Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Oxydation of Polychlorobiphenyls
2.3. Biosurfactant Production
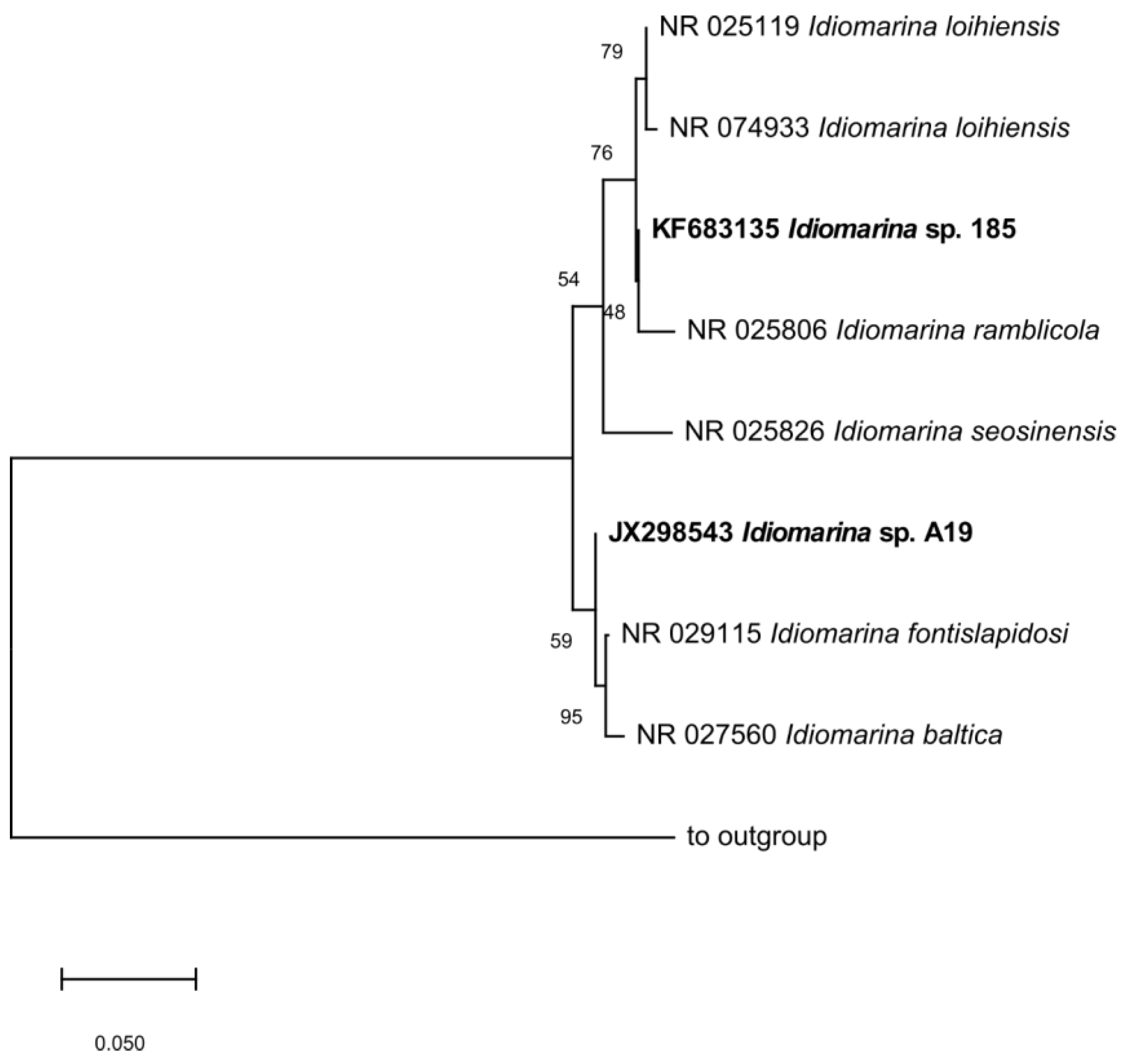
2.4. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Phenotypic Characterization
3.2. Biosurfactant Production
3.3. Oxidation of Polychlorobiphenyls and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perfumo, A.; Banat, I.M.; Marchant, R. Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Lo Giudice, A. The variety and inscrutability of polar environments as a resource of biotechnologically relevant molecules. Microorganisms 2020, 8, 1422. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, R.; Seghal Kiran, G.; Hema, T.A.; Selvin, J.; Rajeetha Raviji, T.; Shanmughapriya, S. Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess Biosyst. Eng. 2009, 32, 825–835. [Google Scholar] [CrossRef]
- Graziano, M.; Rizzo, C.; Michaud, L.; Porporato, E.; De Domenico, E.; Spanò, N.; Giudice, A.L. Biosurfactant production by hydrocarbon-degrading Brevibacterium and Vibrio isolates from the sea pen Pteroeides spinosum (Ellis, 1764). J. Basic Microbiol. 2016, 56, 963–974. [Google Scholar] [CrossRef]
- Kiran, G.S.; Thomas, T.A.; Selvin, J.; Sabarathnam, B.; Lipton, A.P. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour. Technol. 2010, 101, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Lipton, A.N.; Priyadharshini, S.; Anitha, K.; Suárez, L.E.; Arasu, M.V.; Choi, K.C.; Selvin, J.; Al-Dhabi, N.A. Antiadhesive activity of poly-hydroxy butyrate biopolymer from a marine Brevibacterium casei MSI04 against shrimp pathogenic vibrios. Microb. Cell Fact. 2014, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Dhasayan, A.; Kiran, G.S.; Selvin, J. Production and characterisation of glycolipid biosurfactant by Halomonas sp. MB-30 for potential application in enhanced oil recovery. Appl. Biochem. Biotechnol. 2014, 174, 2571–2584. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Hörmann, B.; Gerçe, B.; Syldatk, C.; Hausmann, R.; De Domenico, E.; Giudice, A.L. Bacteria associated with sabellids (Polychaeta: Annelida) as a novel source of surface active compounds. Mar. Pollut. Bull. 2013, 70, 125–133. