A New Method to Speed Up Nannofossil Picking for Monospecific Geochemical Analyses
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Efficiency of the Picking Process
3.2. Possible Dissolution Issues
4. Discussion and Conclusions
| Picking Method | Picking Tool Material | Picking Tool Ø (µm) | #Picked Coccoliths | Type of Sediment Sample | Picking Principle | References |
|---|---|---|---|---|---|---|
| Micromanipulator | Borosilicate glass | 3–20 (inner Ø) | 80–100/h | Wet | Controlled suction and release | This work |
| Manual | Silica | 15–20 (outer Ø) | 5/h | Dry | Electrostatic forces | [41] |
| Micromanipulator | Tungsten | N.A. | 15/30–60 min | Dry | Electrostatic forces | [39] |
| Micromanipulator | Tungsten | N.A. | 20/45–90 min | Dry | Electrostatic forces | [40] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bown, P.R.; Lees, J.A.; Young, J.R. Calcareous Nannoplankton Evolution and Diversity through Time. In Coccolithophores: From Molecular Processes to Global Impact; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 481–508. ISBN 978-3-642-06016-8. [Google Scholar]
- Winter, A.; Jordan, R.W.; Roth, P.H. Biogeography of Living Coccolithophores in Ocean Waters. In Coccolithophores: From Molecular Processes to Global Impact; Winter, A., Siesser, W.G., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 161–177. [Google Scholar]
- Young, J.R.; Geisen, M.; Cros, L.; Kleijne, A.; Sprengel, C.; Probert, I. A Guide to Extant Coccolithophore Taxonomy. In Journal of Nannoplankton Research Special Issue 1; Druckstudio Digital Concept: Bremerhaven, Germany, 2003; pp. 1–132. [Google Scholar]
- Milliman, J.D. Production and Accumulation of Calcium Carbonate in the Ocean: Budget of a Nonsteady State. Glob. Biogeochem. Cycle 1993, 7, 927–957. [Google Scholar] [CrossRef]
- Broecker, W.; Clark, E. Ratio of Coccolith CaCO3 to Foraminifera CaCO3 in Late Holocene Deep Sea Sediments. Paleoceanography 2009, 24, PA3205. [Google Scholar] [CrossRef]
- Balch, W.M. The Ecology, Biogeochemistry, and Optical Properties of Coccolithophores. Annu. Rev. Mar. Sci. 2018, 10, 71–98. [Google Scholar] [CrossRef]
