Selective NO2 Detection by Black Phosphorus Gas Sensor Prepared via Aqueous Route for Ship Pollutant Monitoring
Abstract
1. Introduction
2. Materials and Methods
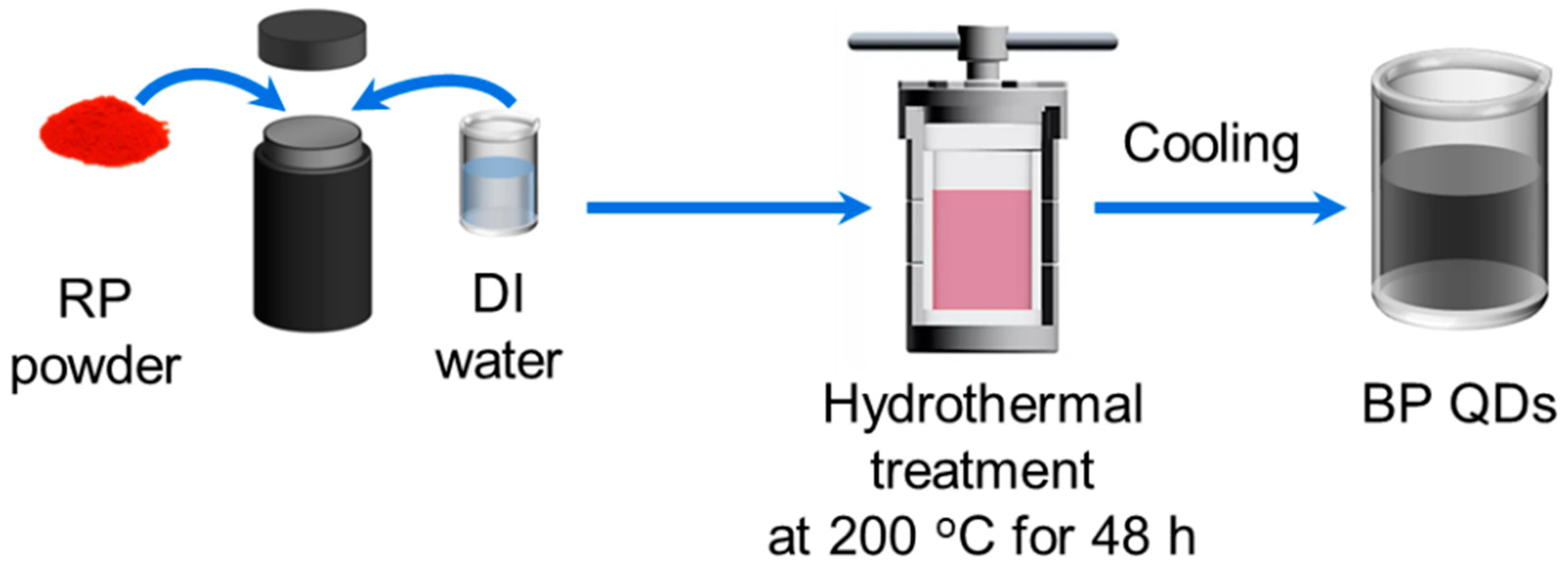
