The First Population Simulation for the Zalophus japonicus (Otariidae: Sea Lions) on Dokdo, Korea
Abstract
:1. Introduction
2. Materials and Methods
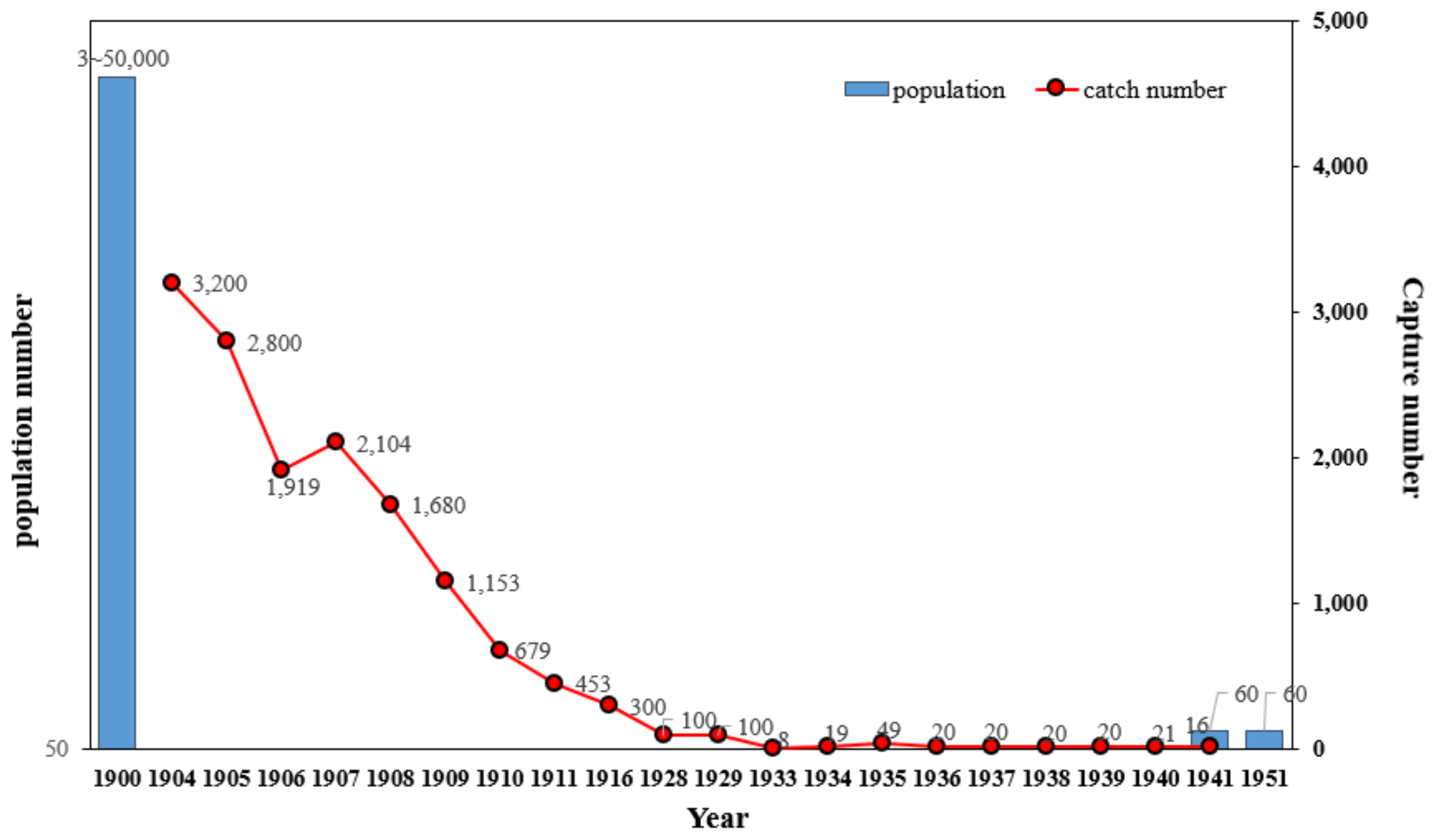
2.1. Historical Hunting Records
2.2. Stage-Structured Model
2.3. Parameter Estimation
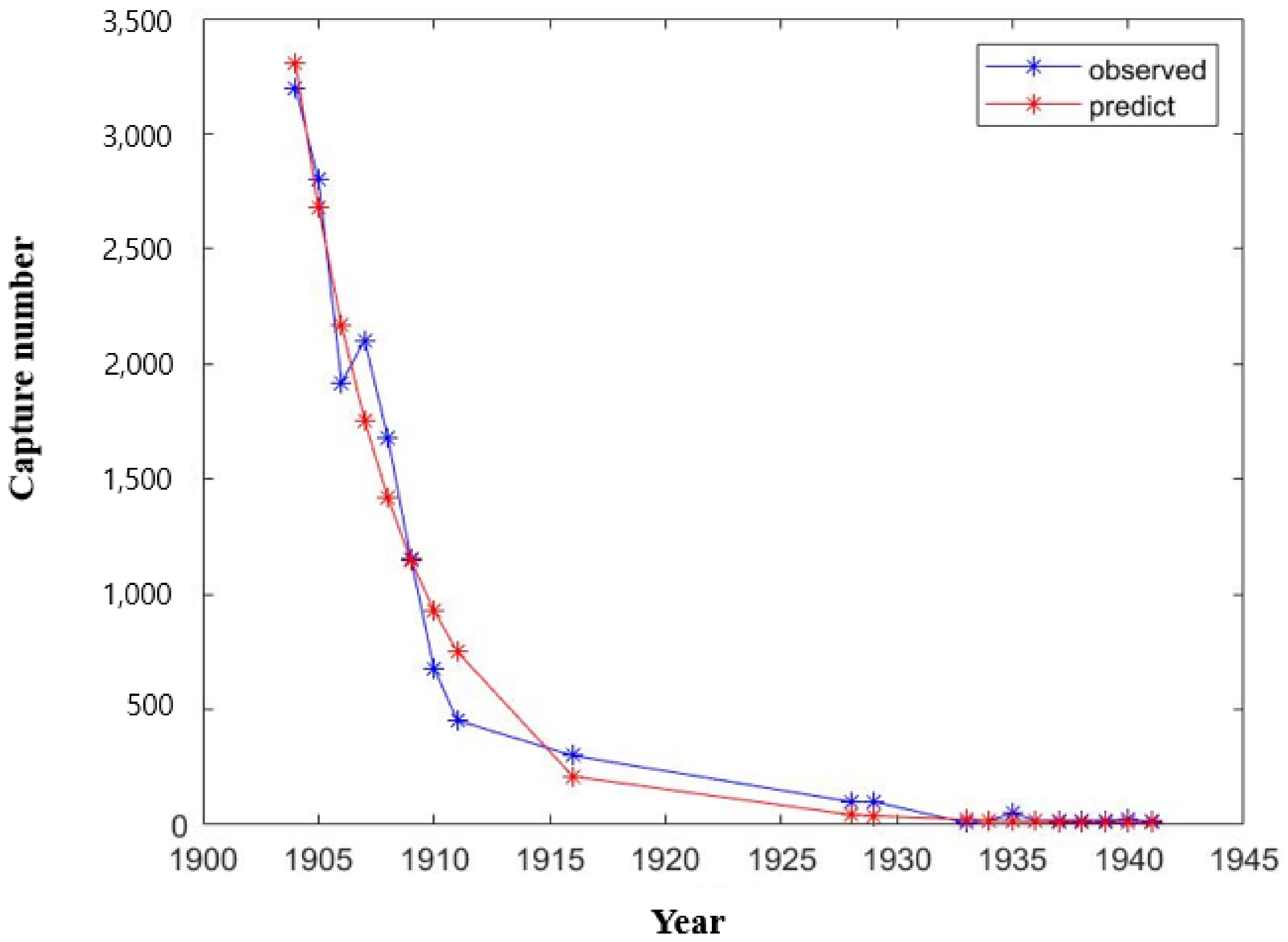
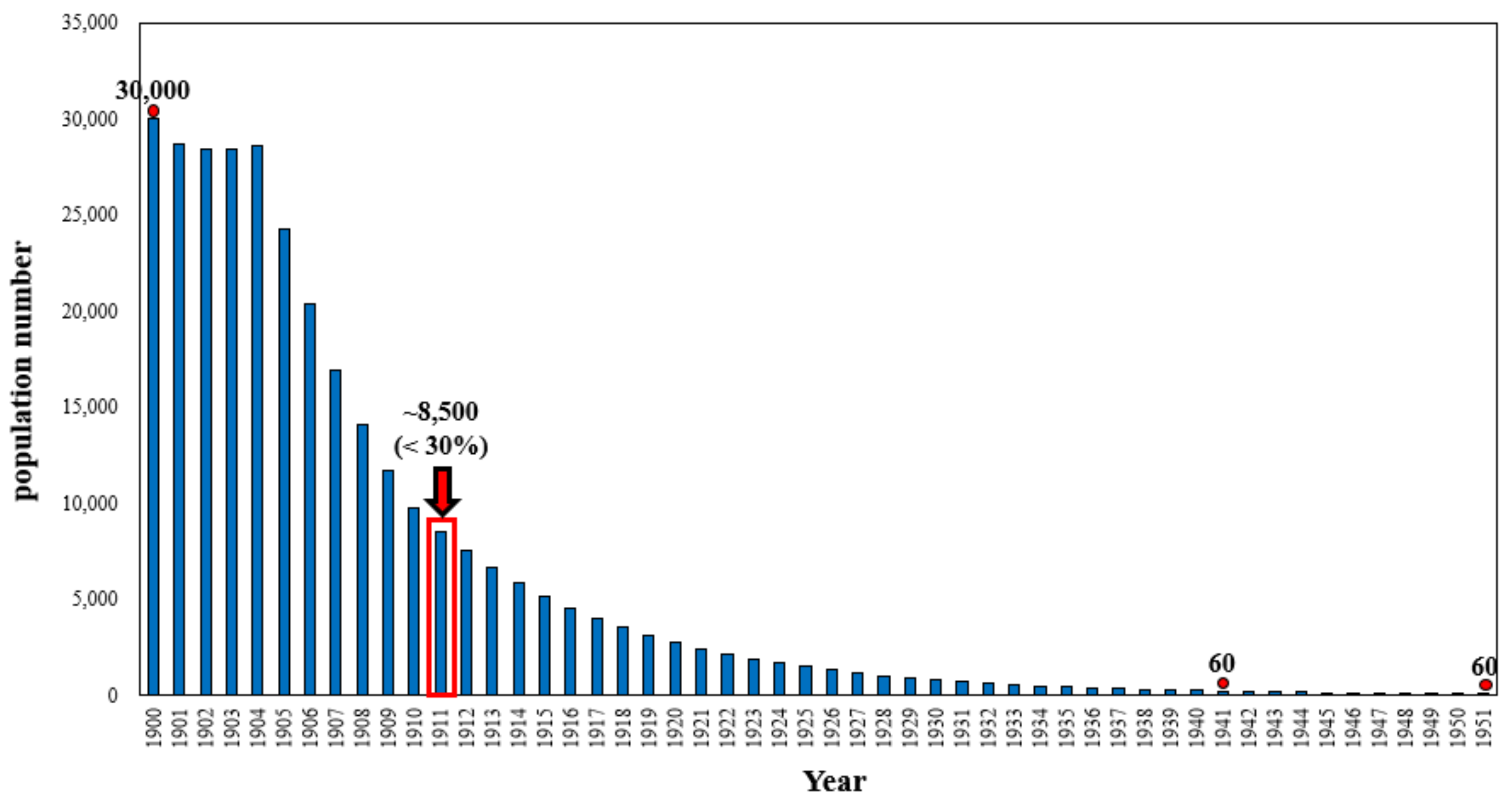
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, D.W. Marine Mammals of the World: Systematics and Distribution; Special Publication Number 4, The Society for Marine Mammalogy; Allen Press: Lawrence, KS, USA, 1998; pp. 29–42. [Google Scholar]
- Heath, C.B.; Perrin, W.F. California, Galapagos, and Japanese sea lions: Zalophus californianus, Z. wollebaeki, and Z. japonicus. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2009; pp. 170–176. [Google Scholar]