- McIntyre, A.; Bé, A.W.H.; Roche, M.B. Modern Pacific Coccolithophorida: A Paleontological Thermometer. Ann. N. Y. Acad. Sci. 1970, 32, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Beaufort, L.; de Garidel-Thoron, T.; Mix, A.C.; Pisias, N.G. ENSO-like Forcing on Oceanic Primary Production during the Late Pleistocene. Science 2001, 293, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Almeida, I.; Ausín, B.; Saavedra-Pellitero, M.; Baumann, K.-H.; Stoll, H.M. Quantitative Reconstruction of Primary Productivity in Low Latitudes during the Last Glacial Maximum and the Mid-to-Late Holocene from a Global Florisphaera Profunda Calibration Dataset. Quat. Sci. Rev. 2019, 205, 166–181. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Mortyn, G.; Antonarakou, A.; Drinia, H. Assessing the Reliability of Foraminiferal Mg/Ca Thermometry by Comparing Field-Samples and Culture Experiments: A Review. Geol. Quart. 2016, 60, 547–560. [Google Scholar] [CrossRef]
- Füger, A.; Kuessner, M.; Rollion-Bard, C.; Leis, A.; Magna, T.; Dietzel, M.; Mavromatis, V. Effect of Growth Rate and PH on Li Isotope Fractionation during Its Incorporation in Calcite. Geochim. Cosmochim. Acta 2022, 323, 276–290. [Google Scholar] [CrossRef]
- Erez, J. The Source of Ions for Biomineralization in Foraminifera and Their Implications for Paleoceanographic Proxies. Rev. Mineral. Geochem. 2003, 54, 115–149. [Google Scholar] [CrossRef]
- Young, J.R.; Davis, S.A.; Bown, P.R.; Mann, S. Coccolith Ultrastructure and Biomineralisation. J. Struct. Biol. 1999, 126, 195–215. [Google Scholar] [CrossRef]
- Brownlee, C.; Wheeler, G.L.; Taylor, A.R. Coccolithophore Biomineralization: New Questions, New Answers. Semin. Cell Dev. Biol. 2015, 46, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Ziveri, P.; Stoll, H.; Probert, I.; Klaas, C.; Geisen, M.; Ganssen, G.; Young, J. Stable Isotope ‘Vital Effects’ in Coccolith Calcite. Earth Planet. Sci. Lett. 2003, 210, 137–149. [Google Scholar] [CrossRef]
