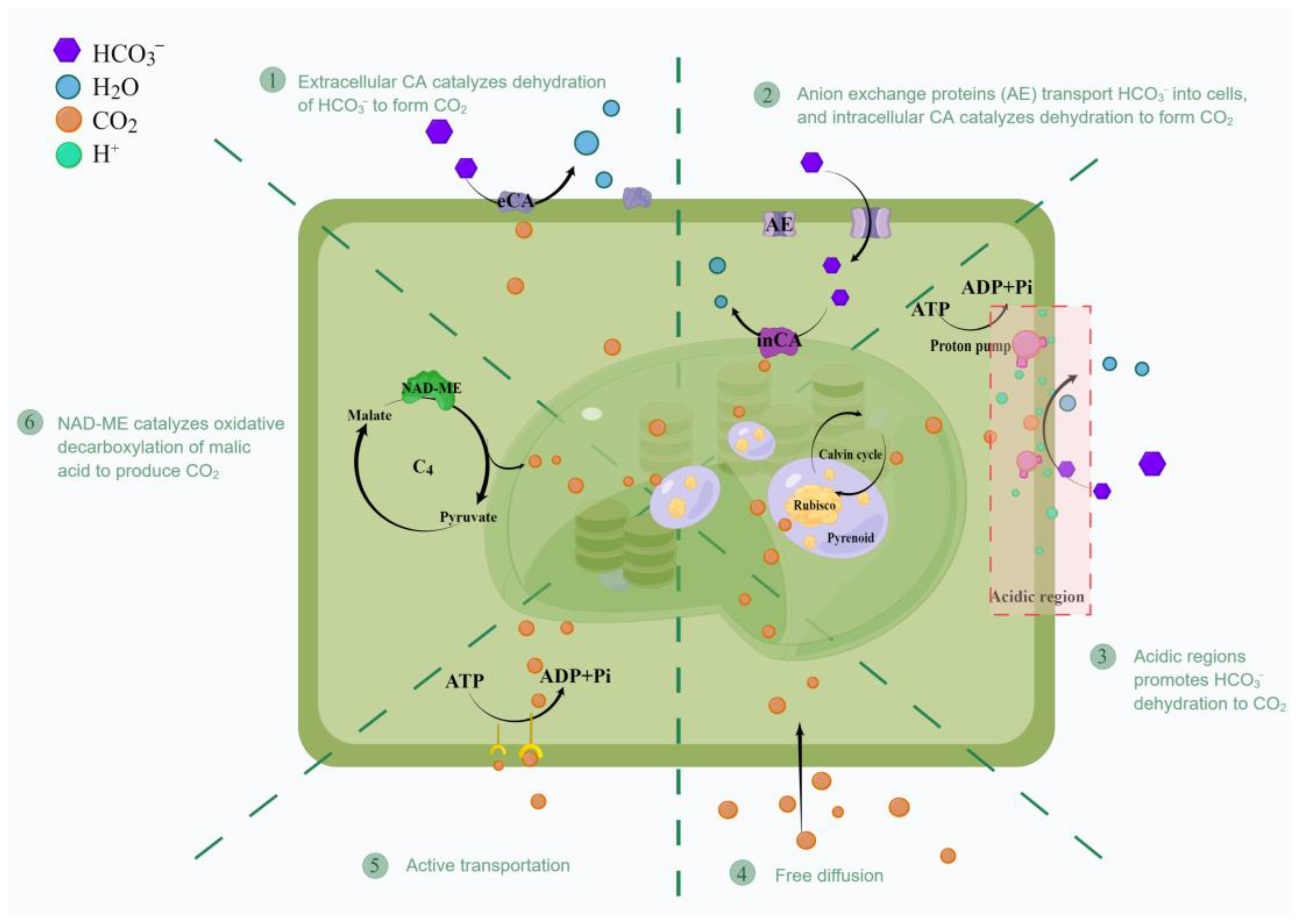
4. Operation of Ulva sp. CCM
5. CCM Gene of Ulva sp.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Björk, M.; Haglund, K.; Ramazanov, Z.; Pedersén, M. Inducible mechanisms for HCO3− utilization and repression of photorespiration in protoplasts and thalli of three species of Ulva (Chlorophyta). J. Phycol. 1993, 29, 166–173. [Google Scholar] [CrossRef]
- Yue, G.; Wang, J.; Zhu, M.; Zhou, B. Progress of inorganic carbon acquisition by algae (I): Origen and methods of the studies. Mar. Sci. 2003, 27, 15–18. (In Chinese) [Google Scholar]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Q.; Valiela, I.; Anderson, D.M.; Keesing, J.K.; Gao, K.; Zhen, Y.; Sun, X.; Wang, Y. Role of C4 carbon fixation in Ulva prolifera, the macroalga responsible for the world’s largest green tides. Commun. Biol. 2020, 3, 494. [Google Scholar] [CrossRef]
- Feng, Z.; Meng, Y.; Lu, W.; Chen, Q.; Yu, K.; Cai, C.; Huo, Y.; Wu, W.; Wei, H.; He, P. Studies on photosynthesis carbon fixation and ocean acidification prevention in Ulva prolifera Ⅰ.Rate of photosynthesis carbon fixation and seawater pH increase. Acta Oceanol. Sin. 2012, 34, 162–168. (In Chinese) [Google Scholar]
- Sand-Jensen, K.; Gordon, D. Differential ability of marine and freshwater macrophytes to utilize HCO3− and CO2. Mar. Biol. 1984, 80, 247–253. [Google Scholar] [CrossRef]
- Gao, G.; Liu, W.; Zhao, X.; Gao, K. Ultraviolet Radiation Stimulates Activity of CO2 Concentrating Mechanisms in a Bloom-Forming Diatom Under Reduced CO2 Availability. Front. Microbiol. 2021, 12, 651567. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, S.; Li, H.; Wu, S.; Gu, W. Enzyme activities suggest that the NAD-ME C4 type CCM exist in Ulva sp. Algal Res. 2020, 47, 101809. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, X.; Shi, S.; Bi, Y.; Moejes, F.W. Comparative transcriptome analysis of four co-occurring Ulva species for understanding the dominance of Ulva prolifera in the Yellow Sea green tides. J. Appl. Phycol. 2019, 31, 3303–3316. [Google Scholar] [CrossRef]
- Huan, L.; Gu, W.; Gao, S.; Wang, G. Photosynthetic activity and proteomic analysis highlights the utilization of atmospheric CO2 by Ulva prolifera (Chlorophyta) for rapid growth. J. Phycol. 2016, 52, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, H.; Yue, Y.; Hu, Y.; Zhai, W.; Xue, L.; Jiao, N.; Zhang, Y. Legacy effects of late macroalgal blooms on dissolved inorganic carbon pool through alkalinity enhancement in coastal ocean. Environ. Sci. Technol. 2023, 57, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, K. Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol. 2012, 160, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Meyer, M.T.; Rickaby, R.E.M. Overcoming adversity through diversity: Aquatic carbon concentrating mechanisms. J. Exp. Bot. 2017, 68, 3689–3695. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Beardall, J. The ins and outs of CO2. J. Exp. Bot. 2016, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Raven, J.A. Cyanobacteria vs green algae: Which group has the edge? J. Exp. Bot. 2017, 68, 3697–3699. [Google Scholar] [CrossRef]