- Sakahira, F.; Niimi, M. Ancient DNA analysis of the Japanese sea lion (Zalophus californianus japonicus Peters, 1866): Preliminary results using mitochondrial control-region sequences. Zoolog. Sci. 2007, 24, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-B.; Kim, M.J.; Hwang, I.; Park, H.-M.; Lee, S.H.; Kim, H.-W. The complete mitochondrial genome of Japanese sea lion, Zalophus japonicus (Carnivora: Otariidae) analyzed using the excavated skeletal remains from Ulleungdo, South Korea. Mitochondrial DNA Part B 2021, 6, 3184–3185. [Google Scholar] [CrossRef] [PubMed]
- Melin, S.R.; Trillmich, F.; Aurioles-Gamboa, D. California, Galapagos, and Japanese Sea Lions: Zalophus californianus, Z. wollebaeki, and Z. japonicus. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–157. [Google Scholar]
- Lee, S.-R.; Kim, Y.-B.; Lee, T. The first molecular evidence of Korean Zalophus japonicus (Otariidae: Sea lions) from the archaeological site of Dokdo island, Korea. Ocean Sci. J. 2019, 54, 497–501. [Google Scholar] [CrossRef]
- Choy, K.; Richards, M.P. Isotopic evidence for diet in the Middle Chulmun period: A case study from the Tongsamdong shell midden, Korea. Archaeol. Anthropol. Sci. 2010, 2, 1–10. [Google Scholar] [CrossRef]
- IIzumi, K.; IIzumi, K.; Koda, Y.; Koike, W.; Nishimoto, T.; Ando, H.; Date, M. Latest Pleistocene Japanese Sea lion(Otariidae) fossil from the riverbed of the Hanamurogawa River west of Kasumigaura Lake, Ibaraki Prefecture. J. Geol. Soc. Japan. Tokyo 2010, 116, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Itoo, T. Miscellaneous on the Japanese sea lion Zalophus californianusc japonicus. Mammal Sci. 1979, 19, 27–39. [Google Scholar] [CrossRef]
- Reynolds, J.E., III.; Marsh, H.; Ragen, T.J. Marine mammal conservation. Endanger. Species Res. 2009, 7, 23–28. [Google Scholar] [CrossRef]
