Establishing an Agenda for Biofouling Research for the Development of the Marine Renewable Energy Industry in Indonesia
Abstract
1. Introduction
1.1. Overview of the Energy Profile and Targets in Indonesia
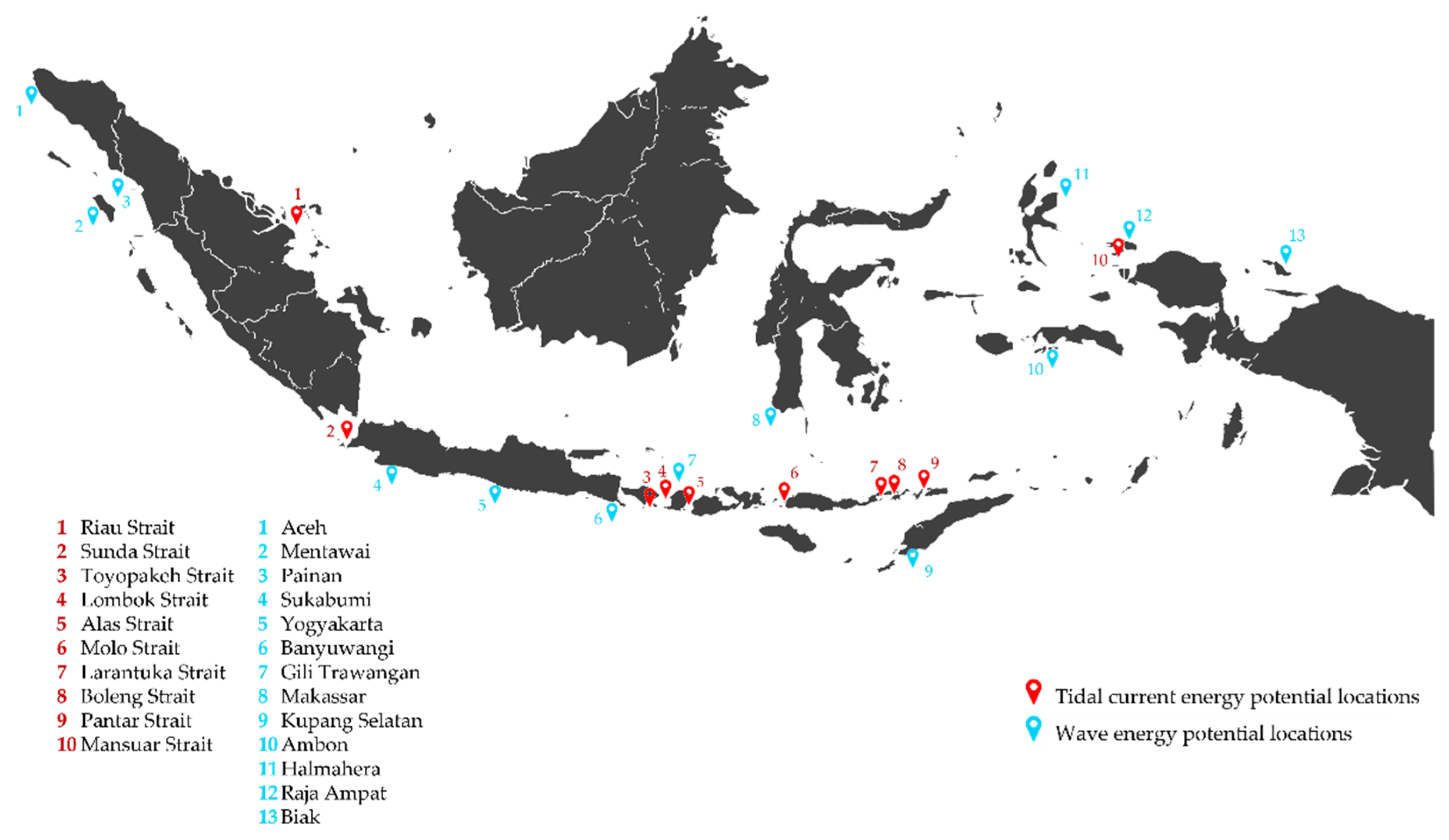
