Abstract
The disposal of mine tailings into the marine environment is considered an essential option to secure the economic efficiency of deep-sea mining, but it might adversely affects the ecosystem. To examine the potential impacts of tailing disposal from polymetallic nodules and polymetallic sulfide mines on phytoplankton communities, addition experiments of crushed fine particles into surface seawater were conducted in the open Indian Ocean and changes in chlorophyll a fluorescence and community composition were analyzed. The addition of tailings had serious adverse effects on phytoplankton fluorescence and photosynthetic activity, regardless of mine type. The adverse effects seemed to mainly be due to the physical properties of the mine tailings. These also showed discriminatory effects on phytoplankton, resulting in great changes in community composition. The results suggest that mine tailings could have significant adverse impacts on phytoplankton assemblages, but the degree of impact greatly varies depending on the phytoplankton groups. The discriminatory impacts would cause changes in biomass, community structure, and thus ecological function.
1. Introduction
As land mineral resources are increasingly depleted, efforts to mine mineral resources from the oceans, which account for two-thirds of the Earth’s area, have been actively pursued. Similar to their adverse effects on land, mining activities can directly affect the marine environment and surrounding organisms. Therefore, the potential impacts of the mining process of seabed mineral resources on ecosystems have been considered [1,2]. Research on the distribution of seabed mineral resources has been conducted for several decades since the early 1970s, and in recent years, efforts for the commercial exploitation of mining technologies have been accelerating. As deep-sea mining sites are located far from land, expensive transportation costs and large amounts of waste to be disposed of on land have become issues. To solve this problem, transporting only useful minerals to land after dressing at mining sites has been suggested as a realistic method [3,4]. However, scientists and environmental organizations have been concerned about the disturbance of the marine environment due to outflow and the in situ disposal of mine tailings and have raised the necessity to identify potentially harmful materials and to assess their impacts on marine environments and ecosystems. With growing concerns from international communities regarding mine tailings, the International Seabed Authority (ISA), an international organization that organizes and controls all mineral-resource-related activities in the seabed, included a regulation on mining discharges in the draft exploitation regulations in 2020. Therefore, understanding the impacts of ocean runoff and the release of tailings on the marine environment and ecosystems is critical for the commercial development of seabed mineral resources and the protection of marine ecosystems.
To date, there has been relatively much research on the effects of mining on benthic ecosystems; however, studies on the effects of the release of mine tailings on marine ecosystems have been rarely conducted. Most of the known effects of tailings on marine environments have been assessed in nearshore shallow waters and coastal fjords with relatively short distances from land [5,6,7]. In coastal waters in which mine tailings have been disposed, shifts in meiofaunal and macrofaunal community structures have been observed, with reduced biodiversity [6,7,8,9]. However, there have been even fewer studies on the impacts of tailing disposal in the open ocean [9]. Furthermore, the effects of effluents from mining activities, as well as tailings disposal, on planktonic organisms have rarely been investigated until now [10,11,12].
After extracting the desired metal from an ore, slurries (composed mainly of fine-particle waste) are produced. The particles also contain low concentrations of targeted minerals and measurable concentrations of non-target minerals, such as arsenic, cobalt, nickel, mercury, lead, and zinc, as well as processing chemicals (e.g., acids, flocculants, and flotation agents) [3]. Therefore, deep-sea tailing disposal would have a direct and wide-area effect on the plankton community in the deep sea, and the intentional or accidental discharge of tailings to surface ocean can affect organisms living in the surface layers of the area. As the open ocean is oligotrophic, tiny phytoplankton, such as Prochloroocccus, Synechococcus, and picoeukaryotes, are dominant and have different ecological characteristics from plankton communities in shallow coastal waters. Moreover, they are less exposed to disturbances and variable environmental conditions. Therefore, as they are more vulnerable to environmental disturbances, their phytoplankton biomass, composition, and diversity are affected by tailing materials, potentially leading to functional changes in marine ecosystems.
Fine particles can remain in the water column for some time and exert unexplored effects on ambient organisms during the on-ship processes of ores mined in the deep sea. Although the particle concentration would decrease along the distance from the source, impact will be unavoidable within a certain range of seawater. However, because on-ship extraction processes have not yet been conducted in deep-sea areas, we could not examine the effects of the tailing plume discharged during the dressing processes. Therefore, the use of manipulation experiments that can simulate the input of tailing products into seawater and measure changes in the ecological traits of target organisms would be a good approach for understanding the putative responses of pelagic ecosystems.