2.1. Aqueous Preparation of BP
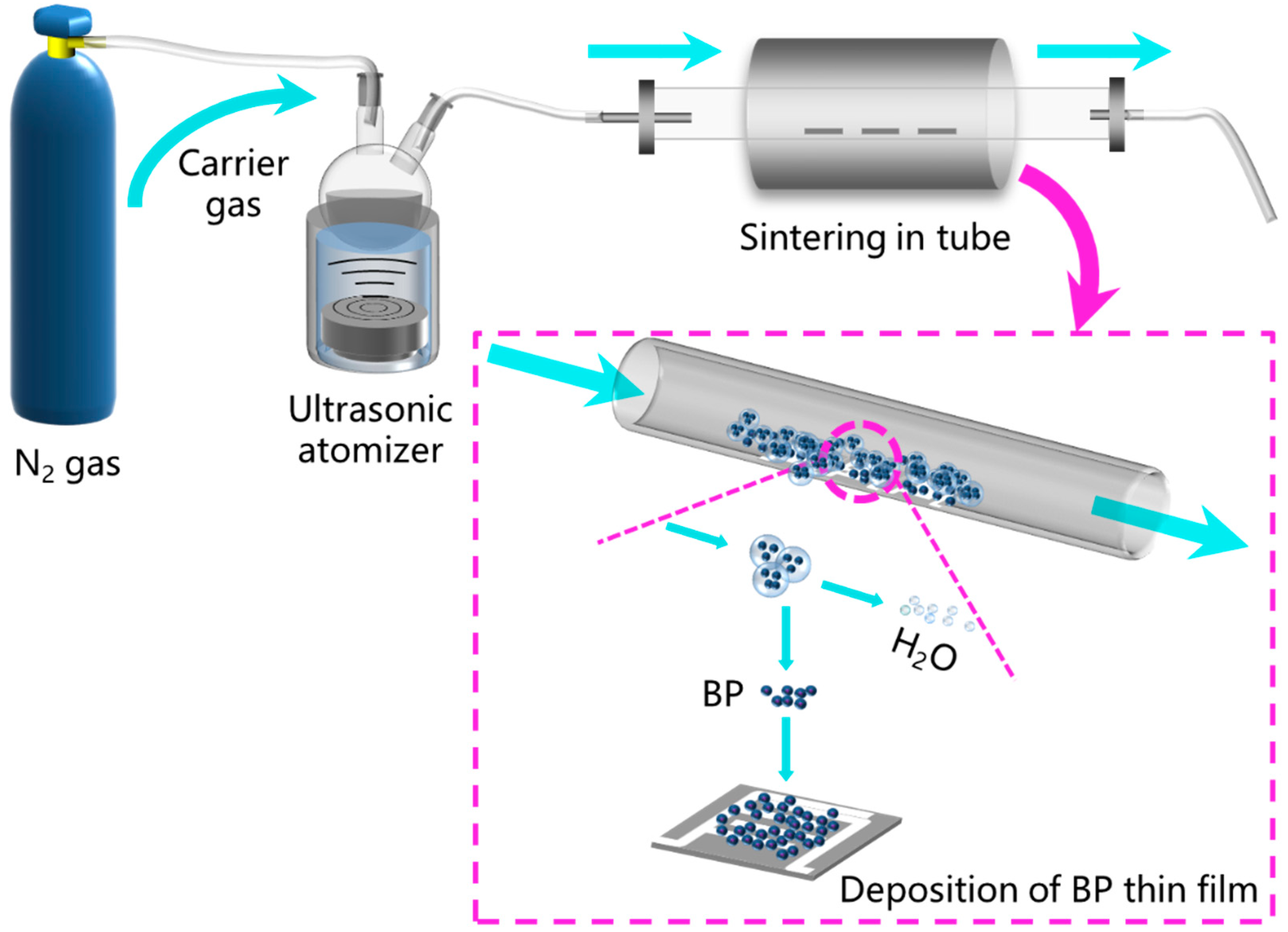
2.2. Deposition of BP Gas Sensors
2.3. Characterization
2.4. Measurement of Gas-Sensing Properties
