Optimization of Algicidal Activity for Alteromonas sp. FDHY-03 against Harmful Dinoflagellate Prorocentrum donghaiense
Abstract
:1. Introduction
2. Materials and Method
2.1. Algal Cultures, Algicidal Bacterium Culture, and Seed Bacteria Culture
2.2. Measurement of Algicidal Rate and Biomass
2.3. Single-Factor Design
2.4. Plackett–Burman Experimental Design
2.5. Central Composite Design
2.6. Statistical Analysis
3. Result
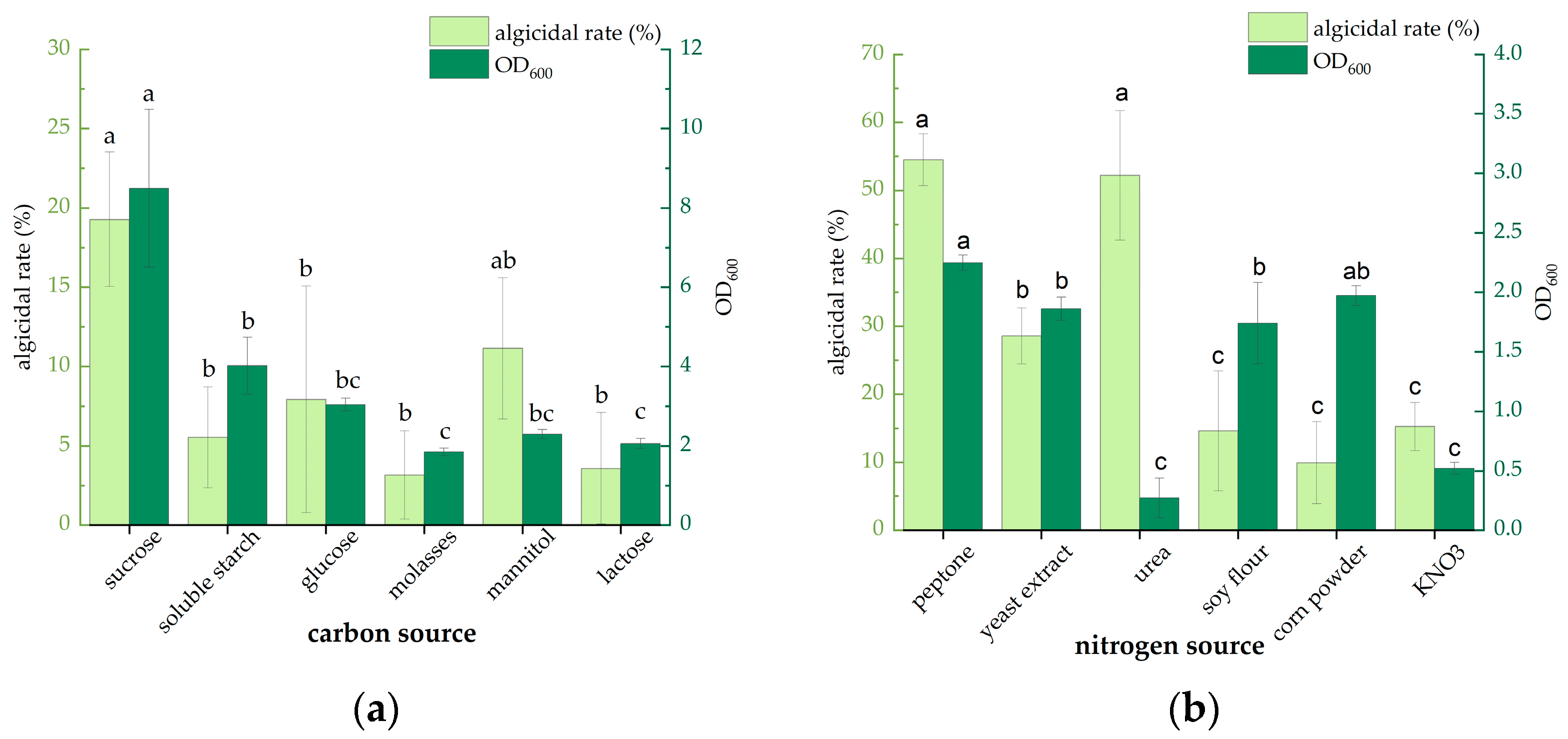
3.1. Optimization of Medium Components on Strain FDHY-03
3.2. Plackett–Burman Design
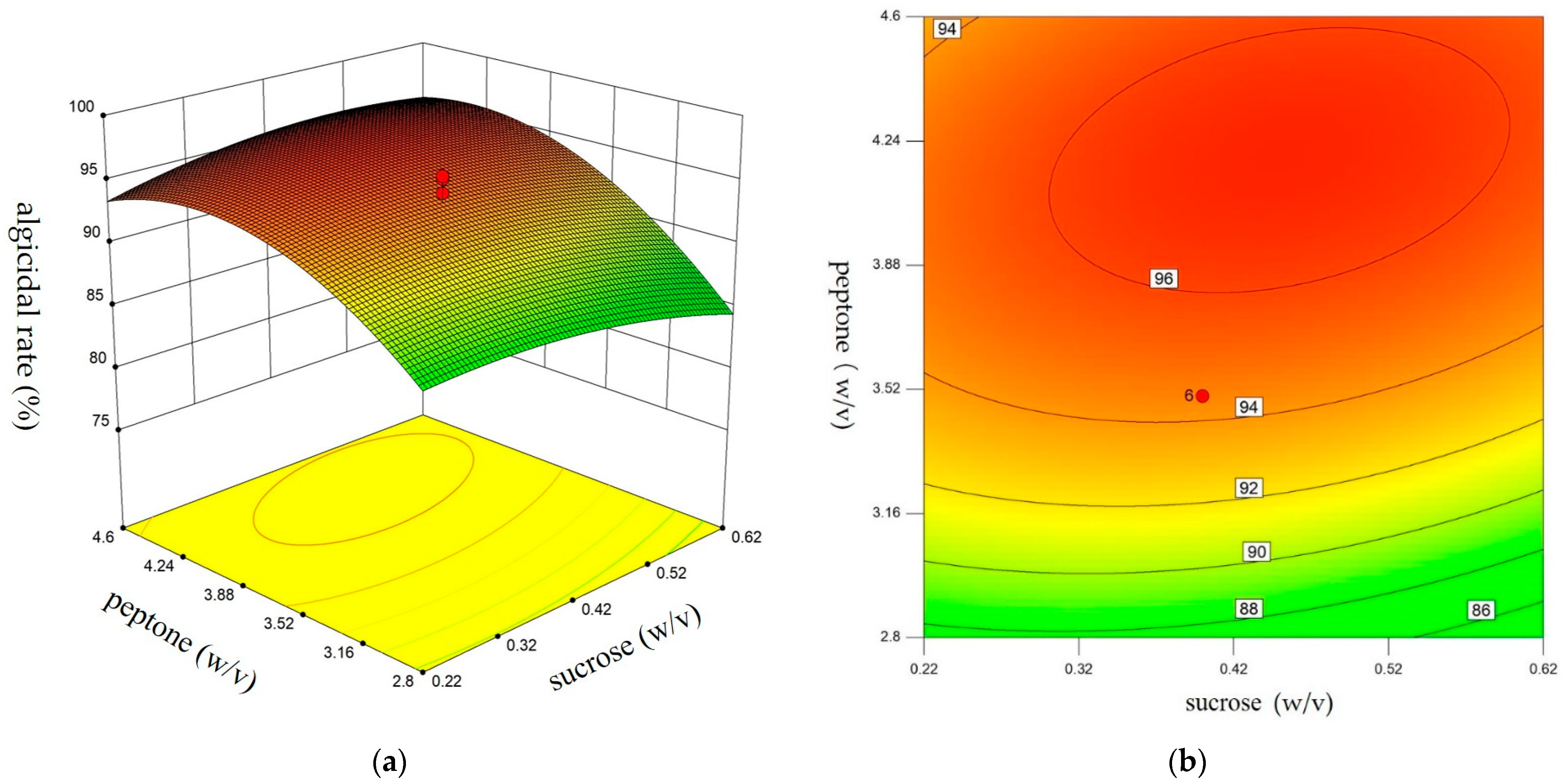
3.3. Central Composite Design
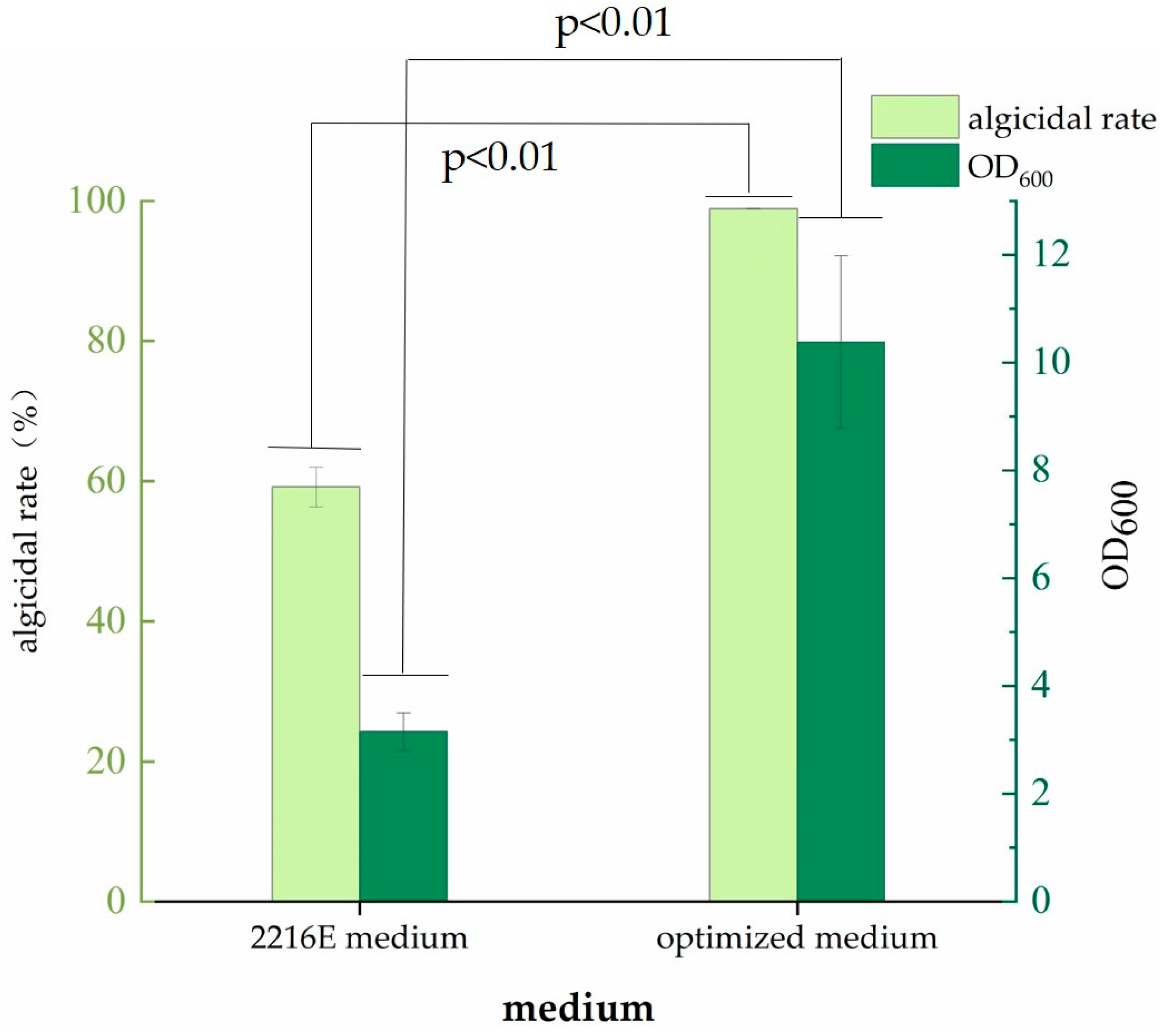
3.4. Verification of the model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Mirza Esmaeili, F.; Mortazavi, M.S.; Dehghan Banadaki, A.; Saraji, F.; Mohebbi Nozar, S.L. Algal blooms historical outbreaks in the northern coastal waters of the Persian Gulf and Oman Sea (1980–2015). Environ. Monit. Assess 2021, 193, 648. [Google Scholar] [CrossRef] [PubMed]
- Mingjiang, Z.; Mingyuan, Z.H.U. Progress of the Project “Ecology and Oceanography of Harmful Algal Blooms in China”. Adv. Earth Sci. 2006, 21, 673–679. [Google Scholar]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manag. 2009, 52, 342. [Google Scholar] [CrossRef] [PubMed]
- Mayali, X.; Azam, F. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004, 51, 139–144. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Guan, C.; Zhang, H.; Guo, J.; Chen, Z.; Cai, G.; Lei, X.; Zheng, W.; Tian, Y.; et al. Towards molecular, physiological, and biochemical understanding of photosynthetic inhibition and oxidative stress in the toxic Alexandrium tamarense induced by a marine bacterium. Appl. Microbiol. Biotechnol. 2014, 98, 4637–4652. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, Y.; Zhang, D.; Chen, S.; Bai, X.; Ma, X.; Xie, Z.; Xu, H. Effect and mechanism of the algicidal bacterium Sulfitobacter porphyrae ZFX1 on the mitigation of harmful algal blooms caused by Prorocentrum donghaiense. Environ. Pollut. 2020, 263, 114475. [Google Scholar] [CrossRef]