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Syldatk, C.; Hausmann, R.; De Domenico, E.; Giudice, A.L. Influence of salinity and temperature on the activity of biosurfactants by polychaete-associated isolates. Environ. Sci. Pollut. Res. 2014, 21, 2988–3004. [Google Scholar] [CrossRef]
- Mapelli, F.; Scoma, A.; Michoud, G.; Aulenta, F.; Boon, N.; Borin, S.; Kalogerakis, N.; Daffonchio, D. Biotechnologies for marine oil spill cleanup: Indissoluble ties with microorganisms. Trends Biotechnol. 2017, 35, 860–870. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Romanenko, L.A.; Chun, J.; Matte, M.H.; Matte, G.R.; Mikhailov, V.V.; Svetashev, V.I.; Huq, A.; Maugel, T.; Colwell, R.R. Idiomarina gen. nov., comprising novel indigenous deep-sea bacteria from the Pacific Ocean, including descriptions of two species, Idiomarina abyssalis sp. nov. and Idiomarina zobellii sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Jean, W.D.; Leu, T.Y.; Lee, C.Y.; Chu, T.J.; Lin, S.Y.; Shieh, W.Y. Pseudidiomarina marina sp. nov. and Pseudidiomarina tainanensis sp. nov. and reclassification of Idiomarina homiensis and Idiomarina salinarum as Pseudidiomarina homiensis comb. nov. and Pseudidiomarina salinarum comb. nov., respectively. Int. J. Syst. Evol. Microbiol. 2009, 59, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, Y.O.; Kim, D.G.; Nam, B.H.; Kong, H.J.; Jung, H.; Lee, S.J.; Kim, D.W.; Kim, D.S.; Chae, S.H. Genome sequence of marine bacterium Idiomarina sp. strain 28-8, isolated from Korean ark shells. Genome Announc. 2013, 1, e00772-13. [Google Scholar] [CrossRef]
- Gupta, H.K.; Singh, A.; Sharma, R. Genome sequence of Idiomarina sp. strain A28L, isolated from Pangong Lake, India. J. Bacteriol. 2011, 193, 5875–5876. [Google Scholar] [CrossRef] [PubMed]
- Mata, J.A.; Bèjar, V.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E.; Llamas, I. Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family Alteromonadaceae. J. Appl. Microbiol. 2008, 105, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for novel halophilic and halotolerant sources of hydrolytic enzymes in brackish, saline and hypersaline lakes of Romania. Microorganisms 2020, 8, 1903. [Google Scholar] [CrossRef] [PubMed]
