Microbial Parameters as Predictors of Heterotrophic Prokaryotic Production in the Ross Sea Epipelagic Waters (Antarctica) during the Austral Summer
Abstract
1. Introduction
2. Materials and Methods
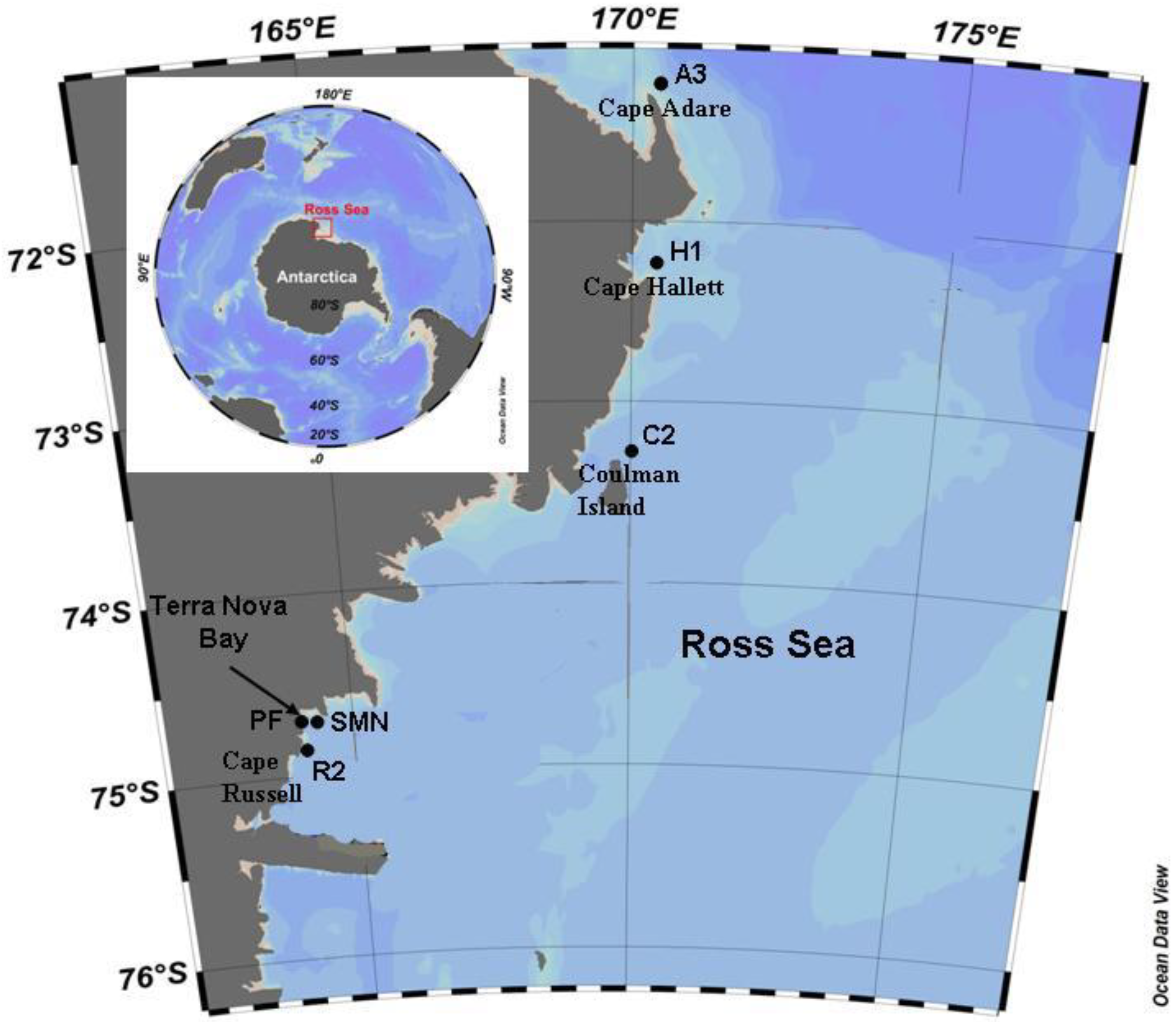
2.1. Study Area and Samplings
2.2. Heterotrophic Prokaryotic Production (HPP) Measurements
2.3. Extracellular Enzymatic Activity (EEA) Measurements
2.4. Total Pico Plankton (TPP) Cell Counts
2.5. Lipopolysaccharides (LPS) Quantitative Determinations
2.6. Statistical Analysis
3. Results
3.1. Microbiological and Environmental Characteristics
3.2. Regression Analysis
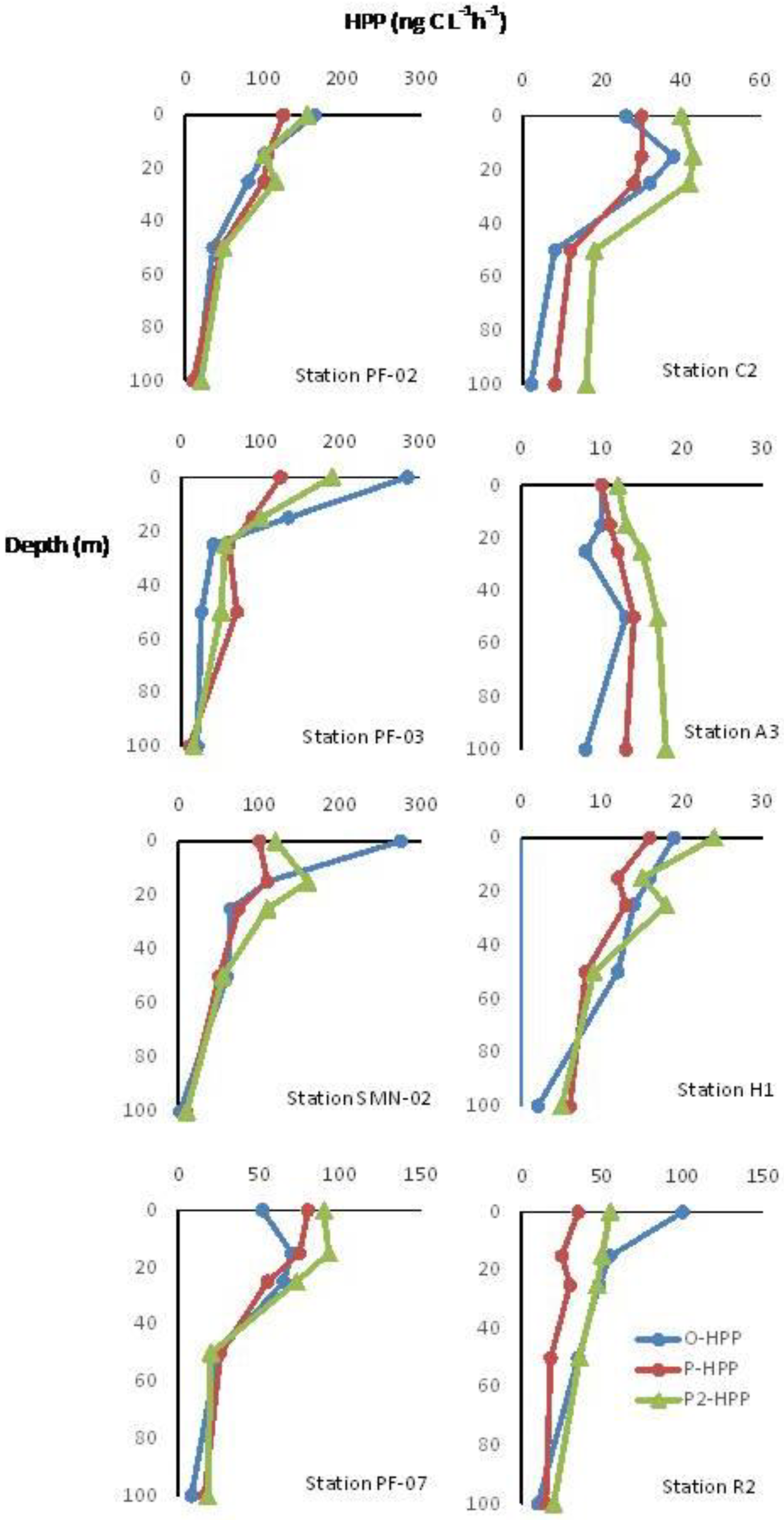
3.3. Observed and Predicted HPP Values
Distribution of O-HPP and P-HPP along TNB and VLT Epipelagic Water Columns
4. Discussion
4.1. Microbiological Scenario
4.2. Regressions and Predictive Models
Post-Regression Tests
4.3. Observed versus Predicted HPP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, B.C.; Azam, F. Major role of bacteria in biogeochemical fluxes in the ocean’s interior. Nature 1988, 332, 441–443. [Google Scholar] [CrossRef]
- Simon, M.; Azam, F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Progr. Ser. 1989, 51, 201–213. [Google Scholar] [CrossRef]
- Hoppe, H.G. Significance of exoenzymatic activities in the ecology of brackish water: Measurements by means of methyl-umbelliferyl-substrates. Mar. Ecol. Progr. Ser. 1983, 11, 299–308. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R. Enzymatic Activity, Bacterial Distribution, and Organic Matter Composition in Sediments of the Ross Sea (Antarctica). Appl. Environ. Microbiol. 1998, 64, 3838–3845. [Google Scholar] [CrossRef]
- Azam, F. Microbial control of oceanic carbon flux: The plot thickens. Science 1998, 280, 694–696. [Google Scholar] [CrossRef]
- Chróst, R.J. Microbial ectoenzymes in aquatic environments. In Aquatic Microbial Ecology; Overbeck, J., Chróst, R.J., Eds.; Springer: New York, NY, USA, 1990; pp. 47–74. [Google Scholar] [CrossRef]
- Chróst, R.J. Microbial Enzymes in Aquatic Environments; Springer: New York, NY, USA, 1991; pp. 1–317. [Google Scholar] [CrossRef]
- Chróst, R.J. Ectoenzymes in aquatic environments: Microbial strategy for substrate supply. Verh. Internat. Verein Limnol. 1991, 24, 2597–2600. [Google Scholar] [CrossRef]