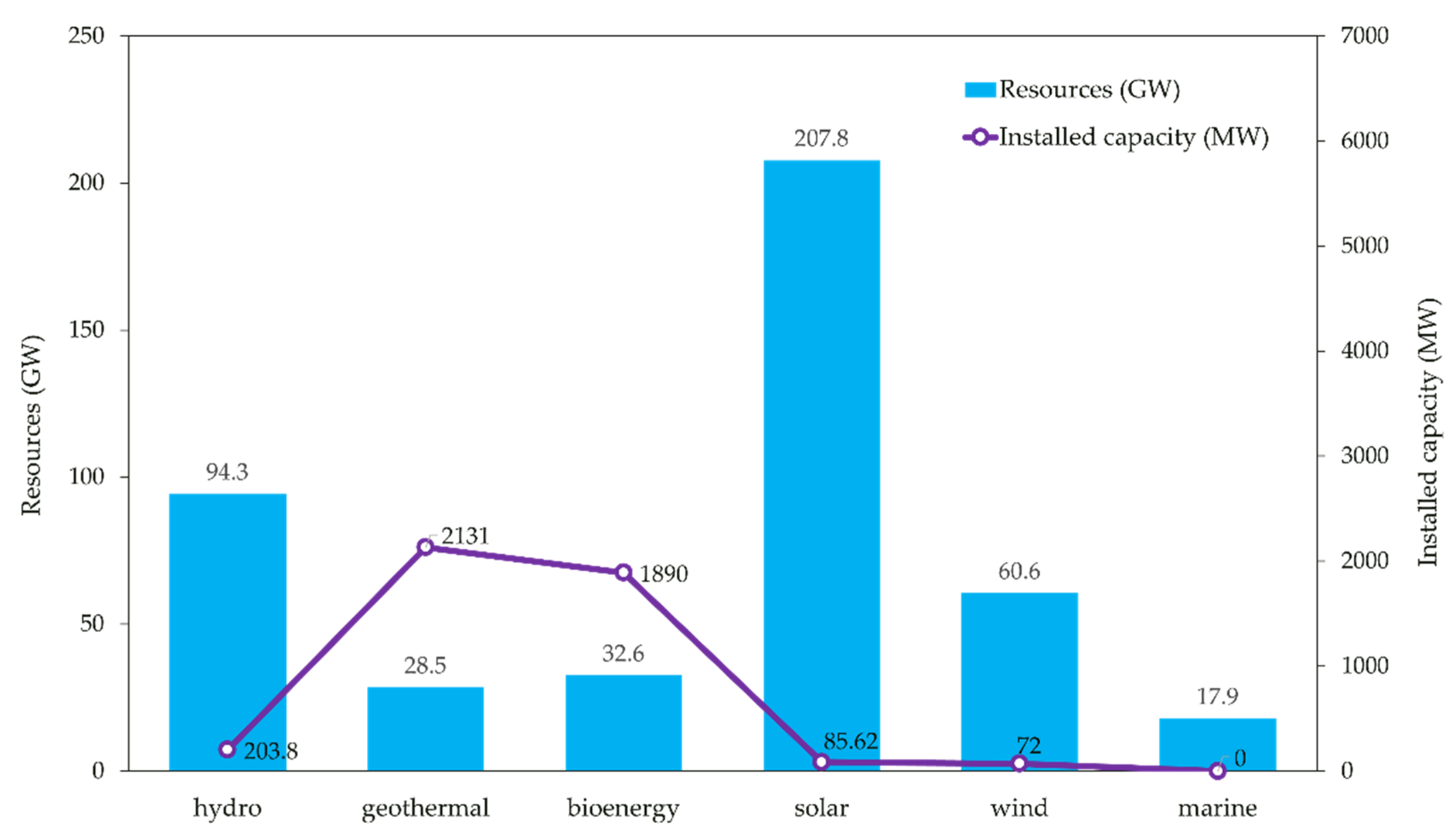
1.2. Overview of Marine Renewable Energy (MRE) Resources and Technologies in Indonesia
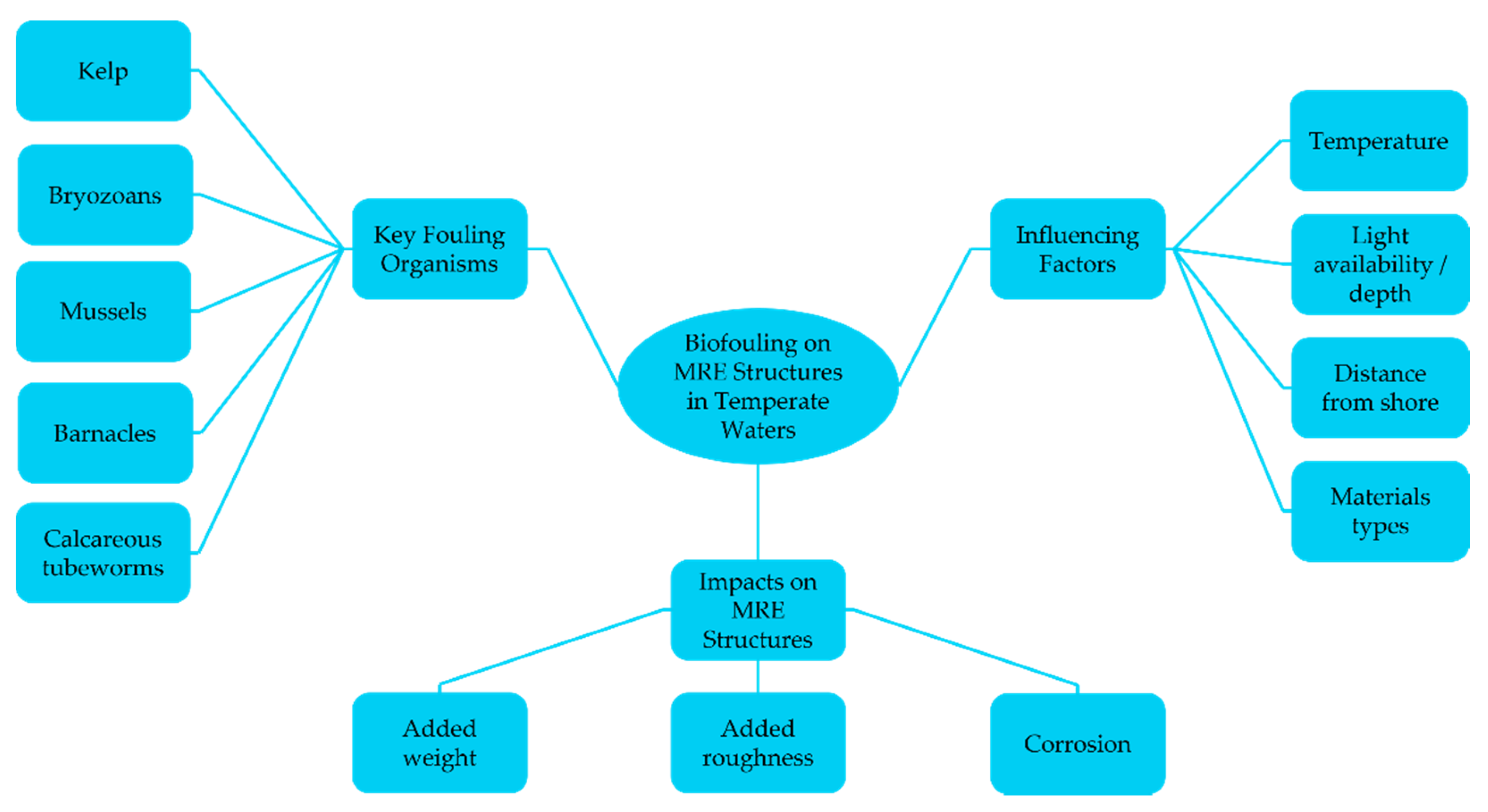
1.3. Introduction to Marine Biofouling and Associated Impacts
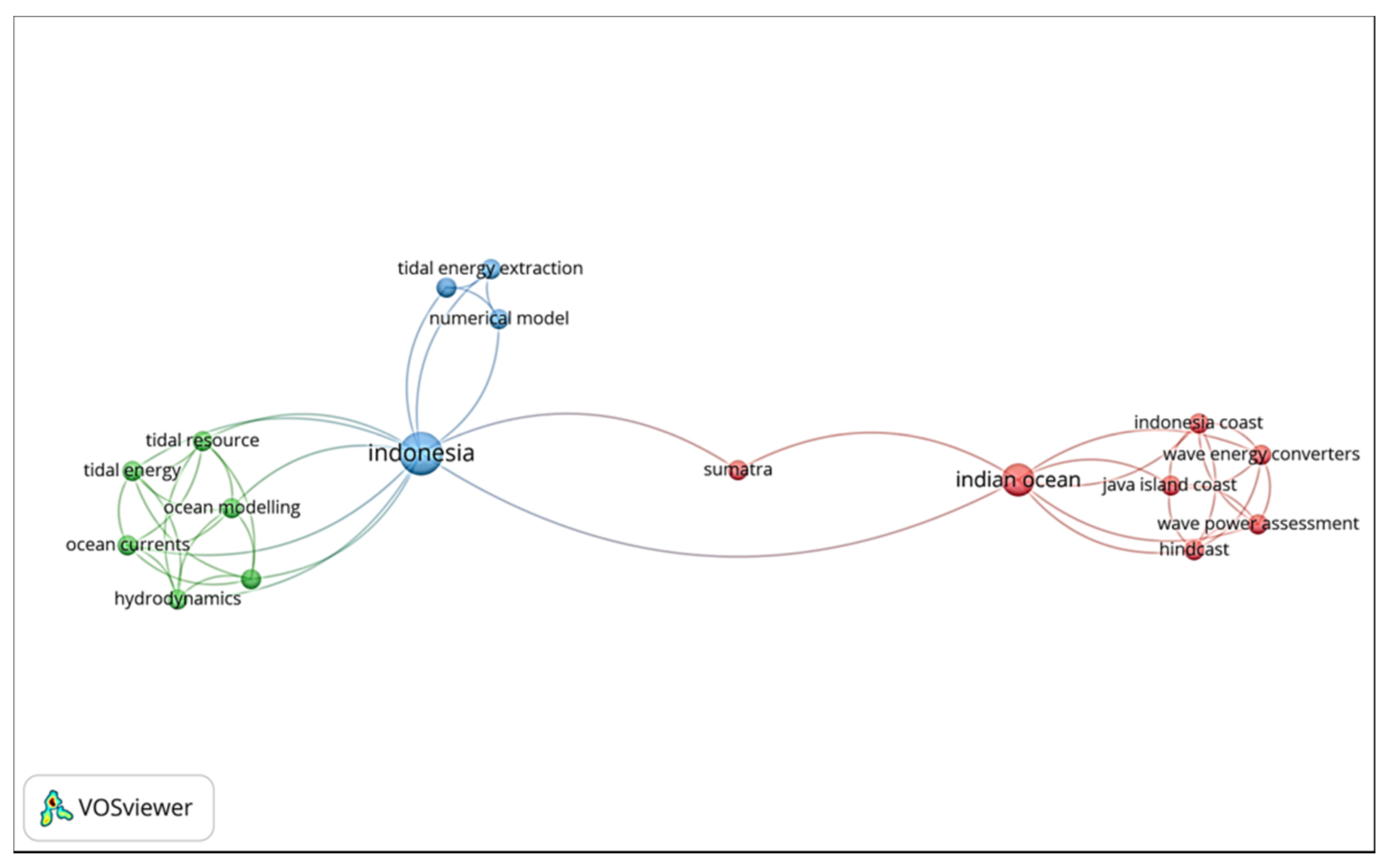