- Kovacs, K.M.; Aguilar, A.; Aurioles, D.; Burkanov, V.; Campagna, C.; Gales, N.; Gelatt, T.; Goldsworthy, S.D.; Goodman, S.J.; Hofmeyr, G.J.G. Global threats to pinnipeds. Mar. Mammal Sci. 2012, 28, 414–436. [Google Scholar] [CrossRef]
- Jackson, P.F.R. Hunt for the Japanese Sea-lion. Environ. Conserv. 1977, 4, 290. [Google Scholar] [CrossRef]
- ME (Ministry of Environment). Study on the Monitoring of Marine Mammals (Pinniped) in the East Sea and Management Plans; NIER (National Institute of Environmental Research): Incheon, Korea, 2007.
- NIBR (National Institute of Biological Resources). Investigation and Network Construction for the Restoration of Endangered Marine Mammals (Pinniped); NIBR: Incheon, Korea, 2010.
- Kim, H.W.; Lee, S.; Sohn, H. A Review on the Status of Pinnipeds in Korea. Korean J. Fish. Aquat. Sci. 2021, 54, 231–239. [Google Scholar]
- Lowry, L. Zalophus japonicus. The IUCN Red List of Threatened Species 2017. Available online: https://www.iucnredlist.org/species/41667/113089431; (accessed on 5 January 2022).