- Talbot, V.; Giuliano, L.; Bruni, V.; Bianchi, M. Bacterial abundance, production and ectoproteolytic activity in the Strait of Magellan. Mar. Ecol. Progr. Ser. 1997, 154, 293–302. [Google Scholar] [CrossRef]
- Piontek, J.; Sperling, M.; Nöthig, E.M.; Engel, A. Regulation of bacterioplankton activity in Fram Strait (Arctic Ocean) during early summer: The role of organic matter supply and temperature. J. Mar. Syst. 2014, 132, 83–94. [Google Scholar] [CrossRef]
- Watson, S.W.; Novitsky, T.J.; Quinby, H.L.; Valois, F.W. Determination of bacterial number and biomass in the marine environment. Appl. Environ. Microbiol. 1977, 33, 940–946. [Google Scholar] [CrossRef]
- Karl, D.M.; Bird, D.F.; Björkman, K.; Houlikan, T.; Shackelford, R.; Tupas, L. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science 1999, 286, 2144–2147. [Google Scholar] [CrossRef]
- Barnett, M.J.; Wadham, J.L.; Jackson, M.; Cullen, D.C. In-field implementation of a recombinant factor C assay for the detection of lipopolysaccharide as a biomarker of extant life within glacial environments. Biosensors 2012, 2, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Bruni, V.; La Ferla, R.; Acosta Pomar, M.L.C.; Salomone, L. Structural differences of the microbial community in two sites of the Terra Nova Bay (Ross Sea, Antarctica): A statistical analysis. Microbiologica 1995, 18, 409–422. [Google Scholar] [PubMed]
- Crisafi, E.; Azzaro, F.; La Ferla, R.; Monticelli, L.S. Microbial biomass and respiratory activity related to the ice-melting upper layers in the Ross Sea (Antarctica). In Ross Sea Ecology; Faranda, F., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 171–180. [Google Scholar]
- Umani, S.F.; Accornero, A.; Budillon, G.; Capello, M.; Tucci, S.; Cabrini, M.; Del Negro, P.; Monti, M.; De Vittor, C. Particulate matter and plankton dynamics in the Ross Sea Polynya of Terra Nova Bay during the austral summer 1997/98. J. Mar. Syst. 2002, 36, 29–49. [Google Scholar] [CrossRef]
- Misic, C.; Povero, P.; Fabiano, M. Ectoenzymatic ratios in relation to particulate organic matter distribution (Ross Sea, Antarctica). Microb. Ecol. 2002, 44, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.S.; La Ferla, R.; Maimone, G. Dynamics of bacterioplankton activities after a summer phytoplankton bloom period in Terra Nova Bay. Antarct. Sci. 2003, 15, 85–93. [Google Scholar] [CrossRef]