This study was conducted to understand the potential effects of tailings from polymetallic nodule (PN) and polymetallic sulfide (PS) deposits on phytoplankton communities in oligotrophic open oceans. The effects were analyzed by monitoring the changes in chlorophyll fluorescence, photosynthetic efficiency, and community compositions for ca. 12 h of incubation after crushed PN and PS were added at different concentrations to the surface seawater.
2. Materials and Methods
2.1. Addition Experiments of Fine-Grained Particles
To understand the potential effects of mine tailings on the phytoplankton community, experiments adding powders of PN and PS were carried out in the Central Indian Ridge area in May and June 2018 (Figure 1). As the mineral extraction process has not yet been determined, the effects of chemicals that could be used during dressing processes were not considered in this study. The PN and PS used in this study were obtained from the Clarion-Clipperton Fracture Zone of the northeast Pacific and Fiji Basin, respectively. Each ore sample was crushed and ground using a milling machine. PN powder passing through a 20 μm sieve and PS powder collected on a 20 μm sieve after passing through a 60 μm sieve were used in this study. As a flotation method is highly likely to be applied in the extraction process, particles with fine sizes of 60 μm or less were used in the experiment.
Figure 1.
A map showing the study area.
Surface seawater was collected using Niskin bottles attached to a CTD rosette sampler. Seawater was filtered through a 200 μm mesh to remove zooplankton. The slurries for each addition experiment (working stock, final concentration of 1 g L−1) were prepared by adding 5 g of the powders to a 5 L polycarbonate bottle containing 5 L seawater. Five-liter experimental bottles with different concentrations of the powders (200 mg L−1, 100 mg L−1, 50 mg L−1, 20 mg L−1, and 10 mg L−1) were prepared by adding appropriate amounts of working stock (1 L, 0.5 L, 0.25 L, 0.1 L, and 0.02 L, respectively) in appropriate amounts of 200 μm-filtered seawater (4 L, 4.5 L, 4.75 L, 4.9 L, and 4.95 L, respectively). Control bottles without any powder were also used. The experiment was performed in triplicate. The bottles were freely floated in an on-deck incubator filled with overflowing surface seawater under natural light.
2.2. Assessment of Phytoplankton Photo-Physiology Using the FIRe System
The responses of phytoplankton to tailings were assessed using the FIRe (Fluorescence Induction and Relaxation) system, which measures photophysiological characteristics at high sensitivity in vivo. The Mini-FIRe system [13] was used in this study. A proxy of chlorophyll a (Chl a) and the efficiency of the conversion of light energy into chemical potential (photosynthesis) can be assessed from parameters measured using the system (Fm and Fv/Fm, respectively) [14]. For the analyses, 10 mL subsamples from the experimental bottles were obtained at five time points (0, 1, 3, 6, and 12 h). Measurements were conducted as described by Bibby et al. [14]. The bottles were incubated for only 12 h to minimize nutrient limitation during the incubation.
2.3. Community Composition of Photosynthetic Eukaryotes
The community composition of photosynthetic eukaryotes was analyzed via plastid 16S rRNA gene sequencing according to the protocol described in a previous study [15]. Analyses were conducted for each of the triplicate samples obtained after 12 h. Five hundred milliliters of the samples were filtered through a 0.2 μm pore-size Supor filter (47 mm; PALL Corp., Washington, DC, USA), and the filters were stored at −80 °C after the addition of 1 mL of an STE buffer (100 mM NaCl, 10 mM Tris-HCl, and 1 mM EDTA; pH 8.0) until DNA extraction. DNA extraction and purification, PCR amplification, MiSeq sequencing, and sequence analyses were conducted as described by Choi et al. [15], with some modifications. After enzymatic treatment in the extraction step, phenol:chloroform:isoamyl alcohol (25:24:1) and subsequent chloroform:isoamyl alcohol (24:1) extractions were applied. Additional column purification was conducted using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol.
2.4. Other Analyses
Seawater temperature and salinity were measured using a CTD (SBE911; Sea-Bird, Bellevue, WA, USA) mounted on a rosette sampler. Chl a was measured using a fluorometer (10AU; Turner Designs, San Jose, CA, USA) according to the protocol of Parsons et al. [16]. ANOVA was analyzed using PASW Statistics 18 (SPSS Inc. Chicago, IL, USA).