- Meyer, M.; Griffiths, H. Origins and diversity of eukaryotic CO2-concentrating mechanisms: Lessons for the future. J. Exp. Bot. 2013, 64, 769–786. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Narendra, K.; Ragalatha, R.; Rao, B.J. Elucidation and genetic intervention of CO2 concentration mechanism in Chlamydomonas reinhardtii for increased plant primary productivity. J. Biosci. 2020, 45, 115. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Fei, X.; Wright, D.A.; Spalding, M.H. Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii. Plant J. Cell Mol. Biol. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Ohnishi, N.; Mukherjee, B.; Tsujikawa, T.; Yanase, M.; Nakano, H.; Moroney, J.V.; Fukuzawa, H. Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga, Chlamydomonas reinhardtii. Plant Cell 2010, 22, 3105–3117. [Google Scholar] [CrossRef]
- Kono, A.; Spalding, M.H. LCI1, a Chlamydomonas reinhardtii plasma membrane protein, functions in active CO2 uptake under low CO2. Plant J. Cell Mol. Biol. 2020, 102, 1127–1141. [Google Scholar] [CrossRef]
- Wang, Y.; Spalding, M.H. Acclimation to very low CO2: Contribution of limiting CO2 inducible proteins, LCIB and LCIA, to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 2040–2050. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lau, C.S.; Walker, C.E.; Rai, A.K.; Prejean, C.I.; Yates, G.; Thomas, E.M.; Spencer, G.L.; David, J.V.; Mackinder, L.C.M.; et al. Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 16915–16920. [Google Scholar] [CrossRef]
- Santhanagopalan, I.; Wong, R.; Mathur, T.; Griffiths, H. Orchestral manoeuvres in the light: Crosstalk needed for regulation of the Chlamydomonas carbon concentration mechanism. J. Exp. Bot. 2021, 72, 4604–4624. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z. Study on Ecophysiological Characteristics and Transcriptome of Enteromorpha prolifera. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2010. [Google Scholar]
- Rautenberger, R.; Fernández, P.A.; Strittmatter, M.; Heesch, S.; Cornwall, C.E.; Hurd, C.L.; Roleda, M.Y. Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida (Chlorophyta). Ecol. Evol. 2015, 5, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, N.; Liang, C.; Mou, S.; Xiao, F.; Xu, J.; Xu, D.; Zhuang, Z. De novo sequencing and analysis of the Ulva linza transcriptome to discover putative mechanisms associated with its successful colonization of coastal ecosystems. BMC Genom. 2012, 13, 565. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Fabre, N.; Reiter, I.M.; Becuwe-Linka, N.; Genty, B.; Rumeau, D. Characterization expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant Cell Environ. 2007, 30, 617–629. [Google Scholar] [CrossRef]
- Moroney, J.V.; Ma, Y.; Frey, W.D.; Fusilier, K.A.; Pham, T.T.; Simms, T.A.; DiMario, R.J.; Yang, J.; Mukherjee, B. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosyn. Res. 2011, 109, 133–149. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant carbonic anhydrases: Structures, locations, evolution, and physiological roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef]
- Yu, J.W.; Price, G.D.; Song, L.; Badger, M.R. Isolation of a putative carboxysomal carbonic anhydrase gene from the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1992, 100, 794–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- So, A.K.; Espie, G.S.; Williams, E.B.; Shively, J.M.; Heinhorst, S.; Cannon, G.C. A novel evolutionary lineage of carbonic anhydrase (epsilon class) is a component of the carboxysome shell. J. Bacteriol. 2004, 186, 623–630. [Google Scholar] [CrossRef]
- de Araujo, C.; Arefeen, D.; Tadesse, Y.; Long, B.M.; Price, G.D.; Rowlett, R.S.; Kimber, M.S.; Espie, G.S. Identification and characterization of a carboxysomal γ-carbonic anhydrase from the cyanobacterium Nostoc sp. PCC 7120. Photosynth. Res. 2014, 121, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Archibald, J.M.; Weber, A.P.; Reyes-Prieto, A. How do endosymbionts become organelles? Understanding early events in plastid evolution. Bioessays 2007, 29, 1239–1246. [Google Scholar] [CrossRef]