- Celussi, M.; Paoli, A.; Crevatin, E.; Bergamasco, A.; Margiotta, F.; Saggiomo, V.; Fonda Umani, S.; Del Negro, P. Short-term under-icevariability of prokaryotic plankton communities in coastalAntarcticwaters (Cape Hallett, Ross Sea). Estuar. Coast. Shelf Sci. 2009, 8, 491–500. [Google Scholar] [CrossRef]
- Zaccone, R.; Misic, C.; Azzaro, F.; Azzaro, M.; Maimone, G.; Mangoni, O.; Fusco, G.; Rappazzo, A.C.; La Ferla, R. Regulation of Microbial Activity Rates by Organic Matter in the Ross Sea during the Austral Summer 2017. Microorganisms 2020, 8, 1273. [Google Scholar] [CrossRef]
- Berkman, P.A.; CattaneoVietti, R.; Chiantore, M.; Howard Williams, C.; Cummings, V.; Kvitek, R. Marine research in the Latitudinal Gradient Project along Victoria Land, Antarctica. Sci. Mar. 2005, 69, 57–63. [Google Scholar] [CrossRef]
- Azzaro, M.; Specchiulli, A.; Maimone, G.; Azzaro, F.; Lo Giudice, A.; Papale, M.; La Ferla, R.; Paranhos, R.; Souza Cabral, A.; Rappazzo, A.C.; et al. Trophic and Microbial Patterns in the Ross Sea Area (Antarctica): Spatial Variability during the Summer Season. J. Mar. Sci. Eng. 2022, 10, 1666. [Google Scholar] [CrossRef]
- Karl, D.M. Microbial processes in the southern oceans. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley: New York, NY, USA, 1993; pp. 1–63. [Google Scholar]
- Wetz, M.S.; Wheeler, P.A. Release of dissolved organic matter by coastal diatoms. Limnol. Oceanogr. 2007, 52, 798–807. [Google Scholar] [CrossRef]
- Strom, L.S.; Benner, R.; Ziegler, S.; Dago, M.J. Planktonic grazers are a potential important source of marine dissolved organic carbon. Limnol. Oceanogr. 1997, 42, 1364–1374. [Google Scholar] [CrossRef]
- Kawasaki, N.; Benner, R. Bacterial release of dissolved organic matter during cell growth and decline: Molecular origin and composition. Limnol. Oceanogr. 2006, 51, 2170–2180. [Google Scholar] [CrossRef]
- Thornton, D.C.O. Dissolved organic matter (DOM) released by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 2014, 49, 20–46. [Google Scholar] [CrossRef]
- Pomeroy, L.R.; Mathews, H.M.; Min, H.S. Excretion of phosphate and soluble organic phosphorus compounds by zooplankton. Limnol. Oceanogr. 1963, 8, 50–55. [Google Scholar] [CrossRef]