3. Results and Discussion
3.1. Environmental Characteristics of the Study Area
The temperature and salinity of the study area ranged from 28.4 to 29.1 °C and from 34.6 to 34.7, respectively, showing characteristics of a tropical open ocean with small variations (Table 1). The Chl a in the surface water was very low (approximately 0.01 μg L−1), indicating an oligotrophic state of the study area (Table 1).

Table 1.
Sampling depth and environmental characteristics of seawater samples and types of ores used in the particle addition experiments.
3.2. Effects of Ore Particles on Fluorescence-Based Phytoplankton Activity
Photochemical properties, measured using fast repetition rate fluorometry (FRRF) or the FIRe system, have been used to assess phytoplankton responses to toxic materials, such as biocides, hydrocarbons, and heavy metals [17,18,19]. In this study, the phytoplankton responses to ore particles assessed using the FIRe system suggested that tailings could exert harmful effects on phytoplankton productivity, especially in cases of high concentrations and long exposure times (Figure 2). Immediately after the particles were added to the seawater sample at a concentration of 1 g L−1 (final concentration), the Fm values, a proxy of Chl a concentration, showed a dramatic decrease to less than 1/3 of the control bottle without particles (ANOVA, p < 0.001) (Figure 2). In contrast, the Fm values at low particle concentrations were not significantly different from those of the control at the beginning (ANOVA, p > 0.05). During the measurements, increasing PN and PS would result in enhanced light diffusion or absorption. To test this possibility, wheat starch powder (Cat. S5127, Sigma-Aldrich, St. Louis, MO, USA) was added to the surface seawater. However, the addition of various concentrations of starch, which has similar mean size to that of PN and PS particles [20], showed no negative effects on both Fm and Fv/Fm (Supplementary Figure S1). On the contrary, Fm tended to slightly increase along with the starch concentration.

Figure 2.
Changes in maximum fluorescence (Fm) measured in samples of different polymetallic nodule (PN, (A)) and polymetallic sulfide (PS, (B)) particle concentrations during the incubation. Asterisks denote that the Fm value measured in each treatment was statistically different from that of the control sample (ANOVA, * p < 0.05; ⁑ p < 0.01; ⁂ p < 0.001). The dotted line represents the value measured in the control sample at the beginning.
However, as the exposure time increased, the Fm values gradually decreased, even at low concentrations (Figure 2). After 6 and 12 h, when no significant changes in Fm values were found in the control samples of the PN and PS treatment experiments, respectively, harmful effects were also found at particle concentrations of less than 10–20 mg L−1 (ANOVA, p < 0.05) (Figure 2). Thus, the magnitude of harmful effects increased with increasing exposure time and particle concentration. In a previous study in the central Atlantic Ocean, consistent with the results of this study, the addition of a fine ground powder slurry of ferromanganese polymetallic crusts (Fe–Mn curst) to surface seawater resulted in 15–25% decrease in CO2 fixation and an increase in the harmful impact along the curst slurry concentrations in a range between 25 and 400 mg L−1 [10].
PN is composed of manganese oxides and fine-grained iron [21]. In contrast, the composition of PS mainly includes iron sulfide, copper sulfide, zinc sulfide, and other metal sulfides [22]. Despite the different compositions of the ores (Table 2), the phytoplankton community showed a tendency to respond similarly to the ore particles (Figure 2).

Table 2.
Chemical composition of polymetallic nodules (PNs) and polymetallic sulfide (PS) used in this study. Values are mean and one standard deviation (parenthesis) from seven replicates.
Despite the significant decrease in the active Chl a, the photosynthetic efficiency of the phytoplankton community, which could be inferred by the Fv/Fm of the FIRe system, did not show significant differences between the control and each particle concentration in most experiments (ANOVA, p > 0.05) (Figure 3). In marine environments, the Fv/Fm variable has been used to assess the potential effects on phytoplankton growth under diverse stresses, such as nutrient limitation and toxicity of copper and polycyclic aromatic hydrocarbons [23,24,25]. The lack of significant changes in Fv/Fm values in most samples in this study suggests that the tolerant or unaffected phytoplankton were not significantly stressed by the introduction of ore particles, regardless of exposure time or concentration. In fact, the phytoplankton composition changes in each treatment sample showed that the effects of the treatments were phytoplankton-group-specific.