- Fujiwara, S.; Fukuzawa, H.; Tachiki, A.; Miyachi, S. Structure and Differential Expression of 2 Genes Encoding Carbonic Anhydrase in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 9779–9783. [Google Scholar] [CrossRef]
- Rawat, M.; Moroney, J.V. Partial characterization of a new isoenzyme of carbonic anhydrase isolated from Chlamydomonas reinhardtii. J. Biol. Chem. 1991, 266, 9719–9723. [Google Scholar] [CrossRef]
- Tachiki, A.; Fukuzawa, H.; Miyachi, S. Characterization of Carbonic Anhydrase Isozyme CA2, Which Is the CAH2 Gene Product, in Chlamydomonas reinhardtii. Biosci. Biotech. Biochem. 1992, 56, 794–798. [Google Scholar] [CrossRef]
- De Clerck, O.; Kao, S.M.; Bogaert, K.A.; Blomme, J.; Foflonker, F.; Kwantes, M.; Vancaester, E.; Vanderstraeten, L.; Aydoqdu, E.; Boesqer, J.; et al. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 2018, 28, 2921–2933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Wang, M.; Moejes, F.; Bi, Y. Characterization and transcriptional analysis of one carbonic anhydrase gene in the green-tide-forming alga Ulva prolifera (Ulvophyceae, Chlorophyta). Phycol. Res. 2020, 68, 90–97. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Shi, S.; Bi, Y.; Chen, N. Molecular cloning and transcriptional regulation of two γ-carbonic anhydrase genes in the green macroalga Ulva prolifera. Genetica 2021, 149, 63–72. [Google Scholar] [CrossRef]
- Lin, M.T.; Stone, W.D.; Chaudhari, V.; Hanson, M.R. Small subunits can determine enzyme kinetics of tobacco Rubisco expressed in Escherichia coli. Nat. Plants 2020, 6, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.J.; Dhandapani, R. Study of ribulose 1,5-bisphosphate carboxylase from Sulfobacillus acidophilus strain NY-1 isolated from Lignite Mines. J. Environ. Sci. Nat. Res. 2020, 18, 356–362. [Google Scholar] [CrossRef]
- Burlacot, A.; Dao, O.; Auroy, P.; Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef]
- Mei, Y.; Li, H.; Xie, J.; Luo, H. Ribulose-1,5-bisphosphate Carboxylase/oxygenase (Rubisco). Plant Physiol. Commun. 2007, 43, 363–368. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, K.; Bao, P.; Bao, Q.; Jia, Y.; Huang, F.; Su, J.; Zhu, Y. Quantitative analyses of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) large-subunit genes (cbbL) in typical paddy soils. FEMS Microbio. Ecol. 2014, 87, 89–101. [Google Scholar] [CrossRef]
- Badger, M.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Price, G.D. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplastbased CO2-concentrating mechanisms in algae. Can. J. Bot. 1998, 76, 1052–1071. [Google Scholar] [CrossRef]
- Meyer, M.T.; Goudet, M.M.M.; Griffiths, H. The Algal Pyrenoid. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms, Advances in Photosynthesis and Respiration 45; Larkum, A., Grossman, A., Raven, J., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 45, pp. 179–203. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Milo, R. The global mass and average rate of Rubisco. Proc. Natl Acad. Sci. USA 2019, 116, 4738–4743. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Wilson, A.T.; Mangan, N.M.; Wingreen, N.S.; Jonikas, M.C. Modelling the pyrenoid-based CO2-concentrating mechanism provides insights into its operating principles and a roadmap for its engineering into crops. Nat. Plants 2022, 8, 583–595. [Google Scholar] [CrossRef]