- Lyu, Y.H.; Zhou, Y.X.; Li, Y.; Zhou, J.; Xu, Y.X. Optimized culturing conditions for an algicidal bacterium Pseudoalteromonas sp. SP48 on harmful algal blooms caused by Alexandrium tamarense. Microbiologyopen 2019, 8, e00803. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Xie, L.; Liu, Y.; Chen, H.; Feng, J.; Ouyang, L. Identification and Optimization of the Algicidal Activity of a Novel Marine Bacterium against Akashiwo sanguinea. Front. Mar. Sci. 2022, 9, 380. [Google Scholar] [CrossRef]
- Bloh, A.H.; Usup, G.; Ahmad, A. Loktanella spp. Gb03 as an algicidal bacterium, isolated from the culture of Dinoflagellate Gambierdiscus belizeanus. Vet. World 2016, 9, 142–146. [Google Scholar] [CrossRef]
- Kong, Y.; Zou, P.; Miao, L.; Qi, J.; Song, L.; Zhu, L.; Xu, X. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ. Sci. Pollut. Res. Int. 2014, 21, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Song, K.; Li, Y.C.; Chen, J. Statistical culture-based strategies to enhance chlamydospore production by Trichoderma harzianum SH2303 in liquid fermentation. J. Zhejiang Univ. Sci. B 2016, 17, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, W.; Tian, Y.; Wang, G.; Zheng, T. Optimization of culture conditions and medium composition for the marine algicidal bacterium Alteromonas sp. DH46 by uniform design. J. Ocean. Univ. China 2013, 12, 385–391. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Zhang, H.; Chen, Z.; Tian, Y.; Xu, H.; Zheng, T.; Zheng, W. Toxicity of algicidal extracts from Mangrovimonas yunxiaonensis strain LY01 on a HAB causing Alexandrium tamarense. J. Hazard. Mater. 2014, 278, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, X.; Shan, L. Optimization of liquid media and biosafety assessment for algae-lysing bacterium NP23. Can. J. Microbiol. 2014, 60, 593–597. [Google Scholar] [CrossRef]
- Wang, Z.W.; Liu, X.L. Medium optimization for antifungal active substances production from a newly isolated Paenibacillus sp. using response surface methodology. Bioresour. Technol. 2008, 99, 8245–8251. [Google Scholar] [CrossRef]