- Rajapitamahuni, S.; Bachani, P.; Sardar, R.K.; Mishra, S. Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J. Appl. Phycol. 2019, 31, 29–39. [Google Scholar] [CrossRef]
- Malavenda, R.; Rizzo, C.; Michaud, L.; Gerçe, B.; Bruni, V.; Syldatk, C.; Hausmann, R.; Giudice, A.L. Biosurfactant production by Arctic and Antarctic bacteria growing on hydrocarbons. Polar Biol. 2015, 38, 1565–1574. [Google Scholar] [CrossRef]
- Gomes, M.B.; Gonzales-Limache, E.E.; Sousa, S.T.P.; Dellagnezze, B.M.; Sartoratto, A.; Silva, L.C.F.; Gieg, L.M.; Valoni, E.; Souza, R.S.; Torres, A.P.R.; et al. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int. Biodeterior. Biodegrad. 2018, 126, 231–242. [Google Scholar] [CrossRef]
- Frame, G.M.; Cochran, J.W.; Bøwadt, S.S. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener specific analysis. J. High Resolut. Chromatogr. 1996, 19, 657–668. [Google Scholar] [CrossRef]
- Papale, M.; Giannarelli, S.; Francesconi, S.; Di Marco, G.; Mikkonen, A.; Conte, A.; Rizzo, C.; De Domenico, E.; Michaud, L.; Lo Giudice, A. Enrichment, isolation and biodegradation potential of psychrotolerant polychlorinated-biphenyl degrading bacteria from the Kongsfjorden (Svalbard Islands, High Arctic Norway). Mar. Poll. Bull. 2017, 114, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Bhawsar, B.D.; Dhakephalkar, P.K.; Chopade, B.A. Assessment of different screening methods for selecting biosurfactant producing marine bacteria. Indian J. Mar. Sci. 2008, 37, 243–250. [Google Scholar]
- Plaza, G.; Zjawiony, I.; Banat, I. Use of different methods for detection of thermophilic biosurfactant-producing bacteria from hydrocarbon contaminated bioremediated soils. J. Pet. Sci. Eng. 2006, 50, 71–77. [Google Scholar] [CrossRef]