- Kevekordes, K.; Holland, D.; Häubner, N.; Jenkins, S.; Koss, R.; Roberts, S.; Raven, J.; Scrimgeour, C.; Shelly, R.; Stojkovic, S.; et al. Inorganic carbon acquisition by eight species of Caulerpa (Caulerpaceae, Chlorophyta). Phycologia 2006, 45, 442–449. [Google Scholar]
- Raven, J.A. Inorganic carbon acquisition by eukaryotic algae: Four current questions. Photosynth. Res. 2010, 106, 123–134. [Google Scholar] [CrossRef]
- Maberly, S.C.; Ball, L.; Raven, J.A.; Sültemeyer, D.F. Inorganic carbon acquisition by chrysophytes. J. Phycol. 2009, 45, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Ding, L.; Luv, Q. Microscopic observation of pyrenoids in Order Ulvales (Chlorophyta) collected from Qingdao coast. J. Ocean Univ. China 2011, 10, 223–228. [Google Scholar] [CrossRef]
- Meyer, M.T.; Whittaker, C.; Griffiths, H. The algal pyrenoid: Key unanswered questions. J. Exp. Bot. 2017, 68, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Beardall, J.; Giordano, M. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth. Res. 2014, 121, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Genkov, T.N.; Skepper, J.N.; Jouhet, J.; Mitchell, M.C.; Spreitzer, R.J.; Griffiths, H. Rubisco small-subunit α-helices control pyrenoid formation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2012, 109, 19474–19479. [Google Scholar] [CrossRef]
- Morita, E.; Abe, T.; Tsuzuki, M.; Fujiwara, S.; Sato, N.; Hirata, A.; Sonoike, K.; Nozaki, H. Presence of the CO2-concentrating mechanism in some species of the pyrenoid-less free-living algal genus Chloromonas (Volvocales, Chlorophyta). Planta 1998, 204, 69–276. [Google Scholar] [CrossRef]
- He, P.; Wu, Q.; Wu, W.; Lu, W.; Zhang, D.; Chen, G.; Zhang, R. Pyrenoid ultrastructure and molecular localization of Rubisco activase in Enteromorpha clathrata. Shuichan Xuebao 2004, 28, 255–260. (In Chinese) [Google Scholar] [CrossRef]
- Villarejo, A.; Martinez, F.; Pino Plumed, M. The induction of CO2 concentrating mechanism in starch-lessnmytant of Chlamydomonas reinhardtii. Physiol. Plantarum 1996, 98, 798–802. [Google Scholar] [CrossRef]
- Cai, C.; Yin, S.; Sun, Z.; Shan, M.; Wang, Q.; Huo, Y.; He, P. Effect of CO2 concentration on Rubisco concentrating in pyrenoids from Enterwomorpha clathrata. Biotech. Bull. 2009, S1, 271–276. (In Chinese) [Google Scholar] [CrossRef]
- Niu, S.; Jiang, G.; Li, Y. Environmental regulations of C3 and C4 plants. Sheng Tai Xue Bao 2004, 2, 308–314. (In Chinese) [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. Models of photosynthesis. Plant Physiol. 2001, 125, 42–45. [Google Scholar] [CrossRef]
- Zhu, X.; Long, S.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass. Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, S.; Yang, B. Transformation of genes of C4 photosynthetic key enzyme into C3 plants. Plant Physiol. Commun. 2008, 44, 187–193. [Google Scholar] [CrossRef]
- Dengler, N.G.; Dengler, R.E.; Donnelly, P.M.; Hattersley, P.W. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): Bundle sheath and mesophyll surface area relationships. Ann. Bot. 1994, 73, 241–255. [Google Scholar] [CrossRef]
- Sage, R.F. C4 photosynthesis in terrestrial plants does not require Kranz anatomy. Trends Plant Sci. 2002, 7, 283–285. [Google Scholar] [CrossRef]
- Xu, J.F.; Zhang, X.; Ye, N.; Zheng, Z.; Mou, S.; Dong, M.; Xu, D.; Miao, J. Activities of principal photosynthetic enzymes in green macroalga Ulva linza: Functional implication of C4 pathway in CO2 assimilation. Sci. China Life Sci. 2013, 56, 571–580. [Google Scholar] [CrossRef]