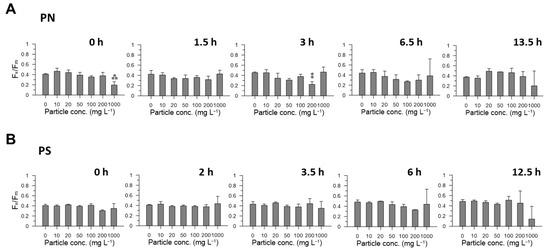
Figure 3.
Changes in photochemical efficiency (Fv/Fm) measured in samples of different polymetallic nodule (PN, (A)) and polymetallic sulfide (PS, (B)) particle concentrations during the incubation. Asterisks denote that the Fv/Fm value measured in each treatment was statistically different from that of the control sample (ANOVA, ⁑ p < 0.01; ⁂ p < 0.001). The dotted line represents the value measured in the control sample at the beginning.
3.3. Effects of Ore Particles on Community Composition of Photosynthetic Eukaryotes
The potential effect of tailings on photosynthetic eukaryotes community composition was assessed using the plastid 16S rRNA gene sequencing approach. Surprisingly, there was a great change in composition after ca. 12 h of treatment, and the change in each phytoplankton group showed a distinct concentration-dependent trend (Figure 4). Haptophyta was the predominant group in the control samples. However, their relative occurrence among the total sequence reads decreased as particle concentration increased. Conversely, the relative percentage of Chlorophyta was very low (<7%) in the control samples and greatly increased up to 58% in the highest concentration samples. Similarly, Cryptophyta occupied negligible fractions (0.2%) in the control and increased to 10% at the highest concentration. The Ochrophyta groups, including Bacillariophyta and Chrysophyceae, did not show distinct changes in particle concentration. Haptophyta is the most dominant photosynthetic eukaryote in the open ocean [15,26]. Thus, the dramatic changes in phytoplankton composition during short-term particle exposure suggest that the discharge of both PN and PS tailings can exert severe effects on phytoplankton community diversity in the oligotrophic open ocean, in addition to phytoplankton photosynthetic activity, as seen above. In this study, the impact on cyanobacterial diversity was not investigated. However, the great decrease in chlorophyll fluorescence indicates that cyanobacteria such as Prochlorococcus and Synechococcus, which are predominant in oligotrophic open oceans, might be severely affected by the particle addition.

Figure 4.
Relative percentages of major phyla as proportions of all photosynthetic eukaryotic sequences obtained in samples of different polymetallic nodule (PN, (A)) and polymetallic sulfide (PS, (B)) particle concentrations after ca. 12 h of incubation. n.d., not determined.
Further analyses of the composition showed that the compositional change was due to the discriminative response of phytoplankton genera (Table 3). The relative proportions of the four dominant taxa belonging to Haptophyta tended to decrease with increasing particle concentrations. Conversely, a taxon affiliated with Phaeocystis was negligible in the control but tended to increase at higher particle concentrations. These differential responses among taxa belonging to Haptophyta were also found in other phyla (Table 3). Most of the taxa belonging to Chlorophyta increased in relative proportions with increasing particle concentrations. However, the prasino-clade-9 taxon showed the opposite trend. Similar trends were observed in diverse orders belonging to the phylum Ochrophyta (Table 3). Thus, the responses of phytoplankton communities to tailings will be different, at least among genera. A wide range of responses to Fe–Mn curst slurry addition found in samples collected along a latitudinal transect from the center of the South Atlantic subtropical gyre to South Georgia Island [10] might have been due to the discriminatory responses of various phytoplankton species in each sample. Changes in composition and diversity due to tailing discharge were relatively frequently observed in benthic communities [6,9]. In particular, long-term deep-sea tailing disposals around Papua New Guinea have led to a decline in the abundance of benthic fauna and consequent changes in community structure [9]. Since tailings discharged into surface seawater rapidly spread and move to other sea areas, tailing input into surface may not affect chronic changes in phytoplankton community composition.

Table 3.
Relative percentages of abundant genus-level taxa obtained in samples of different particle concentrations after ca. 12 h of incubation. The taxonomic classification was annotated according to the PhytoREF database [27]. The intensity of red color represents a scale of the relative percentage.