- Youssef, N.; Duncan, K.; Nagle, D. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Met. 2004, 56, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Walter, V.; Syldatk, C.; Hausmann, R. Screening concepts for the isolation of biosurfactant producing microorganisms. Biosurfactants 2010, 672, 1–13. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef]
- Tajima, F.; Nei, M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1984, 1, 269–285. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Sousa, M.; Melo, V.M.M.; Rodrigues, S.; Sant’ana, H.B.; Goncalves, L.R.B. Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst. Eng. 2012, 35, 897–906. [Google Scholar] [CrossRef]
- Gutierrez, T.; Singleton, D.R.; Berry, D.; Yang, T.; Aitken, M.D.; Teske, A. Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 2013, 7, 2091–2104. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O. Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef]
- Cho, S.H.; Ma, C.W.; Oh, K.H. Optimized production of biosurfactant by the indigenous bacterium, Pseudoalteromonas sp. HK-3 originating from oil-spilled areas. Korean Soc. Biotech. Bioeng. J. 2011, 26, 57–61. [Google Scholar]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef]
- Jaysree, R.C.; Subham, B.; Priyanka, P.S.; Twinkle, G.; Pragya, A.P.; Yekala, K.; Rajendran, N. Isolation of biosurfactant producing bacteria from environmental samples. Pharmacology 2011, 3, 1427–1433. [Google Scholar]
- Okore, C.C.; Mbanefo, O.N.; Onyekwere, B.C.; Onyewenjo, S.; Abba-Father, C.A.M. Isolation and characterization ofbiosurfactants producing bacteria from oil polluted soil. J. Nat. Sci. Res. 2013, 3, 119–122. [Google Scholar]
- Ogbulie, T.E.; Okore, C.C.; Ejele, A.E.; Okwujiako, I.A. Comparative study on identification of biosurfactant producing bacteria using three screening tests. An. Univ. Oradea Fasc. Biol. 2020, XXVII, 179–186. [Google Scholar]

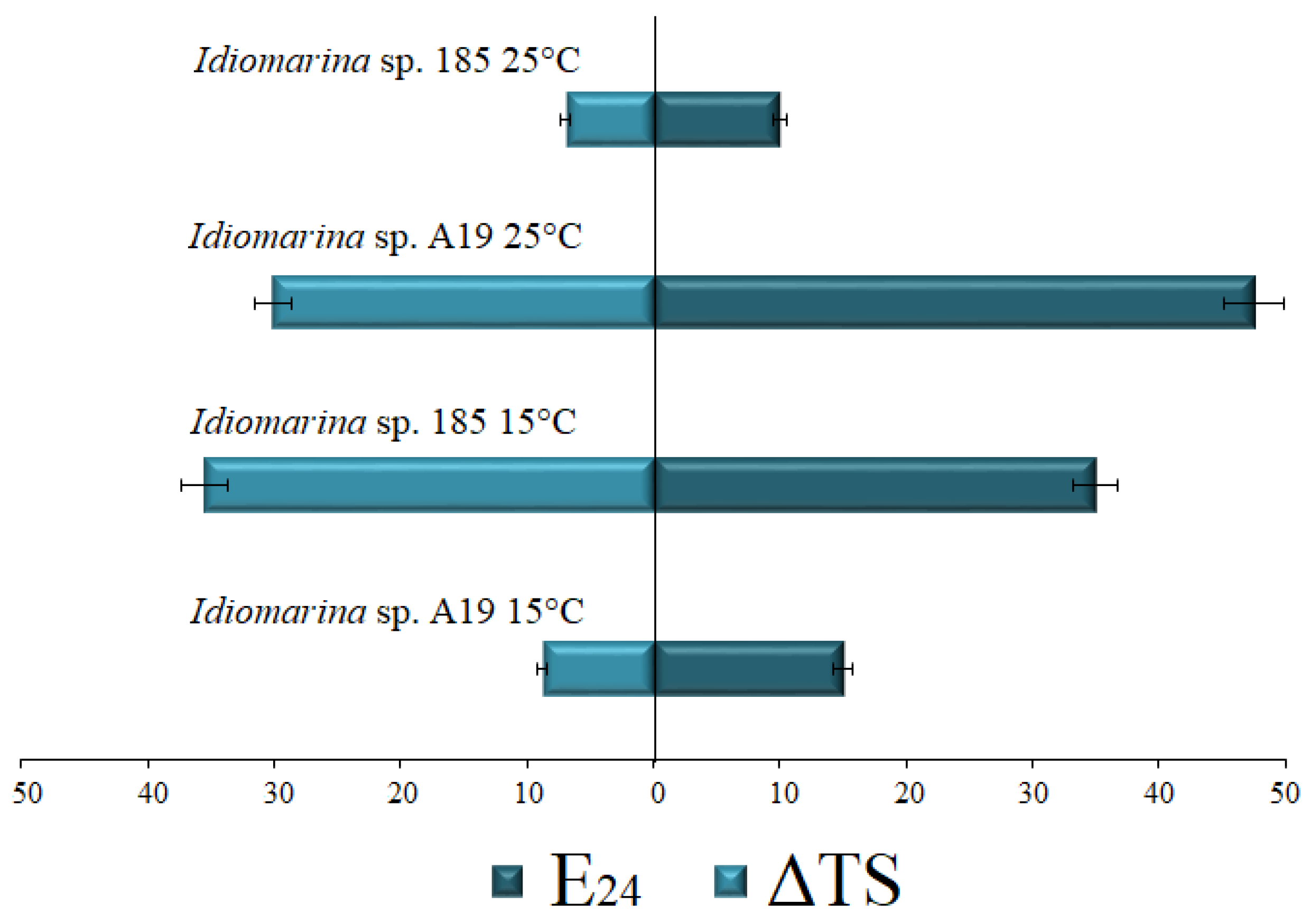
| Strain | Source * | Isolation Medium * | E24 (%) | ST ** (mN/m) | Growth Conditions | Ref |
|---|---|---|---|---|---|---|
| Strain 185 | Enrichment cultures of Antarctic shoreline sediments in seawater plus CO | BH plus CO | 30 | 29.9 | BH, 4 °C, pH 7.6, NaCl 3%, Tetradecane 2% | [18] |