1.4. Overview of Marine Biofouling Studies in Indonesia
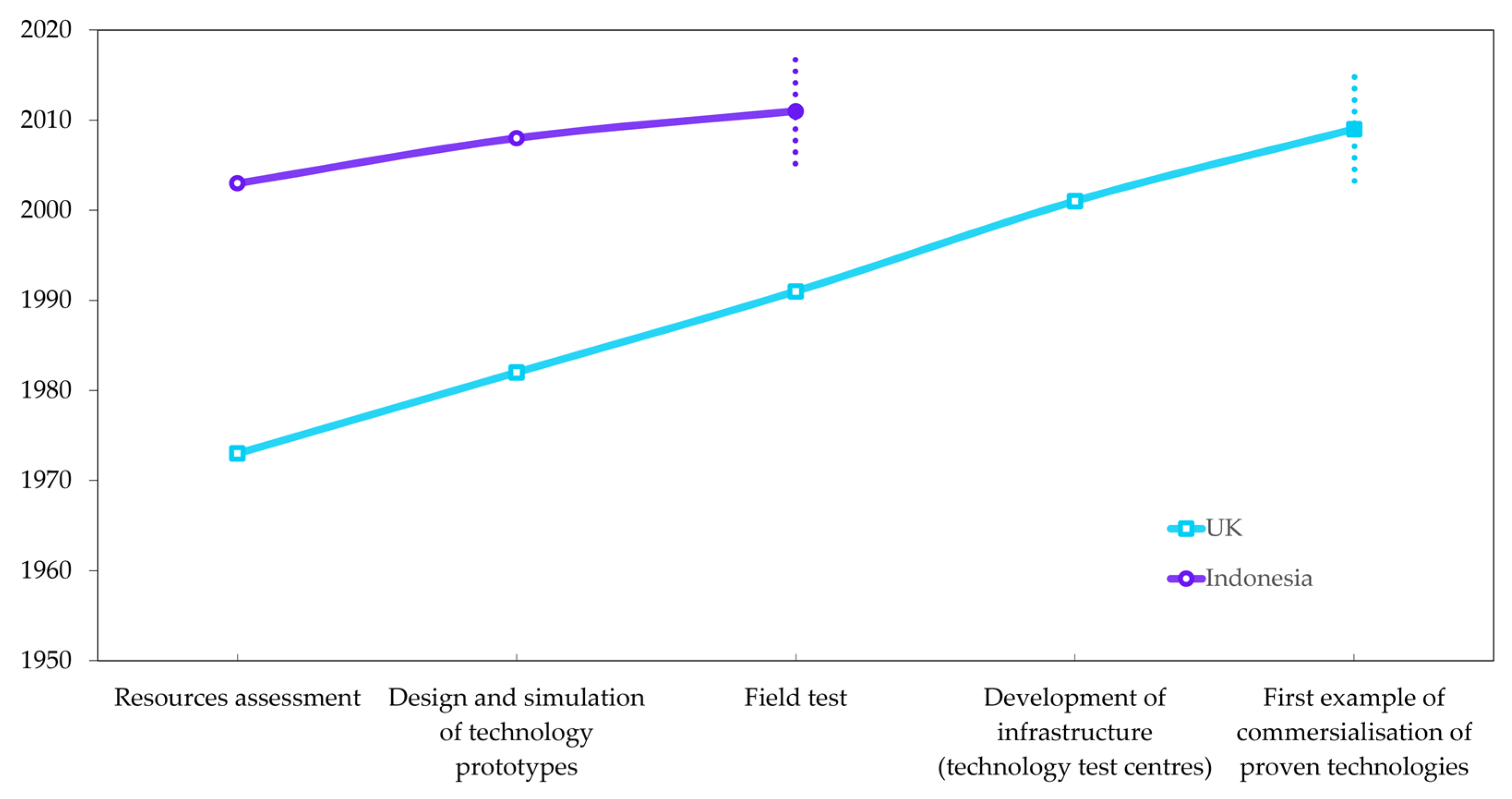
1.5. Knowledge Gaps
- Identifying implications of offshore renewable infrastructure as stepping-stones for non-indigenous marine species [52].