3. Results and Discussion
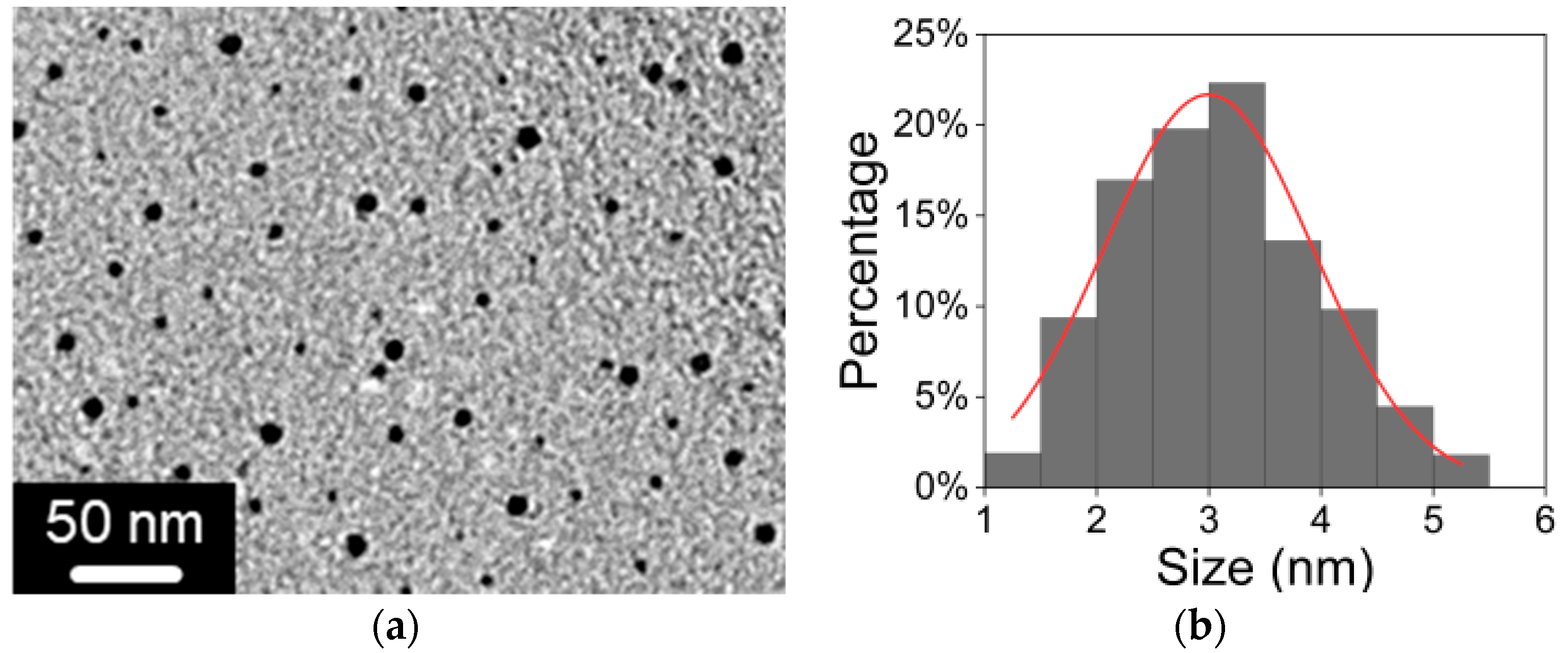
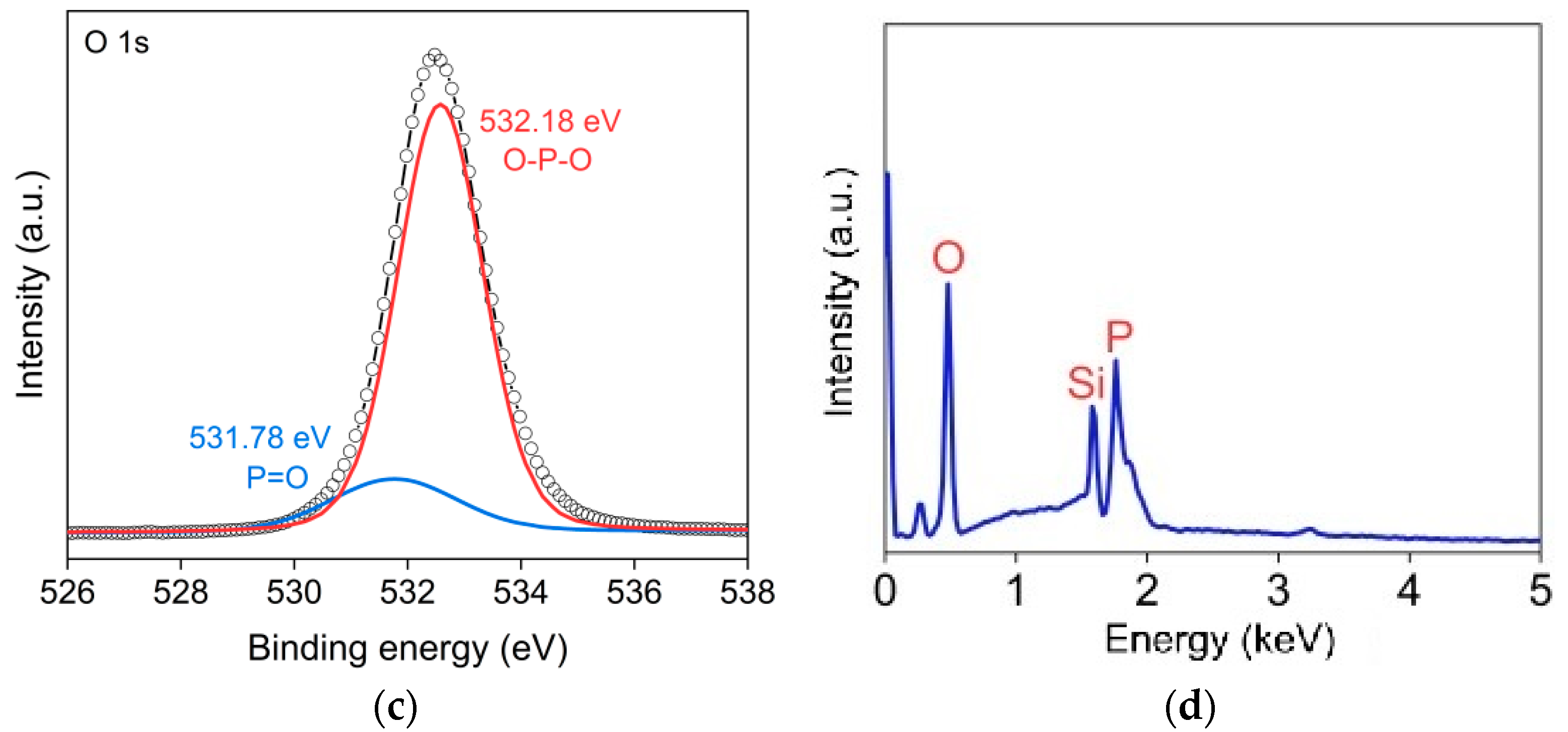
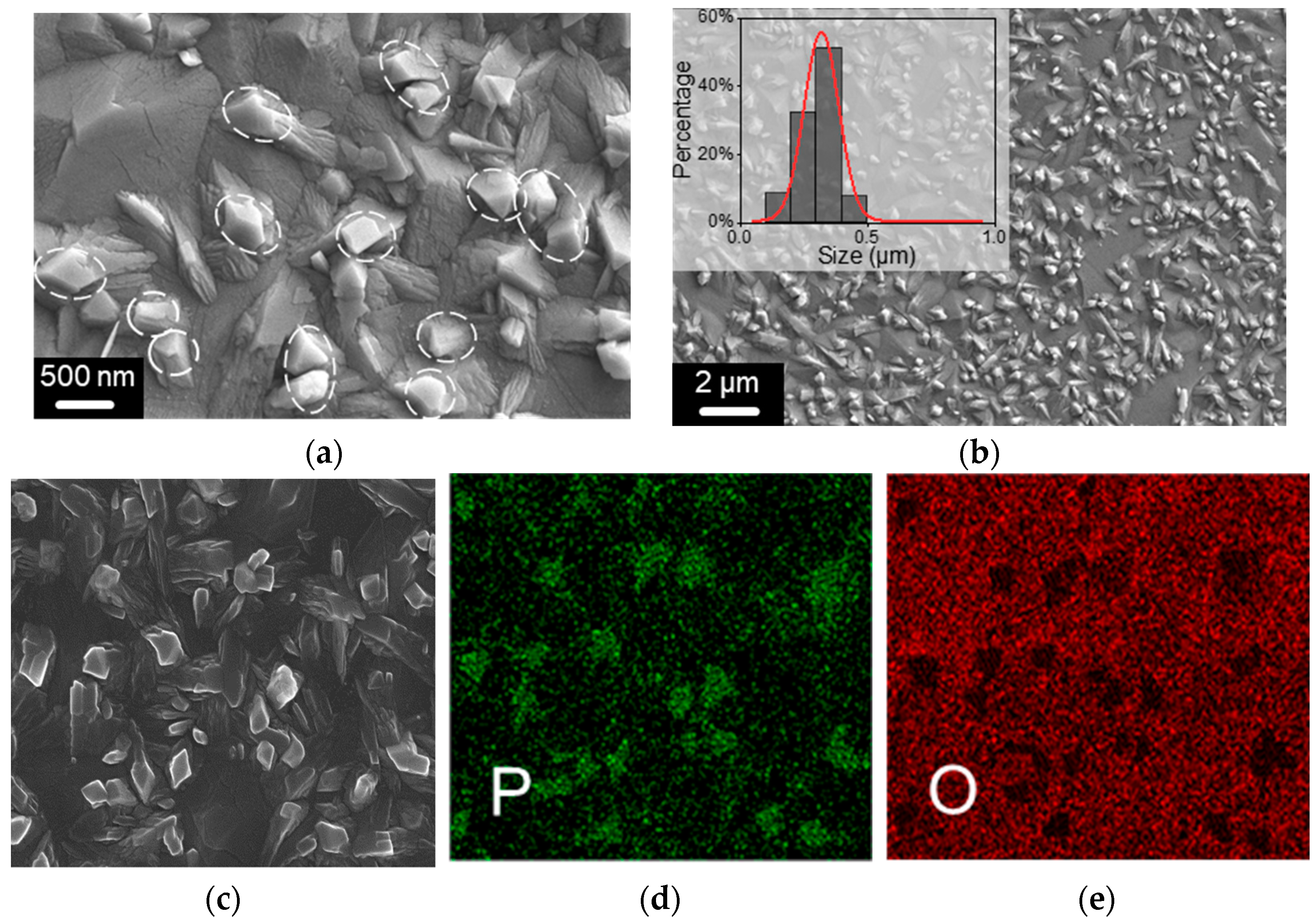
3.1. Characterization of BP QDs