- Steward, G.F.; Smith, D.C.; Azam, F. Abundance and production of bacteria and viruses in the Bering and Chukchi seas. Mar. Ecol. Progr. Ser. 1996, 131, 287–300. [Google Scholar] [CrossRef]
- Shelfort, E.J.; Middelboe, M.; Moller, E.F.; Suttle, C.A. Virus-driven nitrogen cycling enhances phytoplankton growth. Aquat. Microb. Ecol. 2012, 60, 41–46. [Google Scholar] [CrossRef]
- Leventer, A. Particulate flux from sea ice in Antarctic waters. In Sea Ice. An Introduction to Its Physics, Chemistry, Biology and Geology; Thomas, D.N., Dieckmann, G.S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2003; Chapter 10; pp. 303–332. [Google Scholar]
- Povero, P.; Castellano, M.; Ruggieri, N.; Monticelli, L.S.; Saggiomo, V.; Chiantore, M.; Guidetti, M.; CattaneoVietti, R. Water column features and their relationship with sediments and benthic communities along Victoria Land Coast (Ross Sea, summer 2004). Antarct. Sci. 2006, 18, 603–613. [Google Scholar] [CrossRef]
- Kirchman, D.; K’nees, E.; Hodson, R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl. Environ. Microbiol. 1985, 49, 599–607. [Google Scholar] [CrossRef]
- Smith, D.C.; Azam, F. A simple, economical method for measuring bacteria protein synthesis rates in seawater using 3H-leucine. Mar. Microb. Food Webs 1992, 6, 107–114. [Google Scholar]
- Kirchman, D.L. Leucine incorporation as a measure of biomass production bt heterotrophic bacteria. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Cole, J.J., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 509–512. [Google Scholar]
- Ducklow, H.W. Seasonal production and bacterial utilization of DOC in the Ross Sea, Antarctica. In Biogeochemistry of the Ross Sea; Ditullio, G.R., Dunbar, R.B., Eds.; Antarctic Research Series 78; American Geophysical Union: Washington, DC, USA, 2003; pp. 143–158. [Google Scholar]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and count aquatic microflora. Limnol. Oceanogr. 1980, 25, 942–948. [Google Scholar] [CrossRef]
- Watson, S.W.; Hobbie, J.E. Measurement of bacterial biomass as lipopolysaccharides. In Native Aquatic Bacteria: Enumeration, Activity and Ecology; Costerton, J.W., Colwell, R.R., Eds.; ASTM: Philadelphia, PA, USA, 1979; pp. 82–88. [Google Scholar]
- Minitab, LLC. Minitab. 2021. Available online: https://www.minitab.com (accessed on 16 April 2021).