2. Case Studies in Temperate Waters
2.1. Overview of Marine Biofouling Research in Recent Years
2.2. Biofouling on MRE Technologies in Temperate Waters
3. Case Studies in Tropical Waters
3.1. Overview of Marine Biofouling Studies Conducted in Recent Years
3.1.1. Indonesia
3.1.2. Other Tropical Countries
3.2. Biofouling on Offshore Structures in Tropical Waters and Possible Further Research for MRE in Indonesia
4. Discussion: Lessons Learned from the Temperate Water Experience
4.1. The Importance of Biofouling Studies in Marine Renewable Energy Industry
4.2. Biofouling Database for MRE in Temperate Waters
4.3. Possible Biofouling Community and Their Risks on MRE Infrastructure in Indonesian Waters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erahman, Q.F.; Purwanto, W.W.; Sudibandriyo, M.; Hidayatno, A. An assessment of Indonesia’s energy security index and comparison with seventy countries. Energy 2016, 111, 364–376. [Google Scholar] [CrossRef]
- Iswadi, A.; Owen, A.; Garniati, L.; Sugardjito, J. Marine renewable energy: Opportunities and challenges for community development in coastal area of Indonesia. Int. J. Serv. Technol. Manag. 2018, 24, 522–544. [Google Scholar] [CrossRef]
- National Energy Council. Indonesia Energy Outlook 2019; Secretariat General of National Energy Council: Jakarta, Indonesia, 2019. [Google Scholar]
- Publications Office of the European Union. Strategic Plan Book 2020–2024; Ministry of Energy and Mineral Resources: Jakarta, Indonesia, 2020. [Google Scholar]
- Institute for Essential Services Reform. Igniting a Rapid Deployment of Renewable Energy in Indonesia: Lessons Learned from Three Countries; Institute for Essential Services Reform: Jakarta, Indonesia, 2018; Available online: http://iesr.or.id/wp-content/uploads/2019/05/IESR_Research_Igniting-a-Rapid-Deployment-of-RE-in-Indonesia.pdf (accessed on 13 August 2020).
- Yudha, S.W.; Tjahjono, B. Stakeholder mapping and analysis of the renewable energy industry in Indonesia. Energy 2019, 12, 602. [Google Scholar] [CrossRef]
- Hidayati, N.; Mahmudi, M.; Kurniawan, D.; Musa, M.; Setiawan, H. Ocean currents energy for electricity generation and its potential in east java water, Indonesia. J. Environ. Eng. Sustain. Technol. 2016, 3, 104–111. [Google Scholar] [CrossRef][Green Version]
- Firdaus, A.M.; Houlsby, G.T.; Adcock, T.A. Opportunities for Tidal Stream Energy in Indonesian Waters. In Proceedings of the 12th European Wave and Tidal Energy Conference, Cork, Ireland, 2017; p. 887. [Google Scholar]
- Ray, R.D.; Susanto, R.D. Tidal mixing signatures in the Indonesian seas from high-resolution sea surface temperature data. Geophys. Res. Lett. 2016, 43, 8115–8123. [Google Scholar] [CrossRef]
- Sugianto, D.N.; Kunarso, K.; Helmi, M.; Alifdini, I.; Maslukah, L.; Saputro, S.; Yusuf, M.; Endrawati, H. Wave energy reviews in Indonesia. Int. J. Mech. Eng. Technol. 2017, 8, 448–459. [Google Scholar]
- Minister of Energy and Mineral Resource. Indonesia Energy Outlook Association. In Ocean Energy Resources in Indonesia; Ministry of Energy and Mineral Resources: Jakarta, Indonesia, 2014. [Google Scholar]
- Orhan, K.; Mayerle, R.; Pandoe, W.W. Assesment of Energy Production Potential from Tidal Stream Currents in Indonesia. Energy Procedia 2015, 76, 7–16. [Google Scholar] [CrossRef]
- Orhan, K.; Mayerle, R. Assessment of the tidal stream power potential and impacts of tidal current turbines in the Strait of Larantuka, Indonesia. Energy Procedia 2017, 125, 230–239. [Google Scholar] [CrossRef]
- Wahyudie, A.; Susilo, T.B.; Alaryani, F.; Nandar, C.S.A.; Jama, M.A.; Daher, A.; Shareef, H. Wave Power Assessment in the Middle Part of the Southern Coast of Java Island. Energies 2020, 13, 2633. [Google Scholar] [CrossRef]
- Hantoro, R.; Utama, I.K.A.P.; Erwandi, E.; Sulisetyono, A. An experimental investigation of passive variable-pitch vertical-axis ocean current turbine. ITB J. Eng. Sci. 2011, 43, 27–40. [Google Scholar] [CrossRef]
- Hantoro, R.; Septyaningrum, E. Novel design of a vertical axis hydrokinetic turbine-straight-blade cascaded (VAHT-SBC): Experimental and numerical simulation. J. Eng. Technol. Sci. 2018, 50, 73–86. [Google Scholar] [CrossRef]
- Hantoro, R.; Utama, I.K.A.P.; Arief, I.S.; Ismail, A.; Manggala, S.W. Innovation in Vertical Axis Hydrokinetic Turbine—Straight Blade Cascaded (VAHT-SBC) design and testing for low current speed power generation. J. Phys. Conf. Ser. 2018, 1022, 12023. [Google Scholar] [CrossRef]
- Erwandi; Rahuna, D.; Rahuna, D.; Mintarso, C.S.J. The development of the IHL wave-current turbine to convert the ocean energy from both wave and current. In IOP Conference Series: Materials Science and Engineering 2019; IOP Publishing: Bristol, UK, 2019; Volume 588, p. 12008. [Google Scholar] [CrossRef]
- Arif, I.S.; Utama, I.K.A.P.; Hartono, R.; Prananda, J.; Isnaini, R.; Rachmattra, A.T. Analysis Characteristics of Pendulum Oscillation In PLTGL-SB. E3S Web Conf. 2018, 43, 1005. [Google Scholar] [CrossRef]
- Arief, I.S.; Utama, I.K.A.P.; Hantoro, R.; Prananda, J.; Arvisa, T.R.; Kusuma, R.F. Mooring Experimental Study of Motion Response for Pendulum Wave Energy Converters. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 462, p. 12010. [Google Scholar] [CrossRef]