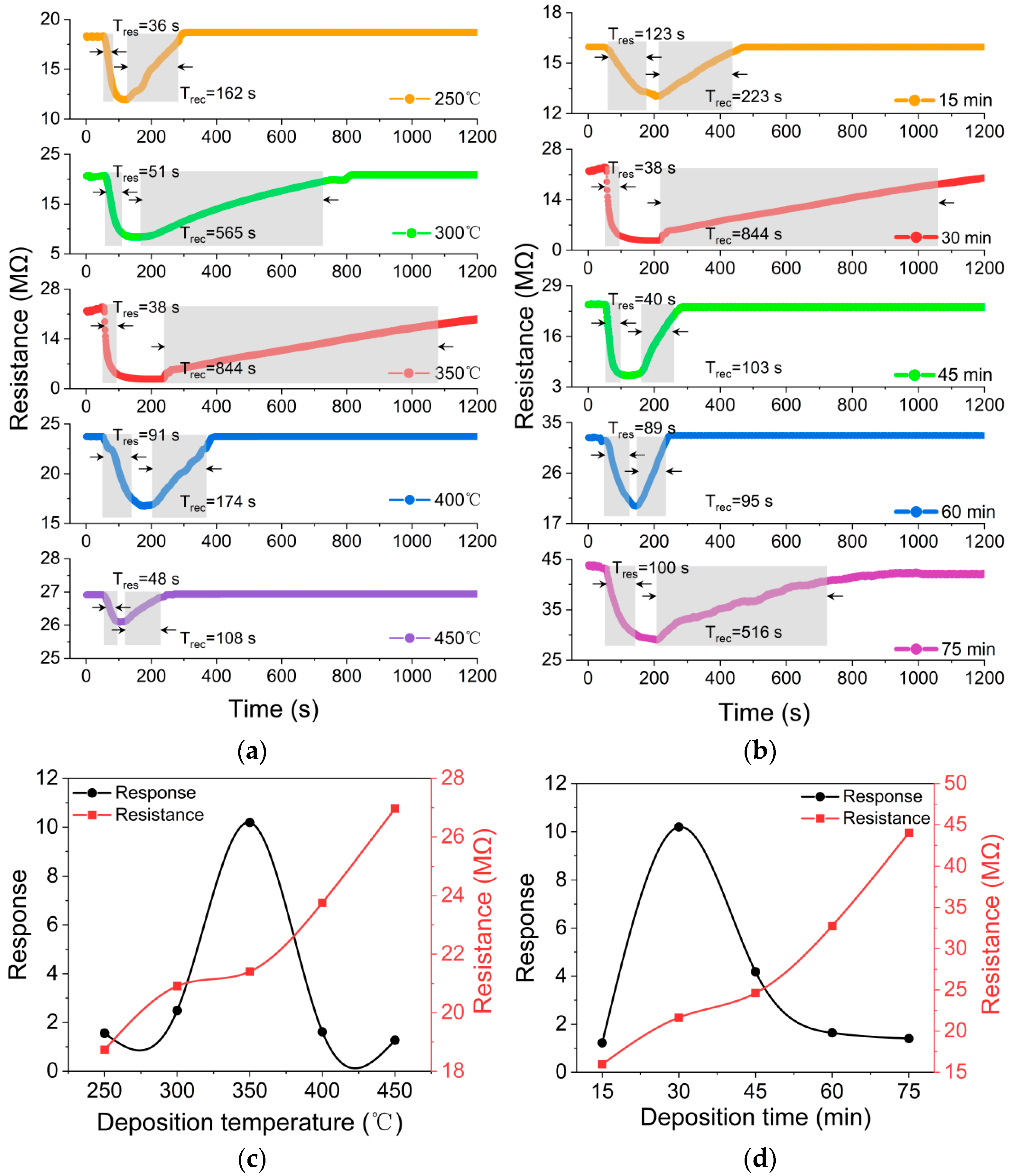
3.2. Characterizations of BP Gas Sensors
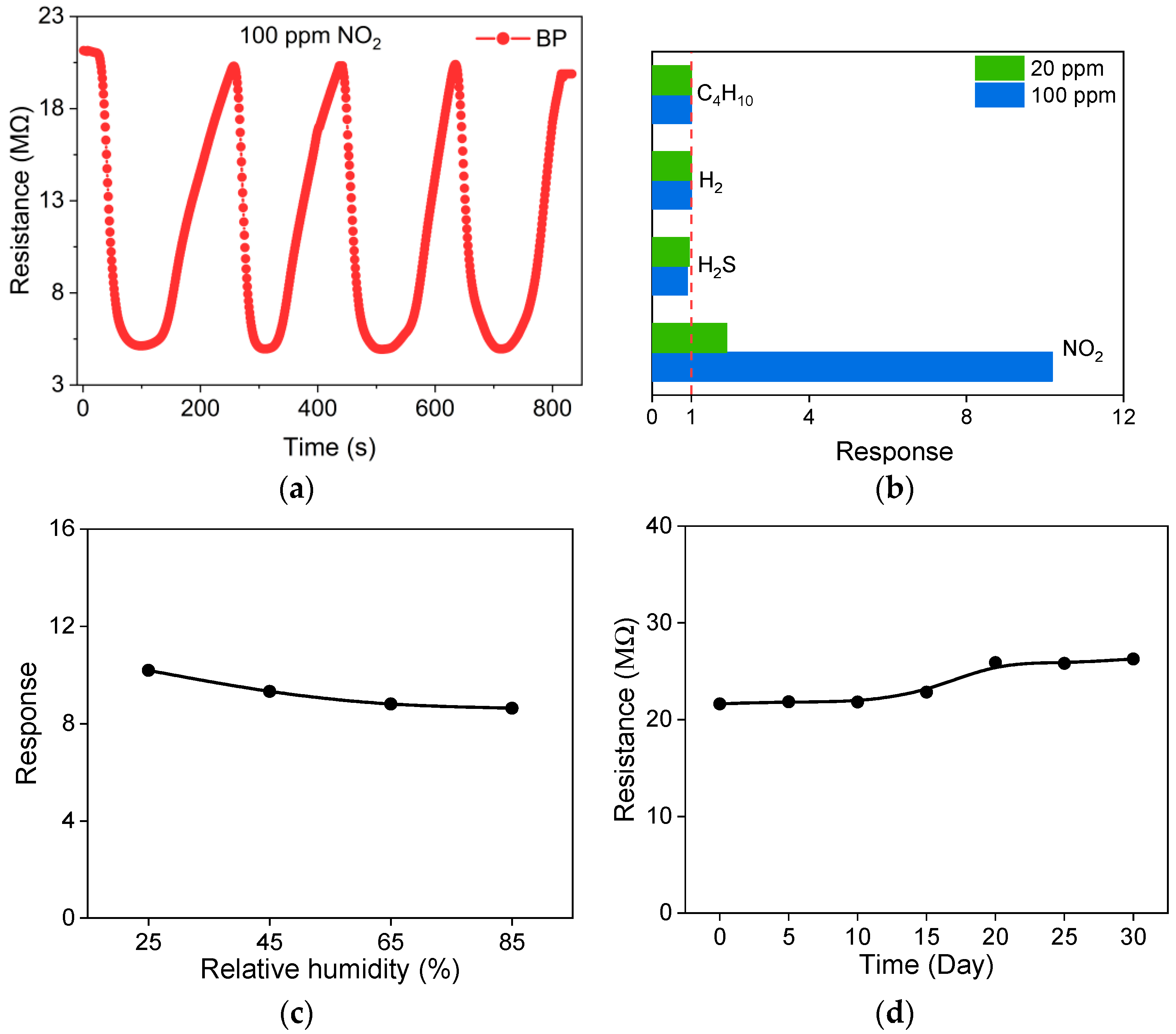
3.3. Gas-sensing Properties
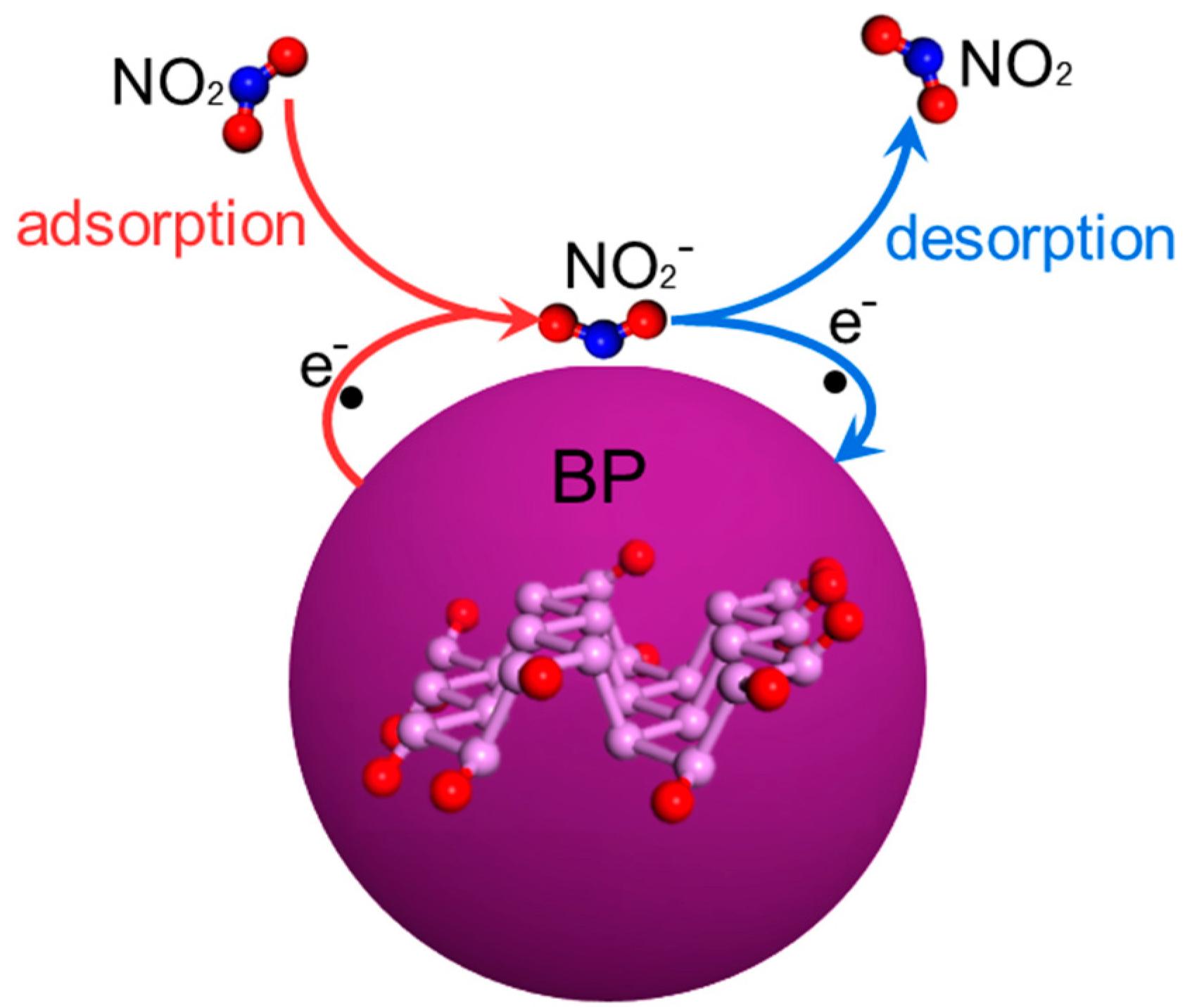
3.4. Gas-Sensing Mechanism
4. Conclusions
- The BP QDs were prepared from RP fine powder under low temperature, without using any toxic or explosive organic solvents.