- Arrigo, K.R.; van Dijken, G.L.; Long, M. Coastal Southern Ocean: A Strong Anthropogenic CO2 Sink. Geophys. Res. Lett. 2008, 35, L21602. [Google Scholar] [CrossRef]
- Kim, B.; Kim, S.-H.; Min, J.-O.; Lee, Y.; Jung, J.; Kim, T.-W.; Lee, J.S.; Yang, E.J.; Park, J.; Lee, S.H.; et al. Bacterial Metabolic Response to Change in Phytoplankton Communities and Resultant Effects on Carbon Cycles in the Amundsen Sea Polynya, Antarctica. Front. Mar. Sci. 2022, 9, 872052. [Google Scholar] [CrossRef]
- Min, J.-O.; Kim, S.-H.; Jung, J.; Jung, U.-J.; Yang, E.J.; Lee, S.H.; Hyun, J.-H. Glacial ice melting stimulates heterotrophic prokaryotes production on the Getz Ice Shelf in the Amundsen Sea, Antarctica. Geophys. Res. Lett. 2022, 49, e2021GL097627. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Shi, Z.; Li, Q.; Li, R. Variability in the empirical leucine-to-carbon conversion factors along an environmental gradient. Acta Oceanol. Sin. 2018, 37, 77–82. [Google Scholar] [CrossRef]
- Giering, S.L.C.; Evans, C. Overestimation of prokaryotic production by leucine incorporation—And how to avoid it. Limnol. Oceanogr. 2022, 67, 726–738. [Google Scholar] [CrossRef]
- Bird, D.F.; Kalff, J. Empirical relationships between bacterial abundance and chlorophyll concentration in fresh and marine waters. Can. J. Fish. Aquat. Sci. 1984, 41, 1015–1023. [Google Scholar] [CrossRef]
- Caruso, G.; Madonia, A.; Bonamano, S.; Miserocchi, S.; Giglio, F.; Maimone, G.; Azzaro, F.; Decembrini, F.; La Ferla, R.; Piermattei, V.; et al. Microbial Abundance and Enzyme Activity Patterns: Response to Changing Environmental Characteristics along a Transect in Kongsfjorden (Svalbard Islands). J. Mar. Sci. Eng. 2020, 8, 824. [Google Scholar] [CrossRef]
- Caruso, G.; Zaccone, R. Estimates of leucine aminopeptidase activity in different marine and brackish environments. J. Appl. Microbiol. 2000, 89, 951–959. [Google Scholar] [CrossRef]
- Zaccone, R.; Caruso, G. Microbial hydrolysis of polysaccharides and organic phosphates in the Northern Adriatic Sea. Chem. Ecol. 2002, 18, 85–94. [Google Scholar] [CrossRef]
- Misic, C.; Povero, P.; Fabiano, M. Relationship between ectoenzymatic activity and availability of organic substrates (Ross Sea, Antarctica): An experimental approach. Polar Biol. 1998, 20, 367–376. [Google Scholar] [CrossRef]
- Shindoh, S.; Obayashi, Y.; Suzuki, S. Induction of Extracellular Aminopeptidase Production by Peptides in Some Marine Bacterial Species. Microbes Environ. 2021, 36, ME20150. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Follstad Shah, J.J.; Findlay, S.G.; Keuehn, K.A.; Moorhead, D.L. Scaling microbial biomass, metabolism and resource supply. Biogeochemistry 2015, 122, 175–190. [Google Scholar] [CrossRef]