- Macleod, A.K.; Stanley, M.S.; Day, J.G.; Cook, E.J. Biofouling community composition across a range of environmental conditions and geographical locations suitable for floating marine renewable energy generation. Biofouling 2016, 32, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Want, A.; Crawford, R.; Kakkonen, J.; Kiddie, G.; Miller, S.; Harris, R.E.; Porter, J.S. Biodiversity characterisation and hydrodynamic consequences of marine fouling communities on marine renewable energy infrastructure in the Orkney Islands Archipelago, Scotland, UK. Biofouling 2017, 33, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Figueira, R.B. Corrosion protection systems and fatigue corrosion in offshore wind structures: Current status and future perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- Bressy, C.; Lejars, M. Marine fouling: An overview. J. Ocean. Technol. 2014, 9, 19–28. [Google Scholar]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.; Abel, P.D.; Arnold, D.W.; Milne, A. Cost–benefit analysis of the use of TBT: The case for a treatment approach. Sci. Total Environ. 2000, 258, 5–19. [Google Scholar] [CrossRef]
- Satheesh, S.; Ba-akdah, M.A.; Al-Sofyani, A.A. Natural antifouling compound production by microbes associated with marine macroorganisms—A review. Electron. J. Biotechnol. 2016, 21, 26–35. [Google Scholar] [CrossRef]
- Ilhan-Sungur, E.; Unsal-Istek, T.; Cansever, N. Microbiologically influenced corrosion of galvanized steel by Desulfovibrio sp. and Desulfosporosinus sp. in the presence of Ag–Cu ions. Mater. Chem. Phys. 2015, 162, 839–851. [Google Scholar] [CrossRef]
- Javed, M.A.; Neil, W.C.; McAdam, G.; Wade, S.A. Effect of sulphate-reducing bacteria on the microbiologically influenced corrosion of ten different metals using constant test conditions. Int. Biodeterior. Biodegrad. 2017, 125, 73–85. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Liengen, T.; Basseguy, R.; Feron, D.; Beech, I.; Birrien, V. Understanding Biocorrosion: Fundamentals and Applications; Elsevier: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Bott, T.R. Industrial Biofouling: Occurrence and Control; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Wu, J.; Zhang, D.; Wang, P.; Cheng, Y.; Sun, S.; Sun, Y.; Chen, S. The influence of Desulfovibrio sp. and Pseudoalteromonas sp. on the corrosion of Q235 carbon steel in natural seawater. Corros. Sci. 2016, 112, 552–562. [Google Scholar] [CrossRef]
- Julistiono, H.; Hidayati, Y.; Yuslaini, N.; Nditasari, A.; Dinoto, A.; Sundjono; Nuraini, L.; Priyotomo, G.; Gunawan, H. Identification of biofilm-forming bacteria from steel panels exposed in sea waters of Jakarta Bay and Madura Strait. Invent. Prosperous Future Through Biol. Res. Trop. Biodivers. Manag. 2018, 2002, 020029. [Google Scholar] [CrossRef]
- Sawall, Y.; Richter, C.; Ramette, A. Effects of Eutrophication, Seasonality and Macrofouling on the Diversity of Bacterial Biofilms in Equatorial Coral Reefs. PLoS ONE 2012, 7, e39951. [Google Scholar] [CrossRef] [PubMed]
- Azmi, F.; Hewitt, C.L.; Campbell, M.L. A hub and spoke network model to analyse the secondary dispersal of introduced marine species in Indonesia. ICES J. Mar. Sci. 2015, 72, 1069–1077. [Google Scholar] [CrossRef]
- Huhn, M.; Zamani, N.; Lenz, M. A ferry line facilitates dispersal: Asian green mussels Perna viridis (Linnaeus, 1758) detected in eastern Indonesia. Bioinvasions Rec. 2015, 4, 23–29. [Google Scholar] [CrossRef]
- Huhn, M.; Madduppa, H.H.; Khair, M.; Sabrian, A.; Irawati, Y.; Anggraini, N.P.; Wilkinson, S.P.; Simpson, T.; Iwasaki, K.; Setiamarga, D.H.E.; et al. Keeping up with introduced marine species at a remote biodiversity hotspot: Awareness, training and collaboration across different sectors is key. Biol. Invasions 2019, 22, 749–771. [Google Scholar] [CrossRef]
- Azmi, F.; Primo, C.; Hewitt, C.L.; Campbell, M.L. Assessing marine biosecurity risks when data are limited: Bioregion pathway and species-based exposure analyses. ICES J. Mar. Sci. 2015, 72, 1078–1091. [Google Scholar] [CrossRef]
- Nugroho, B.; Ganapathisubramani, B.; Utama, I.K.A.P.; Suastika, I.K.; Prasetyo, F.A.; Yusuf, M.; Tullberg, M.; Monty, J.P.; Hutching, N. Managing international collaborative research between academics, industries, and policy makers in understanding the effects of biofouling in ship hull turbulent boundary layers. Trans. R. Inst. Nav. Archit. Part A Int. J. Marit. Eng. 2017, 159, 291–300. [Google Scholar] [CrossRef]
- Yusim, A.K.; Utama, I.K.A.P. An Investigation into The Drag Increase on Roughen Surface due to Marine Fouling Growth. Maj. IPTEK 2017, 28, 73–78. [Google Scholar] [CrossRef]
- Baital, M.S.; Pria Utama, I.K.A. CFD Analysis into the Drag Estimation of Smooth and Roughened Surface Due to Marine Biofouling. Maj. IPTEK 2017, 28, 90–97. [Google Scholar] [CrossRef]
- Hakim, M.L.; Nugroho, B.; Nurrohman, M.N.; Suastika, I.K.; Utama, I.K.A.P. Investigation of fuel consumption on an operating ship due to biofouling growth and quality of anti-fouling coating. In IOP Conference Series: Earth and Environmental Science 2019; IOP Publishing: Bristol, UK, 2019; Volume 339, p. 12037. [Google Scholar] [CrossRef]
- Pratikno, H.; Titah, H.S.; Syahputra, B.E.D. Effect of heat treatment on bio-corrosion rate of steel structure (API 5L) in marine evironment. MATEC Web Conf. 2018, 197, 4002. [Google Scholar] [CrossRef][Green Version]