- Rickaby, R.E.M.; Henderiks, J.; Young, J.N. Perturbing Phytoplankton: Response and Isotopic Fractionation with Changing Carbonate Chemistry in Two Coccolithophore Species. Clim. Past 2010, 6, 771–785. [Google Scholar] [CrossRef]
- Bolton, C.T.; Stoll, H.M.; Mendez-Vicente, A. Vital Effects in Coccolith Calcite: Cenozoic Climate- pCO2 Drove the Diversity of Carbon Acquisition Strategies in Coccolithophores. Paleoceanography 2012, 27, PA4204. [Google Scholar] [CrossRef]
- Hermoso, M.; Chan, I.Z.X.; McClelland, H.L.O.; Heureux, A.M.C.; Rickaby, R.E.M. Vanishing Coccolith Vital Effects with Alleviated Carbon Limitation. Biogeosciences 2016, 13, 301–312. [Google Scholar] [CrossRef]
- McClelland, H.L.O.; Bruggeman, J.; Hermoso, M.; Rickaby, R.E.M. The Origin of Carbon Isotope Vital Effects in Coccolith Calcite. Nat. Commun. 2017, 8, 14511. [Google Scholar] [CrossRef]
- Stoll, H.M.; Ziveri, P. Coccolithophore-Based Geochemical Proxies. In Coccolithophores: From Molecular Processes to Global Impact; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 529–562. [Google Scholar]
- Boye, M.; Vilardell, N.S.I.; Gueguen, B.; Schmidt, S.; Beaufort, L. Geochemistry of the Coccoliths: Proxy of Surface Water Conditions or of Resilience of Coccolithophores Facing Climate Change? Goldschmidt 2019, 2019, 1. [Google Scholar]
- Hermoso, M.; Godbillot, C.; Minoletti, F. Enhancing Our Palaeoceanographic Toolbox Using Paired Foraminiferal and Coccolith Calcite Measurements from Pelagic Sequences. Front. Earth Sci. 2020, 8, 38. [Google Scholar] [CrossRef]
- Stoll, H.M.; Ziveri, P.; Geisen, M.; Probert, I.; Young, J.R. Potential and Limitations of Sr/Ca Ratios in Coccolith Carbonate: New Perspectives from Cultures and Monospecific Samples from Sediments. Philos. Trans. R. Soc. A 2002, 360, 719–747. [Google Scholar] [CrossRef]
- Stoll, H.; Langer, G.; Shimizu, N.; Kanamaru, K. B/Ca in Coccoliths and Relationship to Calcification Vesicle PH and Dissolved Inorganic Carbon Concentrations. Geochim. Cosmochim. Acta 2012, 80, 143–157. [Google Scholar] [CrossRef]