This adverse response could be due to the leaching of toxic substances from the particles [12]. As PN and PS have various types of heavy metals, it is expected that various heavy metals were eluted from the fine particles during the experiments. Heavy metals have been reported to exert toxic effects on diverse diatom and natural plankton communities [28,29,30,31]. Consistently, leachates from metals and metalloids from PSs with different metal components inhibited the growth of the diatom species, Skeletomema marinoi-dohrnii complex, though with different magnitudes of toxic effects according to the type of PS ore [12]. However, when leachate prepared by adding ore particles to 0.2 μm filtered seawater (final particle concentration of 1 g L−1) and leaching for 1–12 h was added to natural seawater (final leachate concentration of 10%) and incubated for 1 h, no significant changes in Chl a fluorescence or photosynthetic efficiency were observed (Figure 5). These results show that the effects of the heavy metals leached from PN and PS during short-term incubation were not significant in this study.

Figure 5.
Maximum fluorescence (Fm) and photochemical efficiency (Fv/Fm) measured in treatment samples after 1 h of incubation. Each treatment sample was made by mixing surface seawater with leachates of polymetallic nodule (PN, (A)) and polymetallic sulfide (PS, (B)) particles (final concentration of 10%). Leachates were obtained by adding each ore particles into 0.2 μm filtered surface seawater (final concentration of 1 g L−1) and mixing them for different time points. Samples denoted as ‘C’ and ‘0′ represent the seawater samples without leachates and with the addition of 0.2 μm filtered seawater used to leach the particles, respectively.
In addition to metal leachates, fine mineral particles could be another cause of the inhibition of phytoplankton growth and composition changes. Currently, clays without known toxic chemicals are used to mitigate harmful algal blooms in many countries [32,33,34,35]. The treatment mainly uses flocculation and removal by the interaction, as well as the physico-biochemical inhibition mechanisms between clay particles and harmful organisms [36]. For example, the addition of phosphatic clay at a concentration up to 0.6 g L−1 to brown tide algae (Aureococcus anophagefferens) samples resulted in a decrease in Chl a fluorescence to 70% after seven hours, and the magnitude of the decrease was positively dependent on clay concentrations [37]. In clay treatments, it is known that the removal efficiencies of clays depend on the clay structure, type, and (thus) their surface chemistry [36,38,39]. Furthermore, numerous studies have been conducted to increase mitigation efficiency by modifying clay surfaces, and several modifications have been shown to have different effects on diatoms, dinoflagellates, and Phaeocystis globosa [36,38,40].
Interestingly, positively charged colloids of aminoclays have been reported to induce cell lysis in harmful algal plankton species such as Cochlorodinium polykrikoides, C. marina and Heterosigma akashiwo but no significant harmful effects on non-harmful phytoplankton such as Nannochloropsis sp., Phaeodactylum sp., and Amphidinium sp. [41]. Thus, electrostatic attraction between clay particles and algal cells seems to be important for the selective flocculation and lysis of algal cells [41].
Given that the interaction between the surface properties of clay particles and algal cells is an important mechanism to suppress harmful algal blooms, the physical interactions between phytoplankton cells and both PN and PS particles would be a major starting point for the significant decrease in photosynthetic activity and change in community composition observed in this study. The surface properties of PN and PS particles and their physical interactions with phytoplankton cells have not been well-studied. However, the trend of the harmful effects being dependent on both the exposure time and ore particle concentrations was similar to that in the clay experiments. Although the influence of harmful materials leached from particles could be excluded, cell lysis by physicochemical interactions between particle-sensitive phytoplankton cells and particles could be regarded as an important mechanism for these influences. The wide range of zeta potentials of marine phytoplankton, which are important for interaction with particles [35], could support the importance of the interaction in the composition change. Furthermore, the abrupt increase or decrease in phytoplankton genera during only a half-day treatment could not be explained without assuming the mass reduction in dominant phytoplankton cells given the slow growth rates (0.1–0.8 day−1) of phytoplankton in the oligotrophic ocean [42,43].
4. Conclusions
The addition of ore particles exerted significant adverse effects on phytoplankton in this study, suggesting that the release of mine tailings into the surface waters of the open ocean will not only cause a significant reduction in primary productivity but also result in functional changes in the ecosystem. In particular, phytoplankton belonging to Haptophyta play an important role in primary production, as well as in carbon and nitrogen cycles, participating in calcium carbonate formation and nitrogen fixing [44,45]. This study provides a better understanding on the potential effects of tailings on microorganisms in the surface waters of the open ocean. However, as it is impossible to completely avoid the harmful effects of tailings and other harmful materials produced by mining activities in the open ocean, further intensive studies are needed to understand the response of marine organisms to tailings and to mitigate the harmful effects of tailings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse10081162/s1, Figure S1: Changes in maximum fluorescence (Fm) and photochemical efficiency (Fv/Fm) measured in samples with different wheat starch concentrations after 1 h of incubation.