- Nuraini, L.; Prifiharni, S.; Priyotomo, G.; Sundjono; Gunawan, H. Evaluation of anticorrosion and antifouling paint performance after exposure under seawater Surabaya–Madura (Suramadu) bridge. In Proceedings of the International Conference on Chemistry, Chemical Process and Engineering, Yogyakarta, Indonesia, 15–16 November 2017; Volume 1823. [Google Scholar] [CrossRef]
- Priyotomo, G.; Nuraini, L.; Gunawan, H.; Triwardono, J.; Sundjono, S.; Prifiharni, S. A Preliminary field study of antifouling paint perfomance after short exposure in Mandara Bali, Indonesia. Int. J. Eng. Trans. A Basics 2021, 34, 976–986. [Google Scholar] [CrossRef]
- Aunurohim; Kurniawan, W.A.; Nurilma, A.D.; Desmawati, I.; Albab, M. Field Optimation of Durio zibethinus as Macro-Antifouling at PT Dok and Shipping, Surabaya, Indonesia. In IOP Conference Series: Earth and Environmental Science 2018; IOP Publishing: Bristol, UK, 2019; Volume 197, p. 12010. [Google Scholar]
- Pratikno, H.; Sulistiyaning Titah, H.; Danesto Rizky Mauludin, M. System Impressed Current Anti Fouling (ICAF) against micro fouling (Bacteria) on ship’s cooling system. MATEC Web Conf. 2018, 177, 1013. [Google Scholar] [CrossRef][Green Version]
- Nall, C.R.; Schläppy, M.-L.; Guerin, A.J. Characterisation of the biofouling community on a floating wave energy device. Biofouling 2017, 33, 379–396. [Google Scholar] [CrossRef]
- Yang, S.-H.; Ringsberg, J.W.; Johnson, E.; Hu, Z. Biofouling on mooring lines and power cables used in wave energy converter systems—Analysis of fatigue life and energy performance. Appl. Ocean Res. 2017, 65, 166–177. [Google Scholar] [CrossRef]
- Loxton, J.; Macleod, A.K.; Nall, C.R.; McCollin, T.; Machado, I.; Simas, T.; Vance, T.; Kenny, C.; Want, A.; Miller, R.G. Setting an agenda for biofouling research for the marine renewable energy industry. Int. J. Mar. Energy 2017, 19, 292–303. [Google Scholar] [CrossRef]
- Adams, T.P.; Miller, R.G.; Aleynik, D.; Burrows, M.T.; Frederiksen, M. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Appl. Ecol. 2014, 51, 330–338. [Google Scholar] [CrossRef]
- Elizabeth, J.C.; Robin, D.P.; Adrian, K.M.; Sarah, F.B. Marine biosecurity: Protecting indigenous marine species. Res. Rep. Biodivers. Stud. 2016, 5, 1. [Google Scholar] [CrossRef]
- Minchin, D.; Cook, E.J.; Clark, P.F. Alien species in British brackish and marine waters. Aquat. Invasions 2013, 8, 3–19. [Google Scholar] [CrossRef]
- Farkas, A.; Song, S.; Degiuli, N.; Martić, I.; Demirel, Y.K. Impact of biofilm on the ship propulsion characteristics and the speed reduction. Ocean Eng. 2020, 199, 107033. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G.; Buisman, E.; et al. Costs and benefits to European shipping of ballast-water and hull-fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Legg, M.; Yücel, M.K.; Garcia de Carellan, I.; Kappatos, V.; Selcuk, C.; Gan, T.H. Acoustic methods for biofouling control: A review. Ocean Eng. 2015, 103, 237–247. [Google Scholar] [CrossRef]
- De Castro, M.C.T.; Vance, T.; Yunnie, A.L.E.; Fileman, T.W.; Hall-Spencer, J.M. Low salinity as a biosecurity tool for minimizing biofouling on ship sea chests. Ocean Sci. 2018, 14, 661–667. [Google Scholar] [CrossRef]
- Duncan, K.E.; Davidova, I.A.; Nunn, H.S.; Stamps, B.W.; Stevenson, B.S.; Souquet, P.J.; Suflita, J.M. Design features of offshore oil production platforms influence their susceptibility to biocorrosion. Appl. Microbiol. Biotechnol. 2017, 101, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Gormley, K.; McLellan, F.; McCabe, C.; Hinton, C.; Ferris, J.; Kline, D.I.; Scott, B.E. Automated image analysis of offshore infrastructure marine biofouling. J. Mar. Sci. Eng. 2018, 6, 2. [Google Scholar] [CrossRef]
- Wells, F.E.; Tan, K.S.; Todd, P.A.; Jaafar, Z.; Yeo, D.C.J. A low number of introduced marine species in the tropics: A case study from Singapore. Manag. Biol. Invasions 2019, 10, 23–45. [Google Scholar] [CrossRef]
- Satheesh, S.; Wesley, S.G. Seasonal changes of motile polychaetes in the fouling assemblage developed on test panels submerged on a tropical coast. J. Mar. Biol. Assoc. United Kingd. 2013, 93, 1525–1531. [Google Scholar] [CrossRef]
- Desai, D.V.; Krishnamurthy, V.; Anil, A.C. Biofouling Community Structure in a Tropical Estuary of Goa on the West Coast of India. ASEAN J. Sci. Technol. Dev. 2018, 35, 37–42. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Liu, W.; Liu, K.; Zhang, S.; He, X.; Huang, Y.; Lin, J.; Mou, J.; Zheng, C.; et al. Fouling community characteristics in subtropical coastal waters of the southwestern East China Sea. Acta Oceanol. Sin. 2017, 36, 70–78. [Google Scholar] [CrossRef]
- Lim, C.S.; Jolkifli, Z.H.; Jair, A.; Karim, N.; Wahab, R.A.; Desai, D.V.; Krishnamurthy, V.; Khandeparker, L.; Mapari, K.; Sawant, S.; et al. An inter-site study of biofouling recruitment on static immersion panels in major ports of South East Asia and India. ASEAN J. Sci. Technol. Dev. 2018, 35, 167–176. [Google Scholar] [CrossRef]
- Yan, T.; Yan, W.X. Fouling of Offshore Structures in China-a Review. Biofouling 2003, 19 (Suppl. S1), 133–138. [Google Scholar] [CrossRef]
- Cao, W.; Yan, T.; Li, Z.; Li, J.; Cheng, Z. Fouling acorn barnacles in China—A review. Chin. J. Oceanol. Limnol. 2013, 31, 699–711. [Google Scholar] [CrossRef]