- Nakamura, K. An essay on the Japanese Sea Lion, Zalophus californianus japonicus, living on the seven islands of Izu. Bull. Kanagawa Prefect. Museum (Nat. Sci.) 1991, 20, 59–66. [Google Scholar]
- Joo, K.H. The History of Extinction of Dokdo Sea Lions; Seohaemunjip: Seoul, Korea, 2016. [Google Scholar]
- Kim, S. Japan [Sea Otter and Fur Seal Fishing Law (臘虎腽肭獸獵法)] and Dokdo Sea Lion Fishing. J. Jpn. Cult. 2016, 70, 25–40. [Google Scholar]
- Liu, S.; Chen, L.; Agarwal, R. Recent progress on stage-structured population dynamics. Math. Comput. Model. 2002, 36, 1319–1360. [Google Scholar] [CrossRef]
- Jung, S.; Choi, I.; Jin, H.; Lee, D.; Cha, H.; Kim, Y.; Lee, J. Size-dependent mortality formulation for isochronal fish species based on their fecundity: An example of Pacific cod (Gadus macrocephalus) in the eastern coastal areas of Korea. Fish. Res. 2009, 97, 77–85. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty; Springer Science Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 1461535980. [Google Scholar]
- Brownell, R.L., Jr.; LeBoeuf, B.J. California sea lion mortality: Natural or artifact. In Biological and Oceanographical Survey of the Santa Barbara Channel Oil spill 1969–1970; Allan Hancock Foundation: Los Angeles, CA, USA, 1971; pp. 287–305. [Google Scholar]
- Aurioles, D.; Sinsel, F. Mortality of California Sea Lion Pups at Los Islotes, Baja California Sur, Mexico. J. Mammal. 1988, 69, 180–183. [Google Scholar] [CrossRef]
- Hernández-Camacho, C.J.; Aurioles-Gamboa, D.; Gerber, L.R. Age-specific birth rates of California sea lions (Zalophus californianus) in the Gulf of California, Mexico. Mar. Mamm. Sci. 2008, 24, 664–676. [Google Scholar] [CrossRef]
- Gerber, L.R.; Hilborn, R. Catastrophic events and recovery from low densities in populations of otariids: Implications for risk of extinction. Mamm. Rev. 2001, 31, 131–150. [Google Scholar] [CrossRef] [Green Version]
- Mangel, M.; Tier, C. Four facts every conservation biologists should know about persistence. Ecology 1994, 75, 607–614. [Google Scholar] [CrossRef]
- Diamond, J.M. The present, past and future of human-caused extinctions. Philos. Trans. R. Soc. London B Biol. Sci. 1989, 325, 469–477. [Google Scholar]
- Villegas-Amtmann, S.; Simmons, S.E.; Kuhn, C.E.; Huckstadt, L.A.; Costa, D.P. Latitudinal range influences the seasonal variation in the foraging behavior of marine top predators. PLoS ONE 2011, 6, e23166. [Google Scholar] [CrossRef] [Green Version]
- Laake, J.L.; Lowry, M.S.; DeLong, R.L.; Melin, S.R.; Carretta, J. V Population growth and status of California sea lions. J. Wildl. Manag. 2018, 82, 583–595. [Google Scholar] [CrossRef]
- Joo, H.; Park, J.W.; Son, S.; Noh, J.; Jeong, J.; Kwak, J.H.; Saux-Picart, S.; Choi, J.H.; Kang, C.; Lee, S.H. Long-term annual primary production in the Ulleung Basin as a biological hot spot in the East/Japan Sea. J. Geophys. Res. Oceans 2014, 119, 3002–3011. [Google Scholar] [CrossRef] [Green Version]
- Kahru, M.; Kudela, R.; Manzano-Sarabia, M.; Mitchell, B.G. Trends in primary production in the California Current detected with satellite data. J. Geophys. Res. Oceans 2009, 114, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Roman, J.; McCarthy, J.J. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 2010, 5, e13255. [Google Scholar] [CrossRef] [Green Version]