Author Contributions
Conceptualization, D.H.C., K.H. and C.M.Y.; Data curation, J.Y., W.Y. and K.R.; Funding acquisition, K.H. and C.M.Y.; Investigation, D.H.C., J.Y., W.Y. and K.R.; Methodology, D.H.C., J.H.N. and J.P.; Project administration, K.H. and C.M.Y.; Resources, J.H.N., Y.L. and J.P.; Supervision, D.H.C.; Visualization, J.Y.; Writing—original draft, D.H.C.; Writing—review and editing, D.H.C., J.H.N., Y.L. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by an in-house research program (PEA0023) of the Korea Institute of Ocean and Science Technology (KIOST).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of 16S rRNA gene sequences presented in this study are available on NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA860738, accessed on 19 August 2022).
Acknowledgments
We are also grateful to the editors of JMSE and anonymous reviewers for their feedback on this submission, which has helped improve the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Miller, K.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An Overview of Seabed Mining Including the Current State of Development, Environmental Impacts, and Knowledge Gaps. Front. Mar. Sci. 2018, 4, 418. [Google Scholar] [CrossRef]
- Washburn, T.W.; Turner, P.J.; Durden, J.M.; Jones, D.O.; Weaver, P.; Van Dover, C.L. Ecological risk assessment for deep-sea mining. Ocean Coast. Manag. 2019, 176, 24–39. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Trannum, H.C.; Evenset, A.; Levin, L.A.; Andersson, M.; Finne, T.E.; Hilario, A.; Flem, B.; Christensen, G.; Schaanning, M.; et al. Submarine and deep-sea mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Mar. Pollut. Bull. 2015, 97, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Vare, L.L.; Baker, M.C.; Howe, J.A.; Levin, L.; Neira, C.; Ramirez-Llodra, E.Z.; Reichelt-Brushett, A.; Rowden, A.; Shimmield, T.M.; Simpson, S.; et al. Scientific Considerations for the Assessment and Management of Mine Tailings Disposal in the Deep Sea. Front. Mar. Sci. 2018, 5, 17. [Google Scholar] [CrossRef]
- Burd, B.J. Evaluation of mine tailings effects on a benthic marine infaunal community over 29 years. Mar. Environ. Res. 2002, 53, 481–519. [Google Scholar] [CrossRef]
- Lee, M.R.; Correa, J.A. Effects of copper mine tailings disposal on littoral meiofaunal assemblages in the Atacama region of northern Chile. Mar. Environ. Res. 2005, 59, 1–18. [Google Scholar] [CrossRef]
- Mevenkamp, L.; Stratmann, T.; Guilini, K.; Moodley, L.; Van Oevelen, D.; Vanreusel, A.; Westerlund, S.; Sweetman, A.K. Impaired Short-Term Functioning of a Benthic Community from a Deep Norwegian Fjord Following Deposition of Mine Tailings and Sediments. Front. Mar. Sci. 2017, 4, 169. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Trannum, H.C.; Andersen, G.S.; Baeten, N.J.; Brooks, S.J.; Escudero-Oñate, C.; Gundersen, H.; Kleiv, R.A.; Ibragimova, O.; Lepland, A.; et al. New insights into submarine tailing disposal for a reduced environmental footprint: Lessons learnt from Norwegian fjords. Mar. Pollut. Bull. 2022, 174, 113150. [Google Scholar] [CrossRef]
- Hughes, D.J.; Shimmield, T.M.; Black, K.D.; Howe, J.A. Ecological impacts of large-scale disposal of mining waste in the deep sea. Sci. Rep. 2015, 5, 9985. [Google Scholar] [CrossRef]
- Dabrowska, A.; Kamennaya, N.A.; Murton, B.J.; Zubkov, M.V. Impact of ferromanganese ore pollution on phytoplankton CO2 fixation in the surface ocean. Mar. Pollut. Bull. 2019, 146, 1002–1006. [Google Scholar] [CrossRef]