- Müller, M.N.; Lebrato, M.; Riebesell, U.; Ramos, J.B.E.; Schulz, K.G.; Blanco-Ameijeiras, S.; Sett, S.; Eisenhauer, A.; Stoll, H.M. Influence of Temperature and CO2 on the Strontium and Magnesium Composition of Coccolithophore Calcite. Biogeosciences 2014, 11, 1065–1075. [Google Scholar] [CrossRef]
- Hermoso, M.; Lefeuvre, B.; Minoletti, F.; de Rafélis, M. Extreme Strontium Concentrations Reveal Specific Biomineralization Pathways in Certain Coccolithophores with Implications for the Sr/Ca Paleoproductivity Proxy. PLoS ONE 2017, 12, e0185655. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Rokitta, S.D.; Rost, B.; Eagle, R.A. Constraints on Coccolithophores under Ocean Acidification Obtained from Boron and Carbon Geochemical Approaches. Geochim. Cosmochim. Acta 2021, 315, 317–332. [Google Scholar] [CrossRef]
- Stoll, H.M.; Schrag, D.P. Coccolith Sr/Ca as a New Indicator of Coccolithophorid Calcification and Growth Rate. Geochem. Geophys. Geosyst. 2000, 1, 1999GC000015. [Google Scholar] [CrossRef]
- Stoll, H.M.; Ziveri, P.; Shimizu, N.; Conte, M.; Theroux, S. Relationship between Coccolith Sr/Ca Ratios and Coccolithophore Production and Export in the Arabian Sea and Sargasso Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 581–600. [Google Scholar] [CrossRef]
- Suchéras-Marx, B.; Giraud, F.; Simionovici, A.; Tucoulou, R.; Daniel, I. Evidence of High Sr/Ca in a Middle Jurassic Murolith Coccolith Species. Peer Community Paleontol. 2021, 1, e25. [Google Scholar] [CrossRef]
- Paull, C.K.; Thierstein, H.R. Stable Isotopic Fractionation among Particles in Quaternary Coccolith-Sized Deep-Sea Sediments. Paleoceanography 1987, 2, 423–429. [Google Scholar] [CrossRef]
- Minoletti, F.; Hermoso, M.; Gressier, V. Separation of Sedimentary Micron-Sized Particles for Palaeoceanography and Calcareous Nannoplankton Biogeochemistry. Nat. Protoc. 2009, 4, 14–24. [Google Scholar] [CrossRef]
- Stoll, H.M.; Ziveri, P. Separation of Monospecific and Restricted Coccolith Assemblages from Sediments Using Differential Settling Velocity. Mar. Micropaleontol. 2002, 46, 209–221. [Google Scholar] [CrossRef]