- Yamada, N.; Fukuda, H.; Ogawa, H.; Saito, H.; Suzumura, M. Heterotrophic bacterial production and extracellular enzymatic activity in sinking particulate matter in the western North Pacific Ocean. Front. Microbiol. 2012, 3, 379. [Google Scholar] [CrossRef] [PubMed]
- Hoch, M.P.; Kirchman, D.L. Seasonal and inter-annual variability in bacterial production and biomass in a temperate estuary. Mar. Ecol. Progr. Ser. 1993, 98, 283–295. [Google Scholar] [CrossRef]
- Adams, H.E.; Crump, B.C.; Kling, G.W. Isolating the effects of storm events on arctic aquatic bacteria: Temperature, nutrients, and community composition as controls on bacterial productivity. Front. Microbiol. 2015, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ducklow, H.W. A decadal (2002–2014) analysis for dynamics of heterotrophic bacteria in an Antarctic coastal ecosystem: Variability and physical and biogeochemical forcings. Front. Mar. Sci. 2016, 3, 214. [Google Scholar] [CrossRef]

| T | HPP | LAP | AP | ß-G | LPS | TPP | ||
|---|---|---|---|---|---|---|---|---|
| °C | ngC L−1h−1 | nM h−1 | nM h−1 | nM h−1 | ng L−1 | ×107 cells L−1 | ||
| TNB | mean | −1.65 | 59.23 | 190.12 | 1.26 | 0.48 | 128.34 | 13.32 |
| (0–100 m) | s.d. | 0.48 | 26.96 | 451.15 | 0.45 | 0.14 | 38.30 | 4.55 |
| 22 January–10 February 2000 | max | −0.89 | 282.79 | 733.05 | 6.73 | 1.85 | 357.87 | 13.90 |
| min | −1.99 | 4.4 | 28.63 | 0.03 | 0.41 | 61.15 | 5.40 | |
| n | 84 | 84 | 81 | 54 | 48 | 83 | 78 | |
| VLT | mean | −1.26 | 11.67 | 6.44 | 0.054 | 0.064 | 70.81 | 6.06 |
| (0–100 m) | s.d. | 0.50 | 2.44 | 1.97 | 0.003 | 0.027 | 3.13 | 4.37 |
| 3–21 February 2004 | max | −0.69 | 125.49 | 50.76 | 2.027 | 0.569 | 212.11 | 32.44 |
| min | −1.90 | 0.49 | 0.12 | 0.017 | 0.003 | 39.41 | 2.65 | |
| n | 78 | 78 | 78 | 78 | 78 | 74 | 78 |
| Matrix A. | TNB 2000 Survey Dataset | Matrix B | VLT 2004 Survey Dataset | ||||||||
| HPP | LAP | β-G | AP | TPP | HPP | LAP | β-G | AP | TPP | ||
| LAP | 0.848 | LAP | 0.853 | ||||||||
| (•) * | (•) | ||||||||||
| β-G | 0.449 | 0.553 | β-G | 0.244 | 0.251 | ||||||
| (0.002) | (•) | (0.049) | (0.027) | ||||||||
| AP | 0.318 | 0.318 | 0.358 | AP | 0.472 | 0.367 | 0.259 | ||||
| (0.022) | (0.022) | (0.016) | (•) | (0.001) | (0.022) | ||||||
| TPP | 0.483 | 0.470 | 0.243 | 0.248 | TPP | 0.415 | 0.360 | 0.304 | 0.485 | ||
| (•) | (•) | n.s. | n.s. | (•) | (0.001) | (0.007) | (•) | ||||
| LPS | 0.531 | 0.533 | 0.439 | 0.288 | 0.138 | LPS | 0.389 | 0.335 | 0.329 | 0.528 | 0.691 |
| (•) | (•) | (0.002) | (0.038) | n.s. | (0.001) | (0.004) | (0.005) | (•) | (•) | ||
| Matrix C | Overall Data (TNB + VLT Datasets) | Matrix D | As Cell-Specific Activity | ||||||||
| HPP | LAP | β-G | AP | TPP | HPPcsa | LAPcsa | β-Gcsa | APcsa | |||
| LAP | 0.826 | LAPcsa | 0.828 | ||||||||
| (•) | (•) | ||||||||||
| β-G | 0.331 | 0.467 | β-Gcsa | 0.441 | 0.511 | ||||||
| (•) | (•) | (•) | (•) | ||||||||
| AP | 0.499 | 0.574 | 0.461 | APcsa | 0.542 | 0.582 | 0.470 | ||||