- The BP thin films deposited from QDs exhibited good gas-sensing performance and optimized sensor properties were obtained from the BP gas sensors deposited at 350 °C for 30 min.
- The optimum response to 100 ppm NO2 was 10.19 with a sensor sensitivity of 0.48. The BP thin film gas sensor also demonstrated excellent recovery ability, repeatability, selectivity and stability. The present BP thin film gas sensor has superior gas-sensing properties to other published devices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminzadegan, S.; Shahriari, M.; Mehranfar, F.; Abramović, B. Factors affecting the emission of pollutants in different types of transportation: A literature review. Energy Rep. 2022, 8, 2508–2529. [Google Scholar] [CrossRef]
- Wang, R.; Bei, N.; Wu, J.; Li, X.; Liu, S.; Yu, J.; Jiang, Q.; Tie, X.; Li, G. Cropland nitrogen dioxide emissions and effects on the ozone pollution in the North China plain. Environ. Pollut. 2022, 294, 118617. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Chen, J. Mitigating nitrogen dioxide air pollution: The roles and effect of national smart city pilots in China. Energy 2023, 263, 125652. [Google Scholar] [CrossRef]
- Costa, S.; Ferreira, J.; Silveira, C.; Costa, C.; Lopes, D.; Relvas, H.; Borrego, C.; Roebeling, P.; Miranda, A.I.; Paulo Teixeira, J. Integrating health on air quality assessment—Review report on health risks of two major european outdoor air pollutants: PM and NO2. J. Toxicol. Environ. Health Part B 2014, 17, 307–340. [Google Scholar] [CrossRef]
- Tao, Y.; Mi, S.; Zhou, S.; Wang, S.; Xie, X. Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environ. Pollut. 2014, 185, 196–201. [Google Scholar] [CrossRef]
- Zeng, W.; Zhao, H.; Liu, R.; Yan, W.; Qiu, Y.; Yang, F.; Shu, C.; Zhan, Y. Association between NO2 cumulative exposure and influenza prevalence in mountainous regions: A case study from southwest China. Environ. Res. 2020, 189, 109926. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, R.N. NO2 increases the risk for childhood asthma: A global concern. Lancet Planet. Health 2019, 3, e155–e156. [Google Scholar] [CrossRef]
- Achakulwisut, P.; Brauer, M.; Hystad, P.; Anenberg, S.C. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: Estimates from global datasets. Lancet Planet. Health 2019, 3, e166–e178. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Pan, J.; Zhou, W.; Li, H.-M.; Liu, S. Fabrication and application of arrays related to two-dimensional materials. Rare Met. 2022, 41, 262–286. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Xuan, J.-Y.; Zhang, Q.; Sun, M.-L.; Jia, F.-C.; Wang, X.-M.; Yin, G.-C.; Lu, S.-Y. Strategies and challenges for enhancing performance of MXene-based gas sensors: A review. Rare Met. 2022, 41, 3976–3999. [Google Scholar] [CrossRef]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A review on Ti3C2Tx-based nanomaterials: Synthesis and applications in gas and humidity sensors. Rare Met. 2021, 40, 1459–1476. [Google Scholar] [CrossRef]
- Galstyan, V.; Moumen, A.; Kumarage, G.W.C.; Comini, E. Progress towards chemical gas sensors: Nanowires and 2D semiconductors. Sens. Actuators B 2022, 357, 131466. [Google Scholar] [CrossRef]
- Erande, M.B.; Pawar, M.S.; Late, D.J. Humidity sensing and photodetection behavior of electrochemically exfoliated atomically thin-layered black phosphorus nanosheets. ACS Appl. Mat. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, X.; Xie, J.; Lu, G.; Zhang, J. Emerging van der Waals junctions based on TMDs materials for advanced gas sensors. Coord. Chem. Rev. 2021, 447, 214151. [Google Scholar] [CrossRef]
- Ghosh, R.; Aslam, M.; Kalita, H. Graphene derivatives for chemiresistive gas sensors: A review. Mater. Today Commun. 2022, 30, 103182. [Google Scholar] [CrossRef]
- Ding, J.; Dai, H.; Chen, H.; Jin, Y.; Fu, H.; Xiao, B. Highly sensitive ethylene glycol gas sensor based on ZnO/rGO nanosheets. Sens. Actuators B 2022, 372, 132655. [Google Scholar] [CrossRef]
- Jibril Ibrahim, A.; Ghani, H.A.; Hussein, E.S.; Abdullaha, S.A.H.; Kadhim, M.M.; Mahdi Rheima, A.; Turki Jalil, A.; Anshul, Y. Metal-decorated BN monolayer as potential chemical sensors for detection of purinethol drug. Inorg. Chem. Commun. 2022, 145, 109919. [Google Scholar] [CrossRef]
- Huang, W.-X.; Li, Z.-P.; Li, D.-D.; Hu, Z.-H.; Wu, C.; Lv, K.-L.; Li, Q. Ti3C2 MXene: Recent progress in its fundamentals, synthesis, and applications. Rare Met. 2022, 41, 3268–3300. [Google Scholar] [CrossRef]