- Halloran, P.R.; Rust, N.; Rickaby, R.E.M. Isolating Coccoliths from Sediment for Geochemical Analysis. Geochem. Geophys. Geosyst. 2009, 10, Q03001. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Mejía, L.M.; Stoll, H. Technical Note: Accelerate Coccolith Size Separation via Repeated Centrifugation. Biogeosciences 2021, 18, 1909–1916. [Google Scholar] [CrossRef]
- Young, J.R.; Geisen, M.; Probert, I. A Review of Selected Aspects of Coccolithophore Biology with Implications for Paleobiodiversity Estimation. Micropaleontology 2005, 51, 267–288. [Google Scholar] [CrossRef]
- Young, J.R.; Bown, P.R.; Lees, J.A. Nannotax3 Website, 2022. Available online: https://www.mikrotax.org/Nannotax3/ (accessed on 12 May 2022).
- Stoll, H.; Shimizu, N.; Arevalos, A.; Matell, N.; Banasiak, A.; Zeren, S. Insights on Coccolith Chemistry from a New Ion Probe Method for Analysis of Individually Picked Coccoliths. Geochem. Geophys. Geosyst. 2007, 8. [Google Scholar] [CrossRef]
- Stoll, H.M.; Shimizu, N. Micropicking of Nannofossils in Preparation for Analysis by Secondary Ion Mass Spectrometry. Nat. Protoc. 2009, 4, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Suchéras-Marx, B.; Giraud, F.; Lena, A.; Simionovici, A. Picking Nannofossils: How and Why. J. Micropalaeontol. 2016, 36, 219–231. [Google Scholar] [CrossRef]
- Prentice, K.; Jones, T.D.; Lees, J.; Young, J.; Bown, P.; Langer, G.; Fearn, S. Eimf Trace Metal (Mg/Ca and Sr/Ca) Analyses of Single Coccoliths by Secondary Ion Mass Spectrometry. Geochim. Cosmochim. Acta 2014, 146, 90–106. [Google Scholar] [CrossRef]
- Suchéras-Marx, B.; Giraud, F.; Daniel, I.; Rivard, C.; Aubry, M.-P.; Baumann, K.-H.; Beaufort, L.; Tucoulou, R.; Simionovici, A. Origin of Manganese in Nannofossil Calcite Based on Synchrotron NanoXRF and XANES. Mar. Micropaleontol. 2021, 163, 101961. [Google Scholar] [CrossRef]
- Bordiga, M.; Cobianchi, M.; Lupi, C.; Pelosi, N.; Venti, N.L.; Ziveri, P. Coccolithophore Carbonate during the Last 450 Ka in the NW Pacific Ocean (ODP Site 1209B, Shatsky Rise). J. Quat. Sci. 2014, 29, 57–69. [Google Scholar] [CrossRef]