- Boukinda Mbadinga, M.L.; Schoefs, F.; Quiniou-Ramus, V.; Birades, M.; Garretta, R. Marine Growth Colonization Process in Guinea Gulf: Data Analysis. J. Offshore Mech. Arct. Eng. 2007, 129, 97–106. [Google Scholar] [CrossRef]
- Elliott, D. Renewable Energy in the UK Past, Present and Future/by David Elliott; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Mueller, M.; Wallace, R. Enabling science and technology for marine renewable energy. Energy Policy 2008, 36, 4376–4382. [Google Scholar] [CrossRef]
- Kasharjanto, A.; Rahuna, D.; Rina, R. Kajian Pemanfaatan Energi Arus Laut Di Indonesia. Wave: J. Ilm. Teknol. Marit. 2017, 11, 75–84. [Google Scholar] [CrossRef]
- Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 495. [Google Scholar] [CrossRef]
- Hadiyanto, H. Fouling Polychaetes in Tanjung Priok Port of Jakarta, Indonesia. ASEAN J. Sci. Technol. Dev. 2018, 35, 79–87. [Google Scholar] [CrossRef]
- Want, A.; Bell, M.C.; Harris, R.E.; Hull, M.Q.; Long, C.R.; Porter, J.S. Sea-trial verification of a novel system for monitoring biofouling and testing anti-fouling coatings in highly energetic environments targeted by the marine renewable energy industry. Biofouling 2021, 37, 433–451. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Research Area | Key Findings |
|---|---|---|
| Julistiono et al. [34] * | Bacterial biofilm (biofilm-forming bacteria) | The study isolated and screened bacterial colonies from Jakarta Bay and Madura Strait for biofilm formulation. Based on the molecular identification of isolates from both locations, it was found that the most active biofouling bacterial isolates in Jakarta Bay and Madura Strait were closely related to Vibrio alginolyticus and Vibrio natriegens, respectively. Both biofilm-forming bacteria were commonly found in the Java Islands waters. |
| Sawall et al. [35] * | Bacterial biofilm | The study identified bacterial composition and abundance patterns of coral reef biofilms from 4 regions in Spermando Archipelago, Indonesia. Strong influences of microhabitat type (exposed or sheltered), season and nutrient availability affected by anthropogenic activities were noted. On the other hand, variations in macrofouling community structure did not have a notable influence. |
| Azmi et al. [36] * | Biological invasion: Geographical aspects | The authors developed a hub—spoke network model to illustrate the level of shipping activity from 32 regions in Indonesia, with Tanjung Priok Port in Jakarta Bay as the main hub. Based on the data available, East Java, Central Java, and North Sumatera appear to have highest traffic frequency, with largest numbers of shipping activity from Tanjung Priok Port, which more likely to encounter the introduced marine species transfer. Regional shipping, with short travel distance (duration) and slow speed vessels used within intra-coastal transport, appear most likely to promote biofouling development reaching spoke ports. |
| Huhn et al. [37] * | Biological invasion | The study found that the Asian green mussel Perna viridis, a native organism in western Indonesia, was transferred to eastern Indonesia by domestic shipping activity. The green mussels collected in eastern Indonesia had lower body condition index than those in western Indonesia. |
| Huhn et al. [38] * | Biological invasion: education and awareness raising | Education and awareness raising among relevant stakeholders are important factors in preventing bioinvasions. The study has produced crucial baseline data on introduced marine species and raised awareness on the impact of vessel activities around Banda Islands to non-native species invasion that could cause biofouling settlement. |
| Azmi et al. [39] * | Marine biosecurity risk management | The study evaluated a bioregion pathway risk model and a species-based exposure risk model that can be utilised in managing limited biological data for marine biosecurity in Indonesia waters. |
| Nugroho et al. [40] ** | Ship drag due to biofouling | Four important research strands were developed: (i) identifying the effect on drag of roughness on ship hulls, evaluated using Laser Doppler Anemometer (LDA); (ii) developing an image-based scanner for recording biofouling development on ship hulls; (iii) validating the LDA technique using a wind tunnel experiment; and (iv) linking the data from the first three strands with ship performance data (GPS coordinate, ship velocity and fuel usage). |
| Yusim et al. [41] ** | Ship drag due to biofouling | This study compared three different roughness models (smooth hull, regular roughness, irregular roughness) due to biofouling that cause ship drag/resistance. |
| Baital et al. [42] ** | Ship drag due to biofouling | A computational fluid dynamics (CFD) simulation indicated that the ship drag due to biofouling would increase by up to 37% per year in the absence of mitigation measures. |
| Hakim et al. [43] * | Ship drag due to biofouling | Biofouling could affect ship resistance and hence power demand. The study found that if the ship operates in normal continuous rating (NCR), biofouling causes fuel consumption to increase by up to 16.7% in addition to the increase in sailing time. On the other hand, if the ship operates in maximum continuous rating (MCR), it will increase fuel consumption by up to 62.5%. |