- Guo, Z.; Ding, W.; Liu, X.; Sun, Z.; Wei, L. Two-dimensional black phosphorus: A new star in energy applications and the barrier to stability. Appl. Mater. Today 2019, 14, 51–58. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, J.; Jing, J.; Li, J.; Shi, F. Preparations, properties and applications of low-dimensional black phosphorus. Chem. Eng. J. 2019, 370, 120–135. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, S.; He, H.; Ling, G.; Zhang, P. Functional black phosphorus nanosheets for cancer therapy. J. Control. Release 2020, 318, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, W.; Xu, M.; Bai, S.; Chen, Y.; Tang, Z.; Wang, C.; Yang, Y.; Zhang, X.; Yuan, Y.; et al. Two-dimensional black phosphorus: Properties, fabrication and application for flexible supercapacitors. Chem. Eng. J. 2021, 412, 128744. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Lang, P.; Yuan, N.; Tang, J. Fabrication and applications of 2D black phosphorus in catalyst, sensing and electrochemical energy storage. J. Alloys Compd. 2021, 850, 156580. [Google Scholar] [CrossRef]
- Zhong, Q. Intrinsic and engineered properties of black phosphorus. Mater. Today Phys. 2022, 28, 100895. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, K.; Sun, R.; Ma, J. Biorefinery-assisted ultra-high hydrogen evolution via metal-free black phosphorus sensitized carbon nitride photocatalysis. Chem. Eng. J. 2022, 446, 137128. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-Q.; Liao, J.; Zhang, X.; Feng, D.; Deng, H.; Ge, C. Infrared-to-visible energy transfer photocatalysis over black phosphorus quantum dots/carbon nitride. Chem. Eng. J. 2022, 431, 133453. [Google Scholar] [CrossRef]
- He, F.; Liu, Z.; Xu, J.; Xiong, Y.; Zhang, X.; Qi, J.; Lin, X.; Chu, C.; Shen, L.; Liu, G.; et al. Black phosphorus nanosheets suppress oxidative damage of stem cells for improved neurological recovery. Chem. Eng. J. 2023, 451, 138737. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Liu, J.; Ren, M.; Xing, D.; Qin, H. Controlling dielectric loss of biodegradable black phosphorus nanosheets by iron-ion-modification for imaging-guided microwave thermoacoustic therapy. Biomaterials 2022, 287, 121662. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Ottaviano, L.; Giancaterini, L.; Fioravanti, G.; Perrozzi, F.; Cantalini, C. Exfoliated black phosphorus gas sensing properties at room temperature. 2D Materials 2016, 3, 025002. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Jung, S.; Jang, S.; Kim, J. Suspended black phosphorus nanosheet gas sensors. Sens. Actuators B 2017, 250, 569–573. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Li, J.; Zhang, R.; Zhao, H.; Wang, Y. Ag decoration-enabled sensitization enhancement of black phosphorus nanosheets for trace NO2 detection at room temperature. J. Hazard. Mater. 2022, 435, 129086. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Koh, H.-J.; Yoo, H.-W.; Jung, H.-T. Tunable chemical sensing performance of black phosphorus by controlled functionalization with noble metals. Chem. Mater. 2017, 29, 7197–7205. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ikram, M.; Lv, H.; Chang, J.; Li, Z.; Ma, L.; Rehman, A.U.; Lu, G.; Chen, J.; et al. Facile synthesis of highly dispersed Co3O4 nanoparticles on expanded, thin black phosphorus for a ppb-level NOX gas sensor. ACS Sens. 2018, 3, 1576–1583. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Ren, H.; Wang, Y.; Zhu, X.; Guo, Y.; Li, X. Room-temperature and humidity-resistant trace nitrogen dioxide sensing of few-layer black phosphorus nanosheet by incorporating zinc oxide nanowire. Anal. Chem. 2020, 92, 11007–11017. [Google Scholar] [CrossRef]
- Liang, T.; Dai, Z.; Liu, Y.; Zhang, X.; Zeng, H. Suppression of Sn2+ and Lewis acidity in SnS2/black phosphorus heterostructure for ppb-level room temperature NO2 gas sensor. Sci. Bull. 2021, 66, 2471–2478. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, B.; Yang, Y.; Li, Q.; Mao, S. Black phosphorus quantum dots modified monolayer Ti3C2TX nanosheet for field-effect transistor gas sensor. Sens. Actuators B 2022, 373, 132696. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef]
- Lee, G.; Jung, S.; Jang, S.; Kim, J. Platinum-functionalized black phosphorus hydrogen sensors. Appl. Phys. Lett. 2017, 110, 242103. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, Z.; Tian, Q.; Wang, J.; Yu, X.; Ho, D. Nitrogen dioxide gas sensor based on liquid-phase-exfoliated black phosphorus nanosheets. ACS Appl. Nano Mater. 2020, 3, 6440–6447. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Zhang, X.; Si, J.; Liu, Y.; Ma, L.; ullah, M.; Ikram, M.; Li, L.; Shi, K. Novel intercalated CuO/black phosphorus nanocomposites: Fabrication, characterization and NO2 gas sensing at room temperature. Mater. Sci. Semicond. Process. 2020, 110, 104961. [Google Scholar] [CrossRef]