- Ziveri, P.; de Bernardi, B.; Baumann, K.-H.; Stoll, H.M.; Mortyn, P.G. Sinking of Coccolith Carbonate and Potential Contribution to Organic Carbon Ballasting in the Deep Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 659–675. [Google Scholar] [CrossRef]
- Bralower, T.J.; Premoli Silva, I.; Malone, M.J. Site 1209. In Proceedings Ocean Drilling Program, Initial Reports; Bralower, T.J., Premoli Silva, I., Malone, M.J., Eds.; Ocean Drilling Program: College Station, TX, USA, 2002; Volume 198, pp. 1–102. [Google Scholar]
- Bordiga, M.; Beaufort, L.; Cobianchi, M.; Lupi, C.; Mancin, N.; Luciani, V.; Pelosi, N.; Sprovieri, M. Calcareous Plankton and Geochemistry from the ODP Site 1209B in the NW Pacific Ocean (Shatsky Rise): New Data to Interpret Calcite Dissolution and Paleoproductivity Changes of the Last 450 Ka. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 371, 93–108. [Google Scholar] [CrossRef]
- Zanoni, M.; Garagna, S.; Redi, C.A.; Zuccotti, M. The 2-Cell Block Occurring during Development of Outbred Mouse Embryos Is Rescued by Cytoplasmic Factors Present in Inbred Metaphase II Oocytes. Int. J. Dev. Biol. 2009, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. Micromachines for Biological Micromanipulation; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-74620-3. [Google Scholar]
- Bordiga, M.; Lupi, C.; Langer, G.; Gianoncelli, A.; Birarda, G.; Pollastri, S.; Bonanni, V.; Bedolla, D.E.; Vaccari, L.; Gariani, G.; et al. New Insights on Marine Microalgae Biomineralization: Silicon Localization in Coccolithophore Carbonate Exoskeleton. Proc. Natl. Acad. Sci. USA 2022. in review. [Google Scholar]
- Hay, W.W. Calcareous Nannofossils. In Oceanic Micropalaeontology; Ramsay, A.T.S., Ed.; Academic Press: London, UK, 1977; pp. 1055–1200. [Google Scholar]
- Green, O.R. Extraction Techniques for Calcareous Nannofossils. In A Manual of Practical Laboratory and Field Techniques in Palaeobiology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 366–374. [Google Scholar]