| Strain A19 | Enrichment culture of polychaete homogenate in ONR7a plus CO | ONR7a plus BP | 20 | 56.5; 60 | MB, 28 °C, pH 7–7.5, NaCl 3% | [8] |
| 36.6 | 42.6 | BH, 25 °C, pH 7–7.5, NaCl 3%, Tetradecane 2% | [9] | |||
| Phenotypic characterization (Idiomarina sp. 185, [18]; Idiomarina sp. A19, this study) | ||||||
| Utilization as carbon source | Strain 185 | Strain A19 | Biochemical tests | Strain 185 | Strain A19 | |
| Arabinose | - | - | Citrate utilization | + | - | |
| Glucose | - | - | Ornithine Decarboxylase | - | - | |
| Inositol, Saccharose | - | - | H2S production | - | - | |
| Maltose | - | - | Tryptophan Deaminase | + | - | |
| Mannitol, Sorbitol | - | - | Indole production | - | - | |
| Mannose | - | - | Amygdaline | - | - | |
| Mellibiose, Rhamnose | - | - | Reduction of nitrates | + | + | |
| N-Acetyl-Glucosamine | - | - | Glucose fermentation | + | - | |
| Potassium gluconate | - | - | ||||
| Biochemical test | Adipic acid | - | + | |||
| Urease | + | - | Malate | - | + | |
| β-galactosidase | - | - | Trisodium Citrate | - | - | |
| Gelatinase | + | - | Phenylacetic acid | - | - | |
| Arginine Dihydrolase | + | - | Capric acid | - | - | |
| Lysine Decarboxylase | - | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, C.; Papale, M.; Lo Giudice, A. Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers. J. Mar. Sci. Eng. 2022, 10, 1135. https://doi.org/10.3390/jmse10081135
Rizzo C, Papale M, Lo Giudice A. Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers. Journal of Marine Science and Engineering. 2022; 10(8):1135. https://doi.org/10.3390/jmse10081135
Chicago/Turabian StyleRizzo, Carmen, Maria Papale, and Angelina Lo Giudice. 2022. "Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers" Journal of Marine Science and Engineering 10, no. 8: 1135. https://doi.org/10.3390/jmse10081135
APA StyleRizzo, C., Papale, M., & Lo Giudice, A. (2022). Idiomarina sp. Isolates from Cold and Temperate Environments as Biosurfactant Producers. Journal of Marine Science and Engineering, 10(8), 1135. https://doi.org/10.3390/jmse10081135