- Ambati, P.; Ayyanna, C. Optimizing medium constituents and fermentation conditions for citric acid production from palmyra jaggery using response surface method. World J. Microbiol. Biotechnol. 2001, 17, 331–335. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Yonten, V.; Aktas, N. Exploring the optimum conditions for maximizing the microbial growth of Candida intermedia by response surface methodology. Prep. Biochem. Biotechnol. 2014, 44, 26–39. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Optimization of fermentative hydrogen production process using genetic algorithm based on neural network and response surface methodology. Int. J. Hydrogen Energy 2009, 34, 255–261. [Google Scholar] [CrossRef]
- Shi, X.; Liu, L.; Li, Y.; Xiao, Y.; Ding, G.; Lin, S.; Chen, J. Isolation of an algicidal bacterium and its effects against the harmful-algal- bloom dinoflagellate Prorocentrum donghaiense (Dinophyceae). Harmful Algae 2018, 80, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Mo, K.; Li, S.; Dongmei, S.; Zhu, J.; Zou, X.; Hu, Y.; Bao, S. Alteromonas portus sp. nov., an alginate lyase-excreting marine bacterium. Int. J. Syst. Evol. Microbiol. 2020, 70, 1516–1521. [Google Scholar] [CrossRef]
- Lee, B.K.; Katano, T.; Kitamura, S.; Oh, M.J.; Han, M.S. Monitoring of algicidal bacterium, Alteromonas sp. strain A14 in its application to natural Cochlodinium polykrikoides blooming seawater using fluorescence in situ hybridization. J. Microbiol. 2008, 46, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y. Algicidal activity of marine Alteromonas sp. KNS-16 and isolation of active compounds. Biosci. Biotechnol. Biochem. 2012, 76, 1452–1458. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuang, Y.; Chen, H.; Lu, S.; Li, Y.; Ge, R.; Chen, C.; Liu, G. Effects of Prorocentrum donghaiense bloom on zooplankton functional groups in the coastal waters of the East China Sea. Mar. Pollut. Bull. 2021, 172, 112878. [Google Scholar] [CrossRef] [PubMed]
- Dou-Ding, L.; Goebel, J. Five red tide species in genus Prorocentrum including the description of Prorocentrum donghaiense Lu SP. nov. from the East China Sea. Chin. J. Oceanol. Limnol. 2001, 19, 337–344. [Google Scholar] [CrossRef]