| (•) | (•) | (•) | (•) | (•) | (•) | ||||||
| TPP | 0.329 | 0.100 | 0.150 | 0.165 | LPScsa | 0.588 | 0.436 | 0.330 | 0.434 | ||
| (•) | n.s. | n.s. | n.s. | (•) | (•) | (•) | (•) | ||||
| LPS | 0.450 | 0.374 | 0.285 | 0.148 | 0.284 | ||||||
| (•) | (•) | (0.002) | n.s. | (•) | |||||||
| R-sq | R-sq | Mallows | Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vars | R-sq | (Adj) | PRESS | (Pred) | Cp | S | LAPcsa | APcsa | ß-Gcsa | LPScsa | TPP |
| Total Dataset | |||||||||||
| 1 | 61.4 | 61.1 | 14.1 | 59.9 | 37.6 | 0.3555 | X | ||||
| 1 | 32.1 | 31.4 | 24.8 | 29.2 | 146.1 | 0.4719 | X | ||||
| 1 | 31.1 | 30.5 | 25.0 | 28.7 | 149.5 | 0.4751 | X | ||||
| 1 | 24.9 | 24.2 | 27.4 | 21.9 | 172.8 | 0.4963 | X | ||||
| 1 | 4.4 | 3.5 | 34.5 | 1.5 | 248.4 | 0.5598 | X | ||||
| 2 * | 70.8 | 70.2 | 10.8 | 69.2 | 5.1 | 0.3110 | X | X | |||
| 3 * | 71.7 | 70.9 | 10.8 | 69.3 | 3.7 | 0.3076 | X | X | X | ||
| 4 * | 72.1 | 72.1 | 10.8 | 69.3 | 4.0 | 0.3066 | X | X | X | X | |
| 5 | 72.1 | 70.8 | 10.9 | 68.9 | 6.0 | 0.3080 | X | X | X | X | X |
| ≤50 nmol L−1 LAP Dataset | LAPcsa | APcsa | ß-Gcsa | LPScsa | TPP | ||||||
| 1 | 67.8 | 67.4 | 9.7 | 66.1 | 14.3 | 0.3298 | X | ||||
| 1 | 32.6 | 31.8 | 20.1 | 30.0 | 120.3 | 0.4768 | X | ||||
| 1 | 29.0 | 28.1 | 21.4 | 25.4 | 131.3 | 0.4896 | X | ||||
| 1 | 25.9 | 25.1 | 22.4 | 21.9 | 140.4 | 0.4999 | X | ||||
| 1 | 25.3 | 24.4 | 22.5 | 21.5 | 142.5 | 0.5022 | X | ||||
| 2 * | 70.1 | 69.4 | 9.2 | 67.0 | 9.3 | 0.3196 | X | X | |||
| 3 * | 71.2 | 70.1 | 9.2 | 67.8 | 7.9 | 0.3155 | X | X | X | ||
| 4 * | 71.9 | 70.5 | 9.2 | 68.0 | 7.9 | 0.3137 | X | X | X | X | |
| 5 | 73.2 | 71.5 | 9.1 | 68.4 | 6.0 | 0.3083 | X | X | X | X | X |
| >50 nmol L−1 LAP Data Set | LAPcsa | APcsa | ß-Gcsa | LPScsa | TPP | ||||||
| 1 | 81.8 | 81.0 | 1.6 | 78.6 | 5.5 | 0.2394 | X | ||||
| 1 | 81.5 | 80.6 | 1.6 | 78.1 | 6.0 | 0.2418 | X | ||||
| 1 | 73.9 | 72.8 | 2.3 | 68.6 | 17.1 | 0.2868 | X | ||||
| 1 | 65.1 | 63.6 | 2.9 | 59.4 | 29.9 | 0.3316 | X | ||||
| 1 | 62.8 | 61.2 | 3.1 | 56.6 | 33.2 | 0.3422 | X | ||||
| 2 * | 85.7 | 84.4 | 1.3 | 82.7 | 1.8 | 0.2169 | X | X | |||
| 3 * | 86.2 | 84.3 | 1.3 | 82.1 | 3.1 | 0.2179 | X | X | X | ||
| 4 * | 86.8 | 84.2 | 1.3 | 81.9 | 4.3 | 0.2188 | X | X | X | X | |
| 5 | 87.0 | 83.5 | 1.4 | 80.6 | 6.0 | 0.2230 | X | X | X | X | X |
| Predictor | Coef | SE | p-Value | VIF |
|---|---|---|---|---|
| Total Dataset (TDS) | ||||
| LAP * | 0.5360 | 0.0270 | 0.000 | 5.24 |
| LPS * | 0.2833 | 0.0230 | 0.000 | 5.24 |
| S | 0.2483 | |||
| R-sq | 97.23% | |||
| R-sq (adj) | 97.19% | |||
| R-sq (pred) | 97.16% | |||
| Mallows’ Cp | 2.00 | |||
| alpha = 0.05 | ||||
| Regression Equation from TDS (TDS-RE) | ||||
| Log HPP = 0.536 log (LAP *) + 0.283 log (LPS *) | ||||
| ≤50 nM h−1LAP dataset (≤50 DS) | ||||
| Constant | 0.6820 | 0.1190 | 0.000 | |