- Lilley, R.M.; Walker, D.A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975, 55, 1087–1092. [Google Scholar] [CrossRef]
- Capó-Bauçà, S.; Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Correlative adaptation between Rubisco and CO2-concentrating mechanisms in seagrasses. Nat. Plants 2022, 8, 706–716. [Google Scholar] [CrossRef]
- Beer, S.; Israel, A.; Drechsler, Z.; Cohen, Y. Photosynthesis in Ulva fasciata. V. Evidence for an inorganic carbon concentrating system, and ribulose-1,5-bisphosphate carboxylase/oxygenase CO2 kinetics. Plant Physiol. 1990, 94, 1542–1546. [Google Scholar] [CrossRef][Green Version]
- Axelsson, L.; Ryberg, H.; Beer, S. Two modes of bicarbonate utilization in the marine green macroalga Ulva lactuca. Plant Cell Environ. 1995, 18, 439–445. [Google Scholar] [CrossRef]
- Gao, G.; Liu, Y.; Li, X.; Feng, Z.; Xu, J. An ocean acidification acclimatised green tide alga is robust to changes of seawater carbon chemistry but vulnerable to light stress. PLoS ONE 2016, 11, e0169040. [Google Scholar] [CrossRef]
- Xu, J.T.; Wang, X.; Zhong, Z.; Yao, D. The mechanism of the characters of inorganic carbon acquisition to temperature in two Ulva species. Sheng Tai Xue Bao 2013, 33, 7892–7897. (In Chinese) [Google Scholar] [CrossRef]
- Lucas, W.J. Photosynthetic assimilation of exogenous HCO3− by aquatic plants. Annu. Rev. Plant Physiol. 1983, 34, 71–104. [Google Scholar] [CrossRef]
- Badger, M.R. The CO2-concentrating mechanism in aquatic phototrophs. Photosynthesis 1987, 10, 219–274. [Google Scholar] [CrossRef]
- Johnston, A.M. The acquisition of inorganic carbon by marine macroalgae. Can. J. Bot. 1991, 69, 1123–1132. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol. Plant 1992, 84, 606–615. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Israel, A.; Hophy, M. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biol. 2002, 8, 831–840. [Google Scholar] [CrossRef]
- Badger, M.R. The role of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth. Res. 2003, 77, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.S.; Bowes, G.; Ross, C.; Zhang, X. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Mercado, J.M.; Gordillo, F.J.; Figueroa, F.L.; Niell, F.X. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. J. Exp. Mar. Biol. Ecol. 1998, 221, 209–220. [Google Scholar] [CrossRef]
- Gao, K.; Aruga, Y.; Asada, K.; Kiyohara, M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. And G. chilensis. J. Appl. Phycol. 1993, 5, 563–571. [Google Scholar] [CrossRef]
- Xu, Z.; Zou, D.; Gao, K. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot. Mar. 2010, 53, 123–129. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Hepburn, C.D.; Pritchard, D.; Currie, K.I.; McGraw, C.M.; Hunter, K.A.; Hurd, C.L. Carbon-Use Strategies in Macroalgae: Differential Responses to Lowered pH and Implications for Ocean Acidification. J. Phycol. 2012, 48, 137–144. [Google Scholar] [CrossRef]
- García-Sánchez, M.J.; Fernández, J.A.; Niell, X. Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 1994, 194, 55–61. [Google Scholar] [CrossRef]
- Mercado, J.M.; Niell, F.X.; Figueroa, F.L. Regulation of the mechanism for HCO3− use by the inorganic carbon level in Porphyra leucosticta Thur in le Jolis (Rhodophyta). Planta 1997, 201, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.M.; Raven, J.A. Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus. Br. Phycol. J. 1990, 25, 75–82. [Google Scholar] [CrossRef]