- Huang, X.; Huang, B.; Chen, J.; Liu, X. Cellular responses of the dinoflagellate Prorocentrum donghaiense Lu to phosphate limitation and chronological ageing. J. Plankton Res. 2016, 38, 83–93. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Chen, G.F.; Wang, Y.Y.; Guo, C.L.; Zhou, J. Physiological and molecular responses of Prorocentrum donghaiense to dissolved inorganic phosphorus limitation. Mar. Pollut. Bull. 2018, 129, 562–572. [Google Scholar] [CrossRef]
- Hanlin, C.; Songhui, L.U.; Chuansong, Z.; Dedi, Z.H.U. A Survey on the red tide of Prorocentrum donghaiense in East China Sea, 2004. Ecol. Sci. 2006, 25, 226–230. [Google Scholar]
- Landa, M.; Blain, S.; Christaki, U.; Monchy, S.; Obernosterer, I. Shifts in bacterial community composition associated with increased carbon cycling in a mosaic of phytoplankton blooms. ISME J. 2016, 10, 39–50. [Google Scholar] [CrossRef]
- Shen, A.; Ishizaka, J.; Yang, M.; Ouyang, L.; Yin, Y.; Ma, Z. Changes in community structure and photosynthetic activities of total phytoplankton species during the growth, maintenance, and dissipation phases of a Prorocentrum donghaiense bloom. Harmful Algae 2019, 82, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal Bacteria: A Review of Current Knowledge and Applications to Control Harmful Algal Blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef]
- Meyer, N.; Bigalke, A.; Kaulfuss, A.; Pohnert, G. Strategies and ecological roles of algicidal bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gan, C.; Huang, J.; Zou, C.; Li, H.; Liu, J.; Yang, W. Variability of Prorocentrum donghaiense response to allelopathic action from Alexandrium pacificum in laboratory culture. J. Oceanol. Limnol. 2021, 39, 1305–1315. [Google Scholar] [CrossRef]
- Quan, H.; Shan, X.; Dai, F. The Community Structure of the Phytoplankton in the Funing Bay. Prog. Fish. Sci. 2015, 36, 1–7. [Google Scholar]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2016, 7, 2087. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liu, G.; Li, X.; Yang, Q.; Xu, Y.; Hu, Z.; Chen, C.Y.; Chang, J.S. Continuous production of algicidal compounds against Akashiwo sanguinea via a Vibrio sp. co-culture. Bioresour. Technol. 2020, 295, 122246. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Wu, J.; Su, L. Fermentation Optimization of B. stearothermophilus Cyclo-dextrin Glucosyl Transferase Produced by Recombinant Bacillus subtilis. Genom. Appl. Biol. 2020, 39, 629–635. [Google Scholar]