- Hauton, C.; Brown, A.; Thatje, S.; Mestre, N.C.; Bebianno, M.J.; Martins, I.; Bettencourt, R.; Canals, M.; Sanchez-Vidal, A.; Shillito, B.; et al. Identifying Toxic Impacts of Metals Potentially Released during Deep-Sea Mining—A Synthesis of the Challenges to Quantifying Risk. Front. Mar. Sci. 2017, 4, 368. [Google Scholar] [CrossRef]
- Fuchida, S.; Yokoyama, A.; Fukuchi, R.; Ishibashi, J.-I.; Kawagucci, S.; Kawachi, M.; Koshikawa, H. Leaching of Metals and Metalloids from Hydrothermal Ore Particulates and Their Effects on Marine Phytoplankton. ACS Omega 2017, 2, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kuzminov, F.I.; Bailleul, B.; Yang, E.J.; Lee, S.; Falkowski, P.G.; Gorbunov, M.Y. Light availability rather than Fe controls the magnitude of massive phytoplankton bloom in the Amundsen Sea polynyas, Antarctica. Limnol. Oceanogr. 2017, 62, 2260–2276. [Google Scholar] [CrossRef]
- Bibby, T.S.; Gorbunov, M.Y.; Wyman, K.W.; Falkowski, P.G. Photosynthetic community responses to upwelling in mesoscale eddies in the subtropical North Atlantic and Pacific Oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1310–1320. [Google Scholar] [CrossRef]
- Choi, D.H.; An, S.M.; Chun, S.; Yang, E.C.; Selph, K.E.; Lee, C.M.; Noh, J.H. Dynamic changes in the composition of photosynthetic picoeukaryotes in the northwestern Pacific Ocean revealed by high-throughput tag sequencing of plastid 16S rRNA genes. FEMS Microbiol. Ecol. 2015, 92, fiv170. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; 173p. [Google Scholar]
- Devilla, R.; Brown, M.; Donkin, M.; Tarran, G.; Aiken, J.; Readman, J. Impact of antifouling booster biocides on single microalgal species and on a natural marine phytoplankton community. Mar. Ecol. Prog. Ser. 2005, 286, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Kong, Q.; Dong, W.; Lin, Z. Toxicity of Naphthenic Acids on the Chlorophyll Fluorescence Parameters and Antioxidant Enzyme Activity of Heterosigma akashiwo. Antioxidants 2021, 10, 1582. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Jin, P.; Agusti, S. Adaptation of Red Sea Phytoplankton to Experimental Warming Increases Their Tolerance to Toxic Metal Exposure. Front. Environ. Sci. 2019, 7, 125. [Google Scholar] [CrossRef]
- Stasiak, M.; Molenda, M.; Opaliński, I.; Błaszczak, W. Mechanical properties of native maize, wheat, and potato starches. Czech J. Food Sci. 2013, 31, 347–354. [Google Scholar] [CrossRef]
- Margolis, S.V.; Burns, R.G. Pacific Deep-Sea Manganese Nodules: Their Distribution, Composition, and Origin. Annu. Rev. Earth Planet. Sci. 1976, 4, 229–263. [Google Scholar] [CrossRef]
- Halbach, P.; Blum, N.; Münch, U.; Plüger, W.; Garbe-Schönberg, D.; Zimmer, M. Formation and decay of a modern massive sulfide deposit in the Indian Ocean. Miner. Deposita 1998, 33, 302–309. [Google Scholar] [CrossRef]
- Saeck, E.; O’Brien, K.; Burford, M. Nitrogen response of natural phytoplankton communities: A new indicator based on photosynthetic efficiency Fv/Fm. Mar. Ecol. Prog. Ser. 2016, 552, 81–92. [Google Scholar] [CrossRef]
- Pérez, P.; Fernández, E.; Beiras, R. Use of Fast Repetition Rate Fluorometry on Detection and Assessment of PAH Toxicity on Microalgae. Water Air Soil Pollut. 2009, 209, 345–356. [Google Scholar] [CrossRef]
- Othman, H.B.; Leboulanger, C.; Le Floc’h, E.; Hadj Mabrouk, H.; Sakka Hlaili, A. Toxicity of benz(a)anthracene and fluoranthene to marine phytoplankton in culture: Does cell size really matter? J. Hazard. Mater. 2012, 243, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Not, F.; Latasa, M.; Scharek, R.; Viprey, M.; Karleskind, P.; Balagué, V.; Ontoria-Oviedo, I.; Cumino, A.; Goetze, E.; Vaulot, D.; et al. Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1456–1473. [Google Scholar] [CrossRef][Green Version]