| Pratikno et al. [44] * | Biocorrosion in artificial seawater | The biocorrosion rate of steel was accelerated by addition of bacteria with 3.7699 millimetres per year (mpy) as highest biocorrosion rate caused by Thiobacillus ferrooxidans. The heat treatment (austempering process) decreased the biocorrosion rate to 3.5046 mpy. |
| Nuraini et al. [45] * | Mitigation measures | Performance of anticorrosion and antifouling paints was compared and evaluated in a field test under Suramadu Bridge. |
| Priyotomo et al. [46] * | Mitigation measures | Preliminary investigation on the performance evaluation of antifouling paint in Mandara Bali found that sea depth variation of up to 3 m did not affect steel surfaces differently after exposure of up to 1-month immersion. |
| Aunurohim et al. [47] * | Mitigation measures | The study investigated natural macro-antifouling system performance using concentrates derived from the durian Durio zibethinus. Field tests conducted in a harbour area found that adhesion percentage of Balanus amphitrite declined with an increase of gel extract concentration, containing D. zibethinus, in the antifouling paint. |
| Pratikno et al. [48] * | Mitigation measures | An impressed current antifouling system was demonstrated to be useful in controlling microfouling development by reducing the bacteria (Pseudomonas fluorescens) population settlement. The population reduction of P. fluorescens was 98.5–99% with increasing time and electrical current. |
| Identified Fouling Organism | Discovery Location | Characteristics of Location | Identified Characteristics of Fouling Organisms | Possible Risks on MRE Infrastructure | |
|---|---|---|---|---|---|
| Group | Common Name/Species | ||||
| Polychaeta: Serpulidae | Calcareous polychaetes | Tanjung Priok Port, Jakarta, Indonesia | Port area | Polychaetes were observed during dry season and monsoon season. The dominant settlement occurred in the early monsoon season in November and December 2013 with coverage percentage more than 50%. However, long-term observation found the influence of its settlement was much lower than barnacle and mollusc [65] | Due to the high adhesion strength to the structure, their settlement might cause abrasion and induce corrosion [72] |
| Crustacea: Cirripedia | Barnacle (Amphibalanus sp.) | Tanjung Priok Port, Jakarta, Indonesia | Port area | Barnacles were observed during dry season and transition to monsoon season. Based on the longer observation, barnacle had high influence on panel structure, particularly in June 2013 [65] | Influence device movement by adding substantial weight to the component structures. Due to the high adhesion strength to the structure, their settlement might also cause abrasion and induce corrosion [72] |
| Mollusca: Bivalvia | Perna viridis | Tanjung Priok Port, Jakarta, Indonesia | Port area | Based on the long-term observation, bivalves colonised dominantly the panel structure after barnacle settlement decreased in the transition period of season [65] | Influence device movement by adding substantial weight to the component structures [72] |
| Cnidaria: Hydrozoa | Hydroids | Northern South China Sea | Offshore floating marine installations | In the offshore area, stalked barnacles and hydroids dominated fouling settlement in depth more than 100 m. Acorn barnacles were also observed but their biomass and diversity declined drastically with increasing distance from the shore [66] | Due to the high adhesion strength of barnacles to the structure, their settlement might cause abrasion and induce corrosion [72] |
| Crustacea: Cirripedia | Stalked barnacles | ||||
| Algae | Seaweeds | Gulf of Guinea | Offshore structure | Barnacles, hydroids, seaweeds were found dominantly on the offshore structure in early years of observation. On some structures, barnacles and corals colonised the surface together after a few periods of years [68] | Due to the high adhesion strength of barnacles to the structure, their settlement might cause abrasion and induce corrosion [72] |
| Cnidaria: Hydrozoa | Hydroids | ||||
| Cnidaria: Anthozoa | Coral | ||||
| Crustacea: Cirripedia | Barnacles | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iswadi, A.; Porter, J.S.; Bell, M.C.; Garniati, L.; Harris, R.E.; Priyotomo, G. Establishing an Agenda for Biofouling Research for the Development of the Marine Renewable Energy Industry in Indonesia. J. Mar. Sci. Eng. 2022, 10, 384. https://doi.org/10.3390/jmse10030384
Iswadi A, Porter JS, Bell MC, Garniati L, Harris RE, Priyotomo G. Establishing an Agenda for Biofouling Research for the Development of the Marine Renewable Energy Industry in Indonesia. Journal of Marine Science and Engineering. 2022; 10(3):384. https://doi.org/10.3390/jmse10030384
Chicago/Turabian StyleIswadi, Agung, Joanne S. Porter, Michael C. Bell, Leuserina Garniati, Robert E. Harris, and Gadang Priyotomo. 2022. "Establishing an Agenda for Biofouling Research for the Development of the Marine Renewable Energy Industry in Indonesia" Journal of Marine Science and Engineering 10, no. 3: 384. https://doi.org/10.3390/jmse10030384
APA StyleIswadi, A., Porter, J. S., Bell, M. C., Garniati, L., Harris, R. E., & Priyotomo, G. (2022). Establishing an Agenda for Biofouling Research for the Development of the Marine Renewable Energy Industry in Indonesia. Journal of Marine Science and Engineering, 10(3), 384. https://doi.org/10.3390/jmse10030384