- Wang, H.; Jiang, K.; Zhu, Z.; Jiang, W.; Yang, Z.; Zhu, S.; Qiu, J.; Yan, X.; He, J.; He, Q.; et al. Optimization of fed-batch fermentation and direct spray drying in the preparation of microbial inoculant of acetochlor-degrading strain Sphingomonas sp. DC-6. 3 Biotech 2018, 8, 294. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Liu, L.; Cheng, J.; Yuan, Y. Medium Optimization for Antifungal Active Substance Production from Streptomyces Lydicus Using Response Surface Methodology. Trans. Tianjin Univ. 2016, 23, 78–86. [Google Scholar] [CrossRef]
- Shu, W.; Zhao, L.; Hou, S.; Yu, Q.J.; Tan, S.; Yin, P. Toxic effect on the membrane system and cell proliferation of Prorocentrum donghaiense caused by the novel algicidal fungus Talaromyces purpurogenus YL13. J. Appl. Phycol. 2016, 29, 275–284. [Google Scholar] [CrossRef]
- Pan, P.; Jin, W.; Li, X.; Chen, Y.; Jiang, J.; Wan, H.; Yu, D. Optimization of multiplex quantitative polymerase chain reaction based on response surface methodology and an artificial neural network-genetic algorithm approach. PLoS ONE 2018, 13, e0200962. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.E.; El-Housseiny, G.S.; Abdelaziz, N.A.; El-Ansary, M.R.; Aboshanab, K.M. Optimized Production of the Allylamine Antifungal “Terbinafine” by Lysinibacillus Isolate MK212927 Using Response Surface Methodology. Infect. Drug Resist. 2020, 13, 3613–3626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Xiao, H.D.; Tang, S.K.; Zhang, Y.Q.; Borrathybay, E.; Cui, X.L.; Li, W.J.; Liu, Y.Q. Alteromonas halophila sp. nov., a new moderately halophilic bacterium isolated from a sea anemone. Antonie Van Leeuwenhoek 2009, 96, 259–266. [Google Scholar] [CrossRef] [PubMed]


| Variables | Low Level | High Level |
|---|---|---|
| −1 | +1 | |
| A Sucrose (%, w/v) | 0.5 | 1.5 |
| B Peptone (%, w/v) | 1 | 1.5 |
| C Medium volume (mL) | 60 | 100 |
| D Rotation speed (rpm) | 100 | 200 |
| E Temperature (°C) | 20 | 30 |