| LAPcsa ** | 0.6285 | 0.0403 | 0.000 | 1.28 |
| APcsa ** | 0.0932 | 0.0374 | 0.015 | 1.28 |
| S | 0.2319 | |||
| R-sq | 80.73% | |||
| R-sq (adj) | 80.29% | |||
| R-sq (pred) | 79.17% | |||
| Mallows’ Cp | 2.53 | |||
| alpha = 0.05 | ||||
| Regression Equation from ≤50-DS (≤50 DS-RE) | ||||
| Log HPPcsa = 0.682 + 0.6285 log (LAPcsa **) + 0.0932 log (APcsa **) | ||||
| >50 nM h−1LAP dataset (>50 DS) | ||||
| Constant | 0.1230 | 0.3320 | 0.714 | |
| LAPcsa ** | 0.3950 | 0.1690 | 0.029 | 3.31 |
| LPScsa** | 0.4540 | 0.1110 | 0.000 | 3.31 |
| S | 0.2212 | |||
| R-sq | 85.15% | |||
| R-sq (adj) | 83.80% | |||
| R-sq (pred) | 81.45% | |||
| Mallows’ Cp | 1.59 | |||
| alpha = 0.05 | ||||
| Regression Equation from >50 DS (>50 DS-RE) | ||||
| Log HPPcsa = 0.1230 + 0.3950 log (LAPcsa **) + 0.4540 log (LPScsa **) | ||||
| Enzyme. | N Observations | Slope | Intercept | R2 |
|---|---|---|---|---|
| LAP | 544 | 1.10 | +0.66 | 0.48 |
| B-G | 567 | 1.04 | −1.79 | 0.46 |
| AP | 391 | 1.05 | −1.40 | 0.45 |
| P-HPP from Regression Equations: | |||
|---|---|---|---|
| O-HPP from: | TDS-RE | ≤50 DS-RE | >50 DS-RE |
| Total dataset (TDS) | 0.263 | 0.544 | 0.810 |
| ≤50 nM h−1 LAP dataset (≤50 DS) | 0.104 | 0.995 * | <0.001 |
| >50 nMh−1 LAP dataset (>50 DS) | 0.912 * | 0.509 | 0.741 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monticelli, L.S.; Caruso, G.; Azzaro, F.; Azzaro, M.; Ferla, R.L.; Maimone, G.; Povero, P.; Cosenza, A.; Zaccone, R. Microbial Parameters as Predictors of Heterotrophic Prokaryotic Production in the Ross Sea Epipelagic Waters (Antarctica) during the Austral Summer. J. Mar. Sci. Eng. 2022, 10, 1812. https://doi.org/10.3390/jmse10121812
Monticelli LS, Caruso G, Azzaro F, Azzaro M, Ferla RL, Maimone G, Povero P, Cosenza A, Zaccone R. Microbial Parameters as Predictors of Heterotrophic Prokaryotic Production in the Ross Sea Epipelagic Waters (Antarctica) during the Austral Summer. Journal of Marine Science and Engineering. 2022; 10(12):1812. https://doi.org/10.3390/jmse10121812
Chicago/Turabian StyleMonticelli, Luis Salvador, Gabriella Caruso, Filippo Azzaro, Maurizio Azzaro, Rosabruna La Ferla, Giovanna Maimone, Paolo Povero, Alessandro Cosenza, and Renata Zaccone. 2022. "Microbial Parameters as Predictors of Heterotrophic Prokaryotic Production in the Ross Sea Epipelagic Waters (Antarctica) during the Austral Summer" Journal of Marine Science and Engineering 10, no. 12: 1812. https://doi.org/10.3390/jmse10121812
APA StyleMonticelli, L. S., Caruso, G., Azzaro, F., Azzaro, M., Ferla, R. L., Maimone, G., Povero, P., Cosenza, A., & Zaccone, R. (2022). Microbial Parameters as Predictors of Heterotrophic Prokaryotic Production in the Ross Sea Epipelagic Waters (Antarctica) during the Austral Summer. Journal of Marine Science and Engineering, 10(12), 1812. https://doi.org/10.3390/jmse10121812