- Liu, J.; Qu, X.; Zhang, C.; Dong, W.; Fu, C.; Wang, J.; Zhang, Q. High-yield aqueous synthesis of partial-oxidized black phosphorus as layered nanodot photocatalysts for efficient visible-light driven degradation of emerging organic contaminants. J. Clean. Prod. 2022, 377, 134228. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, S.; Liu, J.; Liu, H.; Gong, S.; Zhou, D. Tin oxide thin films prepared by aerosol-assisted chemical vapor deposition and the characteristics on gas detection. Sens. Actuators B 2010, 145, 788–793. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhai, Z.; Jin, G.; Jiang, Q.; Zhao, Y.; Luo, C.; Quan, L. Evaluation of depletion layer width and gas-sensing properties of antimony-doped tin oxide thin film sensors. Sens. Actuators B 2015, 220, 1354–1360. [Google Scholar] [CrossRef]
- Liu, J.; Lv, J.; Shi, J.; Wu, L.; Su, N.; Fu, C.; Zhang, Q. Size effects of tin oxide quantum dot gas sensors: From partial depletion to volume depletion. J. Mater. Res. Technol. 2020, 9, 16399–16409. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Pei, X.; Zhang, C.; Ding, C.; Xian, Y. Fluorescent black phosphorus quantum dots as label-free sensing probes for evaluation of acetylcholinesterase activity. Sens. Actuators B 2017, 250, 601–607. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, Y.; Wang, Y.; Duan, J.; Tang, Q. Sonochemistry-assisted black/red phosphorus hybrid quantum dots for dye-sensitized solar cells. J. Power Sources 2019, 410–411, 53–58. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, J.; Zhai, Z.; Yang, Z.; Xia, J.; Deng, S.; Qu, X.; Zhang, H.; Wu, D.; et al. Mo-modified band structure and enhanced photocatalytic properties of tin oxide quantum dots for visible-light driven degradation of antibiotic contaminants. J. Environ. Chem. Eng. 2022, 10, 107091. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Tian, X.; Hong, Y.; Nie, Y.; Su, N.; Jin, G.; Zhai, Z.; Fu, C. Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 2021, 404, 127146. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Sun, Z.; Chen, H.; Guan, J.; Chen, X.; Ji, H.; Du, P.; Yang, S. Black phosphorus revisited: A missing metal-free elemental photocatalyst for visible light hydrogen evolution. Adv. Mater. 2017, 29, 1605776. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Wang, Z.; Yang, Z.; Liu, F.; Xu, Y.; Wang, J.; Yi, Y.; Zhang, H.; Liao, L.; et al. Metal-ion-modified black phosphorus with enhanced stability and transistor performance. Adv. Mater. 2017, 29, 1703811. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zheng, X.; Li, Z. A promising photocatalyst for water-splitting reactions with a stable sandwiched P4O2/black phosphorus heterostructure and high solar-to-hydrogen efficiency. Nanoscale 2020, 12, 6617–6623. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Wang, X.; Jiang, J.; Ostrikov, K.; Ni, Z.; Gu, X.; Xiao, S. Effect of the surface oxide layer on the stability of black phosphorus. Appl. Surf. Sci. 2021, 537, 147850. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Cui, X.; Geng, Y.; Jin, G.; Zhai, Z. Gas-sensing properties and sensitivity promoting mechanism of Cu-added SnO2 thin films deposited by ultrasonic spray pyrolysis. Sens. Actuators B 2017, 248, 862–867. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Liu, J.; Lv, J.; Xiong, H.; Wang, Y.; Jin, G.; Zhai, Z.; Fu, C.; Zhang, Q. Size effect and comprehensive mathematical model for gas-sensing mechanism of SnO2 thin film gas sensors. J. Alloys Compd. 2022, 898, 162875. [Google Scholar] [CrossRef]



| Reference | Composition | Concentration (ppm) | Operating Temperature (°C) | Response |
|---|---|---|---|---|
| [32] | BP flakes | 0.2 | 25 | 1.08 |
| [33] | BP flakes | 200 | N/A | 2.86 |
| [34] | Ag-decorated BP | 0.1 | 22 | 1.67 |
| [35] | Au-incorporated BP | 1 | 25 | 3.33 |
| [36] | Co3O4-PEI-BP | 3 | 25 | 2.86 |
| [37] | ZnO-BP | 0.05 | 25 | 3.85 |
| [38] | SnS2-BP | 0.1 | 25 | 6.08 |
| [39] | MXene-BP | 10 | 20 | 1.24 |
| This work | BP thin film | 100 | 25 | 10.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Sun, Y.; Zhang, K.; Zhang, C.; Liu, J.; Fu, C.; Wang, J. Selective NO2 Detection by Black Phosphorus Gas Sensor Prepared via Aqueous Route for Ship Pollutant Monitoring. J. Mar. Sci. Eng. 2022, 10, 1892. https://doi.org/10.3390/jmse10121892
Wang Y, Wang Y, Sun Y, Zhang K, Zhang C, Liu J, Fu C, Wang J. Selective NO2 Detection by Black Phosphorus Gas Sensor Prepared via Aqueous Route for Ship Pollutant Monitoring. Journal of Marine Science and Engineering. 2022; 10(12):1892. https://doi.org/10.3390/jmse10121892
Chicago/Turabian StyleWang, Yang, Yujia Wang, Yue Sun, Kuanguang Zhang, Chenyang Zhang, Jianqiao Liu, Ce Fu, and Junsheng Wang. 2022. "Selective NO2 Detection by Black Phosphorus Gas Sensor Prepared via Aqueous Route for Ship Pollutant Monitoring" Journal of Marine Science and Engineering 10, no. 12: 1892. https://doi.org/10.3390/jmse10121892
APA StyleWang, Y., Wang, Y., Sun, Y., Zhang, K., Zhang, C., Liu, J., Fu, C., & Wang, J. (2022). Selective NO2 Detection by Black Phosphorus Gas Sensor Prepared via Aqueous Route for Ship Pollutant Monitoring. Journal of Marine Science and Engineering, 10(12), 1892. https://doi.org/10.3390/jmse10121892