- Karekar, M.; Joshi, G. Photosynthetic Carbon Metabolism in Marine Algae. Bot. Mar. 1973, 16, 216–220. [Google Scholar] [CrossRef]
- Colman, B. The effect of temperature and oxygen on the CO2 compensation point of the marine alga Ulva lactuca. Plant Cell Environ. 1984, 7, 619–621. [Google Scholar] [CrossRef]
- Beer, S.; Israel, A. Photosynthesis of Ulva sp: III. O(2) Effects, Carboxylase Activities, and the CO(2) Incorporation Pattern. Plant Physiol. 1986, 81, 937–938. [Google Scholar] [CrossRef]
- Lu, D. Progress on photosynthetic carbon metabolism types in marine macroalgae. Chin. J. Nat. 2013, 35, 264–273. (In Chinese) [Google Scholar]
- Xu, J.; Fan, X.; Zhang, X.; Xu, D.; Mou, S.; Cao, S.; Zheng, Z.; Miao, J.; Ye, N. Evidence of coexistence of C3 and C4 photosynthetic pathways in a green-tide-forming alga, Ulva prolifera. PLoS ONE 2012, 7, e37438. [Google Scholar] [CrossRef] [PubMed]
- Reidenbach, L.B.; Fernández, P.A.; Leal, P.P.; Noisette, F.; McGraw, C.M.; Revill, A.T.; Hurd, C.L.; Kübler, J.E. Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS ONE 2017, 12, e0188389. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, E.J.; Edwards, M.S.; Lee, K.; Jeong, H.J.; Kim, K.Y. Species-specific responses of temperate macroalgae with different photosynthetic strategies to ocean acidification: A mesocosm study. Algae 2016, 31, 243–256. [Google Scholar] [CrossRef]
- Kang, J.W.; Chung, I.K. The effects of eutrophication and acidification on the ecophysiology of Ulva pertusa Kjellman. J. Appl. Phycol. 2017, 29, 2675–2683. [Google Scholar] [CrossRef]
- Liu, C.; Zou, D. Responses of elevated CO2 on photosynthesis and nitrogen metabolism in Ulva lactuca (Chlorophyta) at different temperature levels. Mar. Biol. Res. 2015, 11, 1043–1052. [Google Scholar] [CrossRef]
- Gao, G.; Beardall, J.; Bao, M.; Wang, C.; Ren, W. Ocean acidification and nutrient limitation synergistically reduce growth and photosynthetic performances of a green tide alga Ulva linza. Biogeosciences 2018, 15, 3409–3420. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Ma, J.; Zhang, X.; Fan, X.; Zhang, Y.; Wang, W.; Sun, K.; Ye, N. Elevated CO2 accelerated the bloom of three Ulva species after one life cycle culture. J. Appl. Phycol. 2021, 33, 3963–3973. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; He, P. Comparative research on inorganic carbon acquisition by the macroalgae Ulva prolifera (Chlorophyta) and Pyropia yezoensis (Rhodophyta). J. Appl. Phycol. 2016, 28, 491–497. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.L.; Zheng, M.; Zhuo, P.L.; Xu, N. Photoperiod mediates the effects of elevated CO2 on the growth and physiological performance in the green tide alga Ulva prolifera. Mar. Environ. Res. 2018, 141, 24–29. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Niell, F.X.; Figueroa, F.L. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Biomed Life Sci. 2001, 213, 64–70. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Figueroa, F.L.; Niell, F.X. Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 2003, 218, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Björk, M.; Haglund, K.; Ramazanov, Z.; Garcia-Reina, G.; Pedersén, M. Inorganic-carbon assimilation in the green seaweed Ulva rigida C. Ag. (Chlorophyta). Planta 1992, 187, 152–156. [Google Scholar] [CrossRef]
- Drechsler, Z.; Beer, S. Utilization of Inorganic Carbon by Ulva lactuca. Plant Physiol. 1991, 97, 1439–1444. [Google Scholar] [CrossRef]