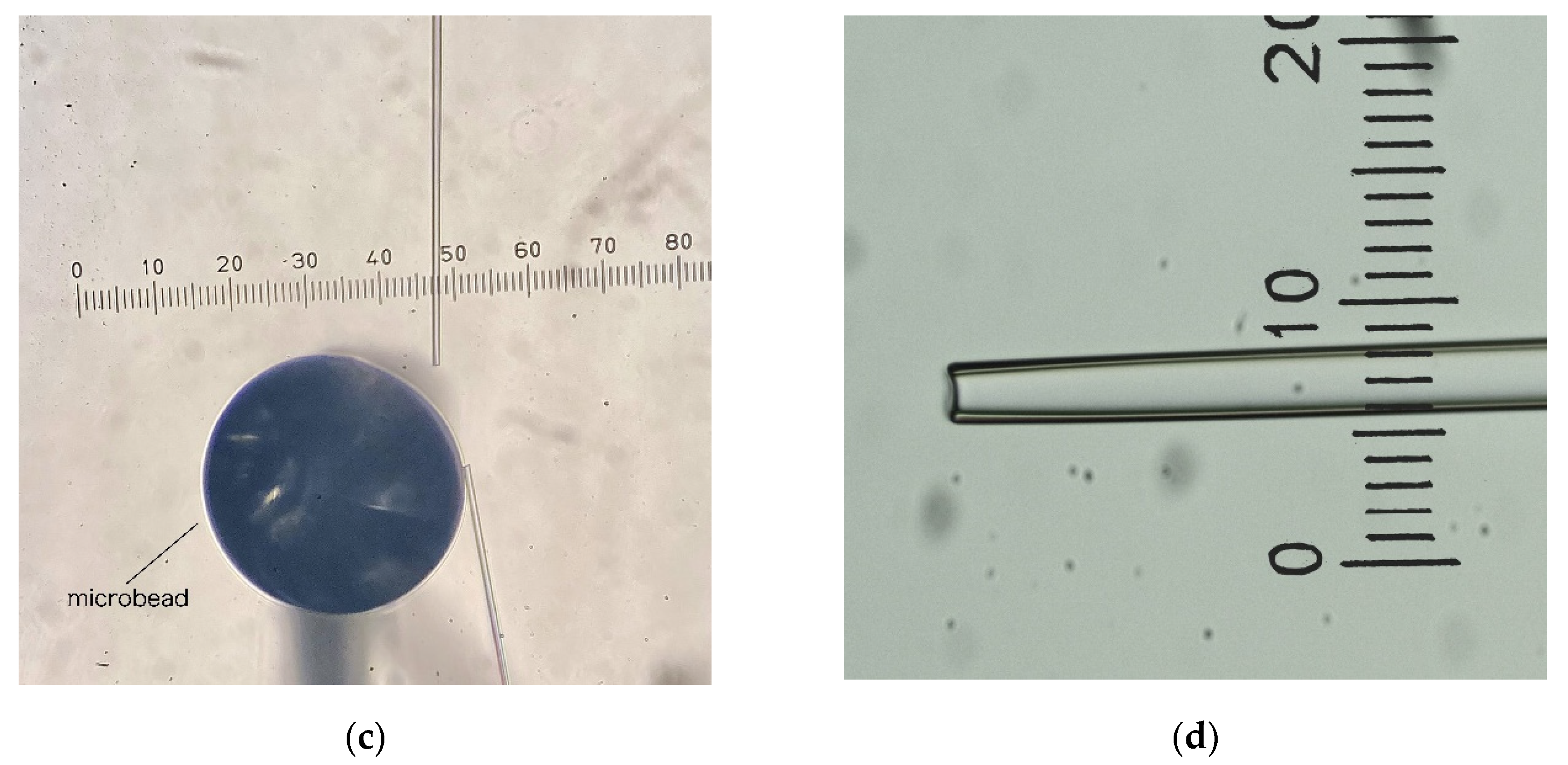

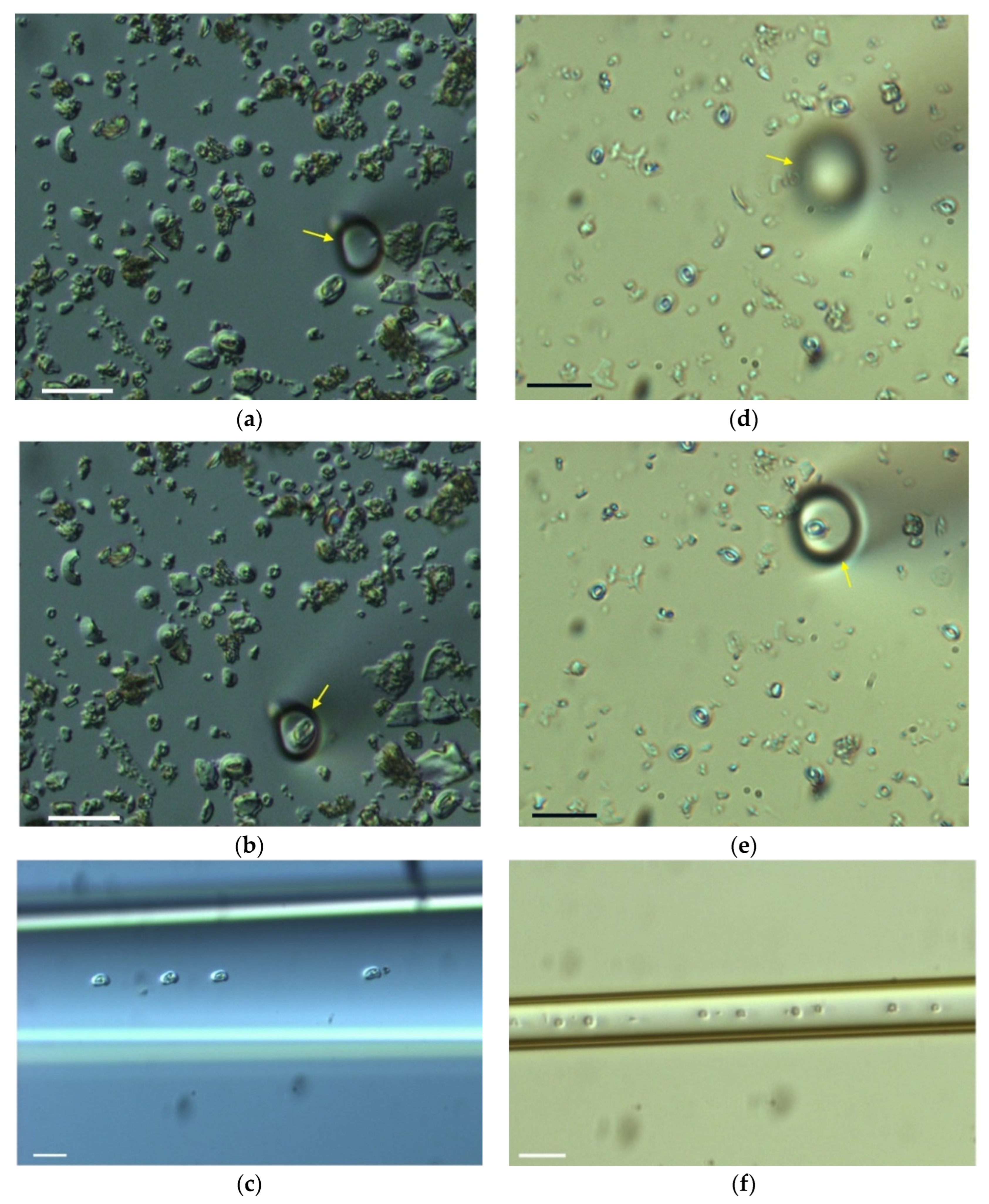
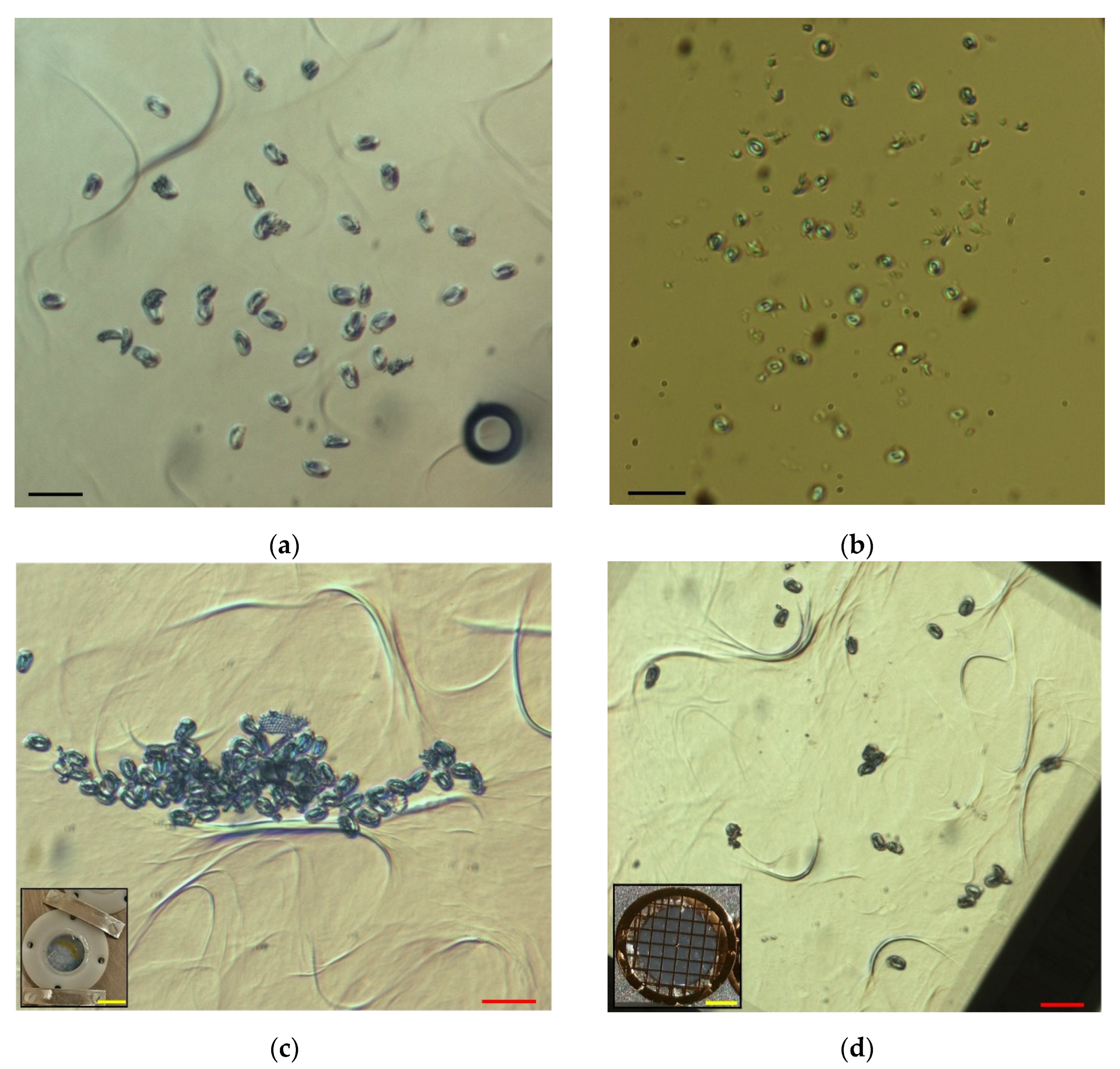

| #Attempt | #Picked Coccoliths | #Lost Coccoliths | #Deposited Coccoliths | Min | Efficiency (#Coccoliths/h) |
|---|---|---|---|---|---|
| 1 H.c. | 30 | 5 | 25 | 10 | 150 |
| 2 H.c. | 16 | 2 | 14 | 7 | 120 |
| 3 H.c. | 20 | 5 | 15 | 11 | 81 |
| 4 H.c. | 36 | 6 | 30 | 15 | 120 |
| 5 H.c. | 23 | 4 | 19 | 18 | 66 |
| 6 H.c. | 33 | 3 | 30 | 16 | 114 |
| 7 H.c. | 18 | 1 | 17 | 10 | 102 |
| 1 G.o. | 37 | 8 | 29 | 11 | 156 |
| 2 G.o. | 40 | 6 | 34 | 17 | 120 |
| 3 G.o. | 31 | 5 | 26 | 13 | 120 |
| 4 G.o. | 27 | 4 | 23 | 9 | 150 |
| 5 G.o. | 23 | 2 | 21 | 8 | 156 |
| 6 G.o. | 32 | 5 | 27 | 14 | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordiga, M.; Lupi, C.; Zanoni, M.; Bianco, S.; Cabrini, M.; Fiorentino, G.; Garagna, S.; Zuccotti, M.; Di Giulio, A. A New Method to Speed Up Nannofossil Picking for Monospecific Geochemical Analyses. J. Mar. Sci. Eng. 2022, 10, 1829. https://doi.org/10.3390/jmse10121829
Bordiga M, Lupi C, Zanoni M, Bianco S, Cabrini M, Fiorentino G, Garagna S, Zuccotti M, Di Giulio A. A New Method to Speed Up Nannofossil Picking for Monospecific Geochemical Analyses. Journal of Marine Science and Engineering. 2022; 10(12):1829. https://doi.org/10.3390/jmse10121829
Chicago/Turabian StyleBordiga, Manuela, Claudia Lupi, Mario Zanoni, Stefania Bianco, Marina Cabrini, Giulia Fiorentino, Silvia Garagna, Maurizio Zuccotti, and Andrea Di Giulio. 2022. "A New Method to Speed Up Nannofossil Picking for Monospecific Geochemical Analyses" Journal of Marine Science and Engineering 10, no. 12: 1829. https://doi.org/10.3390/jmse10121829
APA StyleBordiga, M., Lupi, C., Zanoni, M., Bianco, S., Cabrini, M., Fiorentino, G., Garagna, S., Zuccotti, M., & Di Giulio, A. (2022). A New Method to Speed Up Nannofossil Picking for Monospecific Geochemical Analyses. Journal of Marine Science and Engineering, 10(12), 1829. https://doi.org/10.3390/jmse10121829