- Decelle, J.; Romac, S.; Stern, R.F.; Bendif, E.M.; Zingone, A.; Audic, S.; Guiry, M.D.; Guillou, L.; Tessier, D.; Le Gall, F.; et al. PhytoREF: A reference database of the plastidial 16S rRNA gene of photosynthetic eukaryotes with curated taxonomy. Mol. Ecol. Resour. 2015, 15, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- French, M.S.; Evans, L.V. The effects of copper and zinc on growth of the fouling diatoms amphora and amphiprora. Biofouling 1988, 1, 3–18. [Google Scholar] [CrossRef]
- Hollibaugh, J.; Seibert, D.; Thomas, W. A comparison of the acute toxicities of tenheavy metals to phytoplankton from Saanich Inlet, B.C., Canada. Estuar. Coast. Mar. Sci. 1980, 10, 93–105. [Google Scholar] [CrossRef]
- Nayar, S.; Goh, B.; Chou, L. Environmental impact of heavy metals from dredged and resuspended sediments on phytoplankton and bacteria assessed in in situ mesocosms. Ecotoxicol. Environ. Saf. 2004, 59, 349–369. [Google Scholar] [CrossRef]
- Alprol, A.E.; Heneash, A.M.M.; Soliman, A.M.; Ashour, M.; Alsanie, W.F.; Gaber, A.; Mansour, A.T. Assessment of Water Quality, Eutrophication, and Zooplankton Community in Lake Burullus, Egypt. Diversity 2021, 13, 268. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Sengco, M.R.; Anderson, D.M. Using clay to control harmful algal blooms: Deposition and resuspension of clay/algal flocs. Harmful Algae 2005, 4, 123–138. [Google Scholar] [CrossRef]
- Kim, H.G. Mitigation and controls of HABs. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 327–338. [Google Scholar]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30, S131–S143. [Google Scholar] [CrossRef]
- Sengco, M.R.; Anderson, D.M. Controlling Harmful Algal Blooms through Clay Flocculation1. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef]
- Yu, Z.; Sengco, M.R.; Anderson, D.M. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. J. Appl. Phycol. 2004, 16, 101–110. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Yu, Z.; Song, X.; Qiu, L. Flocculation of harmful algal cells using modified clay: Effects of the properties of the clay suspension. J. Appl. Phycol. 2015, 28, 1623–1633. [Google Scholar] [CrossRef]
- Ren, X.; Yu, Z.; Qiu, L.; Cao, X.; Song, X. Effects of Modified Clay on Phaeocystis globosa Growth and Colony Formation. Int. J. Environ. Res. Public Health 2021, 18, 10163. [Google Scholar] [CrossRef]
- Hagström, J.A.; Sengco, M.R.; Villareal, T.A. Potential Methods for Managing Prymnesium parvum Blooms and Toxicity, with Emphasis on Clay and Barley Straw: A Review1. JAWRA J. Am. Water Resour. Assoc. 2010, 46, 187–198. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Jin, E.; Jung, S.W.; Kim, Y.-M.; Chang, K.S.; Yang, J.-W.; Kim, S.W.; Kim, Y.-O.; Shin, H.-J. Utilizing the algicidal activity of aminoclay as a practical treatment for toxic red tides. Sci. Rep. 2013, 3, 1292. [Google Scholar] [CrossRef]
- Lessard, E.; Murrell, M. Microzooplankton herbivory and phytoplankton growth in the northwestern Sargasso Sea. Aquat. Microb. Ecol. 1998, 16, 173–188. [Google Scholar] [CrossRef]
- Goericke, R.; Welschmeyer, N.A. Response of Sargasso Sea phytoplankton biomass, growth rates and primary production to seasonally varying physical forcing. J. Plankton Res. 1998, 20, 2223–2249. [Google Scholar] [CrossRef]
- De La Rocha, C.L.; Passow, U. Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 639–658. [Google Scholar] [CrossRef]
- Zehr, J.P.; Shilova, I.; Farnelid, H.M.; Marin, M.D.C.M.; Turk-Kubo, K.A. Unusual marine unicellular symbiosis with the nitrogen-fixing cyanobacterium UCYN-A. Nat. Microbiol. 2017, 2, 16214. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).