- Drechsler, Z.; Sharkia, R.; Cabantchik, Z.I.; Beer, S. Bicarbonate uptake in the marine macroalga Ulva sp. is inhibited by classical probes of anion exchange by red blood cells. Planta 1993, 191, 34–40. [Google Scholar] [CrossRef]
- Jennings, M.L. Kinetics and mechanism of anion transport in red blood cells. Annu. Rev. Physiol. 1985, 47, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Sharkia, R.; Beer, S.; Cabantchik, Z.I. A membrane-located polypeptide of Ulva sp. which may be involved in HCO3− uptake is recognized by antibodies raised against the human red-blood-cell anion-exchange protein. Planta 1994, 194, 247–249. [Google Scholar] [CrossRef]
- Hellblom, F.; Axelsson, L. External HCO3− dehydration maintained by acid zones in the plasma membrane is an important component of the photosynthetic carbon uptake in Ruppia cirrhosa. Photosynth. Res. 2003, 77, 173–181. [Google Scholar] [CrossRef]
- Hellblom, F.; Beer, S.; Bjork, M.; Axelsson, L. A buffer sensitive inorganic carbon utilisation system in Zostera marina. Aquat. Bot. 2001, 69, 55–62. [Google Scholar] [CrossRef]
- Mercado, J.M.; Niell, F.X.; Silva, J.; Santos, R. Use of light and inorganic carbon acquisition by two morphotypes of Zostera noltii Hornem. J. Exp. Mar. Bio. Ecol. 2003, 297, 71–84. [Google Scholar] [CrossRef]
- Mercado, J.M.; Andría, J.R.; Pérez-Lloréns, J.L.; Vergara, J.J.; Axelsson, L. Evidence for a plasmalemma-based CO2 concentrating mechanism in Laminaria saccharina. Photosynth. Res. 2006, 88, 259–268. [Google Scholar] [CrossRef]
- Axelsson, L.; Mercado, J.M.; Figueroa, F.L. Utilization of HCO3− at high pH by the brown macroalga Laminaria saccharina. Eur. J. Phycol. 2000, 35, 53–59. [Google Scholar] [CrossRef]
- Larsson, C.; Axelsson, L. Bicarbonate uptake and utilization in marine macroalgae. Br. Phycol. Bull. 1999, 34, 79–86. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Wang, G. Expression analysis of phosphoenolpyruvate carboxykinase in Porphyra haitanensis (Rhodophyta) sporophytes and gametophytes. Phycol. Res. 2013, 61, 172–179. [Google Scholar] [CrossRef]
- Priyam, A.; Woodcroft, B.; Rai, V.; Munagala, A.; Moghul, I.; Ter, F.; Gibbins, M.A.; Moon, H.; Leonard, G.; Rumpf, W.; et al. Sequenceserver: A Modern Graphical User Interface for Custom BLAST Databases, Mol. Biol. Evol. 2015, 36, 2922–2924. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Fan, B.; Miao, G. Research Progress on the CO2 Concentrating Mechanism and Its Regulation in Chlamydomonas. J. Anhui. Agric. Sci. 2021, 49, 7. (In Chinese) [Google Scholar]
- Kaplan, A.; Ronen-Tarazi, M.; Tchernov, D.; Bonfil, D.J.; Zer, H.; Schatz, D.; Vardi, A.; Hassidim, M.; Reinhold, L. The inorganic carbon-concentrating mechanism in cyanobacteria: Induction and ecological significance. Can. J. Bot. 1998, 76, 917–924. [Google Scholar] [CrossRef]
- Toyokawa, C.; Yamano, T.; Fukuzawa, H. Pyrenoid Starch Sheath Is Required for LCIB Localization and the CO2-Concentrating Mechanism in Green Algae. Plant Physiol. 2020, 182, 1883–1893. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D.; Long, B.M.; Woodger, F.J. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 2006, 57, 249–265. [Google Scholar] [CrossRef]
- Duanmu, D.; Miller, A.R.; Horken, K.M.; Weeks, D.P.; Spalding, M.H. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3− transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2009, 106, 5990–5995. [Google Scholar] [CrossRef]
- Moulin, P.; Andría, J.R.; Axelsson, L.; Mercado, J.M. Different mechanisms of inorganic carbon acquisition in red macroalgae (Rhodophyta) revealed by the use of TRIS buffer. Aquat. Bot. 2011, 95, 31–38. [Google Scholar] [CrossRef]