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.B.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as marine ecosystem engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Kiszka, J.J.; Heithaus, M.R.; Wirsing, A.J. Behavioural drivers of the ecological roles and importance of marine mammals. Mar. Ecol. Prog. Ser. 2015, 523, 267–281. [Google Scholar] [CrossRef]
- Baisre, J.A. Shifting baselines and the extinction of the Caribbean monk seal. Conserv. Biol. 2013, 27, 927–935. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, Z.G.; Zhang, C.I. Abundance estimation of the finless porpoise, Neophocaena asiaeorientalis, using models of the detection function in a line transect. Korean J. Fish. Aquat. Sci. 2007, 40, 201–209. [Google Scholar]
- Park, K.J.; Sohn, H.; An, Y.R.; Kim, H.W.; An, D.H. A new abundance estimate for the finless porpoise Neophocaena asiaeorientalis on the west coast of Korea: An indication of population decline. Fish. Aquat. Sci. 2015, 18, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Jinhai, D.; Feng, S. Estimates of historical population size of harbor seal (Phoca largha) in Liaodong Bay. Mar. Sci. 1991, 3, 26–31. [Google Scholar]
- Yan, H.-K.; Wang, N.; Wu, N.; Lin, W. Abundance, habitat conditions, and conservation of the largha seal (Phoca largha) during the past half century in the Bohai Sea, China. Mammal Study 2018, 43, 1–9. [Google Scholar] [CrossRef]
- Won, C.; Yoo, B.-H. Abundance, seasonal haul-out patterns and conservation of spotted seals Phoca largha along the coast of Bak-ryoung Island, South Korea. Oryx 2004, 38, 109–112. [Google Scholar] [CrossRef] [Green Version]
- NIBR (National Institute of Biological Resources). Red Data Book of Endangered Mammals in Korea; NIBR: Incheon, Korea, 2012.


| Parameter | Description | Value | Reference |
|---|---|---|---|
| Birth rate | 0.5 | estimated | |
| The proportion of immature to mature | 1/5 | Heath and Perrin (2009) | |
| The natural mortality of the immature individuals (year−1) | 0.5 | estimated | |
| The natural mortality of the mature individuals (year−1) | 0.1 | estimated | |
| The fishing mortality of the immature individuals (year−1) | 0.0138 | estimated | |
| The fishing mortality of the mature individuals (year−1) | 0.2428 | estimated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Cho, G.; Kim, S.; Hwang, I.; Im, S.-O.; Park, H.-M.; Kim, N.-Y.; Kim, M.-J.; Lee, D.; Kwak, S.-N.; et al. The First Population Simulation for the Zalophus japonicus (Otariidae: Sea Lions) on Dokdo, Korea. J. Mar. Sci. Eng. 2022, 10, 271. https://doi.org/10.3390/jmse10020271
Lee Y-J, Cho G, Kim S, Hwang I, Im S-O, Park H-M, Kim N-Y, Kim M-J, Lee D, Kwak S-N, et al. The First Population Simulation for the Zalophus japonicus (Otariidae: Sea Lions) on Dokdo, Korea. Journal of Marine Science and Engineering. 2022; 10(2):271. https://doi.org/10.3390/jmse10020271
Chicago/Turabian StyleLee, Yoon-Ji, Giphil Cho, Sangil Kim, Inseo Hwang, Seong-Oh Im, Hye-Min Park, Na-Yeong Kim, Myung-Joon Kim, Dasom Lee, Seok-Nam Kwak, and et al. 2022. "The First Population Simulation for the Zalophus japonicus (Otariidae: Sea Lions) on Dokdo, Korea" Journal of Marine Science and Engineering 10, no. 2: 271. https://doi.org/10.3390/jmse10020271
APA StyleLee, Y.-J., Cho, G., Kim, S., Hwang, I., Im, S.-O., Park, H.-M., Kim, N.-Y., Kim, M.-J., Lee, D., Kwak, S.-N., & Lee, S.-H. (2022). The First Population Simulation for the Zalophus japonicus (Otariidae: Sea Lions) on Dokdo, Korea. Journal of Marine Science and Engineering, 10(2), 271. https://doi.org/10.3390/jmse10020271