| F pH | 6 | 9 |
| G Fermentation time (h) | 24 | 36 |
| H Inoculum amount (%, v/v) | 1 | 2 |
| Run | A Sucrose | B Bacterial Peptone | C Medium Volume | D Rotation Speed | E Temperature | F pH | G Fermentation Time | H Inoculum Amount | Algicidal Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 35.5948 |
| 2 | +1 | +1 | −1 | +1 | +1 | +1 | +1 | −1 | 56.7844 |
| 3 | +1 | +1 | +1 | −1 | −1 | −1 | −1 | −1 | 19.9814 |
| 4 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 56.2268 |
| 5 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | 44.5167 |
| 6 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | 59.2937 |
| 7 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | 25.1394 |
| 8 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | 32.2491 |
| 9 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | 45.4926 |
| 10 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 39.777 |
| 11 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | 30.7156 |
| 12 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | 40.8922 |
| Variables | −α | Low Level | 0 | High Level | +α |
|---|---|---|---|---|---|
| −1.68179 | −1 | 0 | +1 | +1.68179 | |
| A Sucrose (%, w/v) | 0.06 | 0.2 | 0.4 | 0.6 | 0.74 |
| B Peptone (%, w/v) | 2.24 | 2.75 | 3.5 | 4.25 | 4.76 |
| D Rotation speed (rpm) | 199.55 | 220 | 250 | 280 | 300.45 |
| Run | A | B | D | Algicidal Rate (%) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 96.2307 |
| 2 | 0 | 0 | 0 | 93.0814 |
| 3 | +1 | −1 | +1 | 72.4895 |
| 4 | 0 | 0 | 0 | 93.9241 |
| 5 | +α | 0 | 0 | 91.1674 |
| 6 | 0 | 0 | 0 | 95.1617 |
| 7 | 0 | −α | 0 | 77.5527 |
| 8 | +1 | −1 | −1 | 89.0295 |
| 9 | 0 | +α | 0 | 97.6371 |
| 10 | −1 | +1 | −1 | 88.0169 |
| 11 | 0 | 0 | −α | 88.9620 |
| 12 | 0 | 0 | 0 | 91.7300 |
| 13 | −1 | +1 | +1 | 90.9058 |
| 14 | +1 | +1 | −1 | 89.7609 |
| 15 | −1 | −1 | −1 | 86.2785 |
| 16 | 0 | 0 | 0 | 96.4557 |
| 17 | −1 | −1 | +1 | 80.3094 |
| 18 | −α | 0 | 0 | 92.3994 |
| 19 | 0 | 0 | +α | 83.0661 |
| 20 | +1 | +1 | +1 | 92.4051 |
| Source | Sum of Square | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 1698.50 | 8 | 212.31 | 11.85 | 0.0333 |