- Stepien, C.C. Impacts of geography, taxonomy and functional group on inorganic carbon use patterns in marine macrophytes. J. Ecol. 2015, 103, 1372–1383. [Google Scholar] [CrossRef]



| Species | References |
|---|---|
| Ulva australis | [95] |
| U. pertusa | [96,97] |
| U. lactuca | [98] |
| U. linza | [68,99,100] |
| U. prolifera | [4,74,100,101,102] |
| U. rigida | [1,103,104] |
| U. compressa | [100] |
| U. pulchra | [1] |
| U. reticulata | [1] |
| Protein | NCBI Accession Number | Copy Number in U. prolifera genome | References |
|---|---|---|---|
| alpha carbonic anhydrases | CAH1 [BAA14232] | 5 | [24,25,118] |
| CAH2 [CAA38360.1] | 5 | ||
| CAH3 [EDP00852.1] | 5 | ||
| beta carbonic anhydrases | CAH6 [AAR82947.1] | 0 | |
| CAH8 [ABS87675.1] | 0 | ||
| gamma carbonic anhydrase | CAG2 [XP_001701594] | 3 | |
| carboxysomal-located carbonic anhydrase | ccaA/icfA [P27134.1] | 0 | [119] |
| phosphoribosyl aminoimidazole carboxylase | purK [AAB05791] | 1 | |
| NADH dehydrogenase | ndhB [CAA46161.1] | 0 | |
| nuclear transcriptional regulators of CCM elements | CIA5 [AAG37909.1] | 2 | [24,25,118,120] |
| CIA5 [AF317732_1] | 2 | ||
| LCR1 [BAD13492.1] | 1 | ||
| low-CO2-inducible proteins | LCIA [BAD16681.1] | 1 | |
| LCIB [BAD16682.1] | 3 | ||
| LCIB [EDP04243.1] | 3 | ||
| LCIC [BAD16683.1] | 3 | ||
| Lci2 [AAC31958.1] | 1 | ||
| low-CO2-inducible membrane protein | [KAF5834422.1] | 1 | |
| LCIA [XP_001703387.1] | 0 | ||
| chloroplast carrier protein 1 | CCP1 [EDP04147.1] | 27 | [25,118] |
| chloroplast proton extrusion protein | CemA [XP_001696592] | 1 | |
| pyruvate orthophosphate dikinase | PPDK [JN222388.1] | 4 | [68] |
| PPDK [JN936854.1] | 2 | ||
| ribulose-1, 5-biphosphate carboxylase | RuBPCase [AAR19268.1] | 2 | |
| high and medium affinity HCO3− transporters | SbtA [UOW71290.1] | 0 | [24,121] |
| BicA [Q14SY0.1] | 1 | ||
| putative ABC transporter/high light-activated 3 | MRP1/HLA3 [AAL35383.1] | 26 | [25,118,122] |
| HLA3 [XP_001700040.1] | 26 | ||
| plasma membrane-type H+-ATPase | [AQM50087.1] | 12 | [73,123,124] |
| [P19456.2] | 7 | ||
| bestrophin-like protein | BSTs [NP_191691.2] | 7 | [22] |
| proton gradient regulation 5 | PGR5 [OAP09444.1] | 1 | [44] |
| proton gradient regulation like protein | PGRL1 [XP_001692513.1] | 1 | |
| flavodiiron protein B | FlvB [AMJ52190.1] | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhao, C.; Zhao, S.; Dai, W.; Liu, J.; Zhang, J.; Xu, J.; He, P. Diversity of CO2 Concentrating Mechanisms in Macroalgae Photosynthesis: A Case Study of Ulva sp. J. Mar. Sci. Eng. 2023, 11, 1911. https://doi.org/10.3390/jmse11101911
Sun J, Zhao C, Zhao S, Dai W, Liu J, Zhang J, Xu J, He P. Diversity of CO2 Concentrating Mechanisms in Macroalgae Photosynthesis: A Case Study of Ulva sp. Journal of Marine Science and Engineering. 2023; 11(10):1911. https://doi.org/10.3390/jmse11101911
Chicago/Turabian StyleSun, Jingyi, Chunyan Zhao, Shuang Zhao, Wei Dai, Jinlin Liu, Jianheng Zhang, Juntian Xu, and Peimin He. 2023. "Diversity of CO2 Concentrating Mechanisms in Macroalgae Photosynthesis: A Case Study of Ulva sp." Journal of Marine Science and Engineering 11, no. 10: 1911. https://doi.org/10.3390/jmse11101911
APA StyleSun, J., Zhao, C., Zhao, S., Dai, W., Liu, J., Zhang, J., Xu, J., & He, P. (2023). Diversity of CO2 Concentrating Mechanisms in Macroalgae Photosynthesis: A Case Study of Ulva sp. Journal of Marine Science and Engineering, 11(10), 1911. https://doi.org/10.3390/jmse11101911