| A | 281.61 | 1 | 281.61 | 15.72 | 0.0287 |
| B | 393.61 | 1 | 393.61 | 21.97 | 0.0184 |
| C | 255.24 | 1 | 255.24 | 14.25 | 0.0326 |
| D | 596.70 | 1 | 596.70 | 33.31 | 0.0103 |
| E | 8.63 | 1 | 8.63 | 0.48 | 0.5376 |
| F | 17.85 | 1 | 17.85 | 1.00 | 0.3917 |
| G | 137.14 | 1 | 137.14 | 7.65 | 0.0698 |
| H | 7.71 | 1 | 7.71 | 0.43 | 0.5586 |
| Source | Sum of Square | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 780.81 | 10 | 78.08 | 17.18 | 0.0001 |
| A | 1.11 | 1 | 1.11 | 0.24 | 0.6326 |
| B | 326.34 | 1 | 326.34 | 71.82 | <0.0001 |
| C | 51.18 | 1 | 51.18 | 11.26 | 0.0084 |
| AB | 8.64 | 1 | 8.64 | 1.90 | 0.2013 |
| AC | 14.62 | 1 | 14.62 | 3.22 | 0.1064 |
| BC | 98.30 | 1 | 98.30 | 21.63 | 0.0012 |
| A2 | 25.79 | 1 | 25.79 | 5.68 | 0.0411 |
| B2 | 114.50 | 1 | 114.50 | 25.20 | 0.0007 |
| C2 | 169.09 | 1 | 169.09 | 37.31 | 0.0002 |
| ABC | 13.33 | 1 | 13.33 | 2.93 | 0.1209 |
| Lack of Fit | 23.64 | 4 | 5.91 | 1.71 | 0.2826 |
| Cor Total | 821.71 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Shi, X.; Guo, Y.; Lv, P.; Zhong, Y.; Xie, H.; Chen, J. Optimization of Algicidal Activity for Alteromonas sp. FDHY-03 against Harmful Dinoflagellate Prorocentrum donghaiense. J. Mar. Sci. Eng. 2022, 10, 1274. https://doi.org/10.3390/jmse10091274
Wang Q, Shi X, Guo Y, Lv P, Zhong Y, Xie H, Chen J. Optimization of Algicidal Activity for Alteromonas sp. FDHY-03 against Harmful Dinoflagellate Prorocentrum donghaiense. Journal of Marine Science and Engineering. 2022; 10(9):1274. https://doi.org/10.3390/jmse10091274
Chicago/Turabian StyleWang, Qianqian, Xinguo Shi, Yisong Guo, Pin Lv, Yuying Zhong, Hui Xie, and Jianfeng Chen. 2022. "Optimization of Algicidal Activity for Alteromonas sp. FDHY-03 against Harmful Dinoflagellate Prorocentrum donghaiense" Journal of Marine Science and Engineering 10, no. 9: 1274. https://doi.org/10.3390/jmse10091274
APA StyleWang, Q., Shi, X., Guo, Y., Lv, P., Zhong, Y., Xie, H., & Chen, J. (2022). Optimization of Algicidal Activity for Alteromonas sp. FDHY-03 against Harmful Dinoflagellate Prorocentrum donghaiense. Journal of Marine Science and Engineering, 10(9), 1274. https://doi.org/10.3390/jmse10091274





