Geostatistical Determination of Soil Noise and Soil Phosphorus Spatial Variability
Abstract
:1. Introduction
2. Materials and Methods
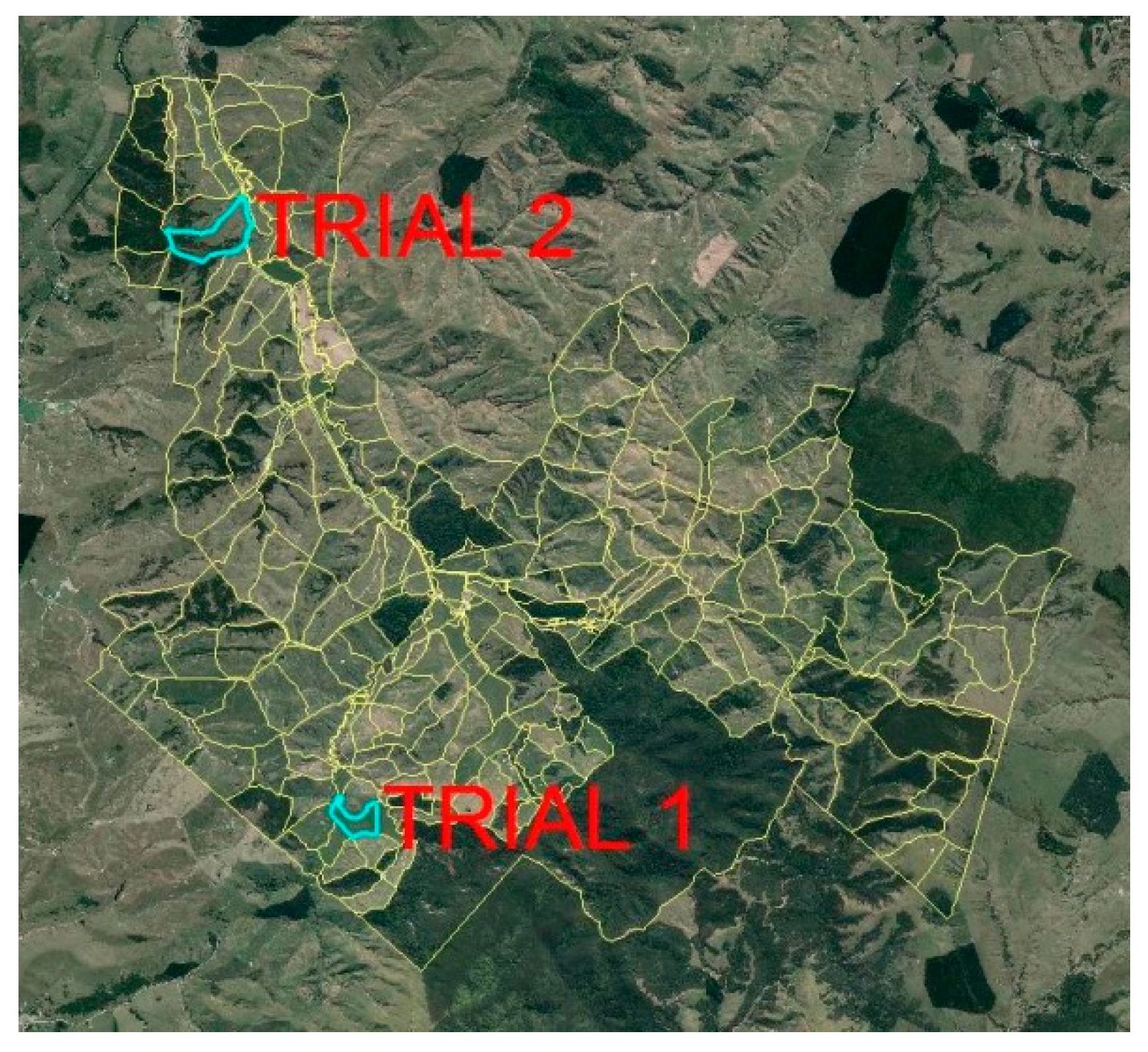
2.1. Site Selection
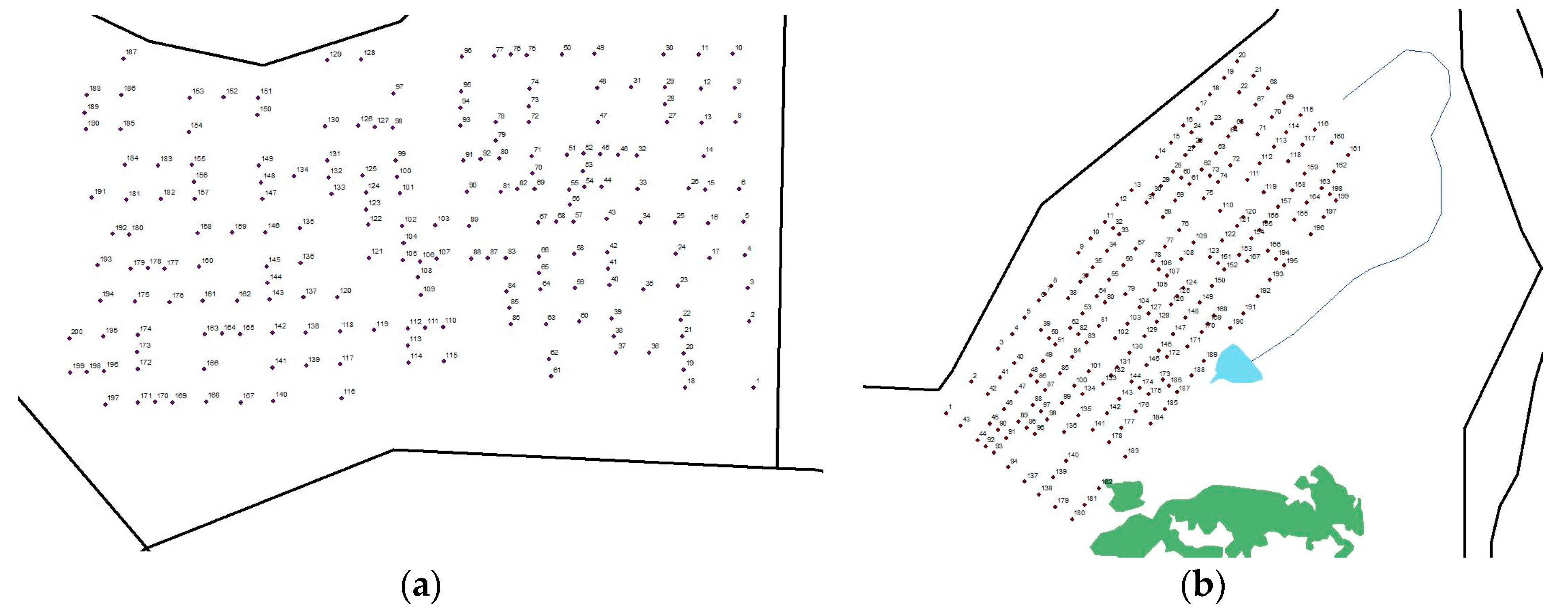
2.2. Core Collection Protocol
2.3. Sectioning of Soil Cores
2.4. Measurement of Olsen P
absorbance at 712 nm = 476.19 × absorbance (for 1 cm cuvette cell)
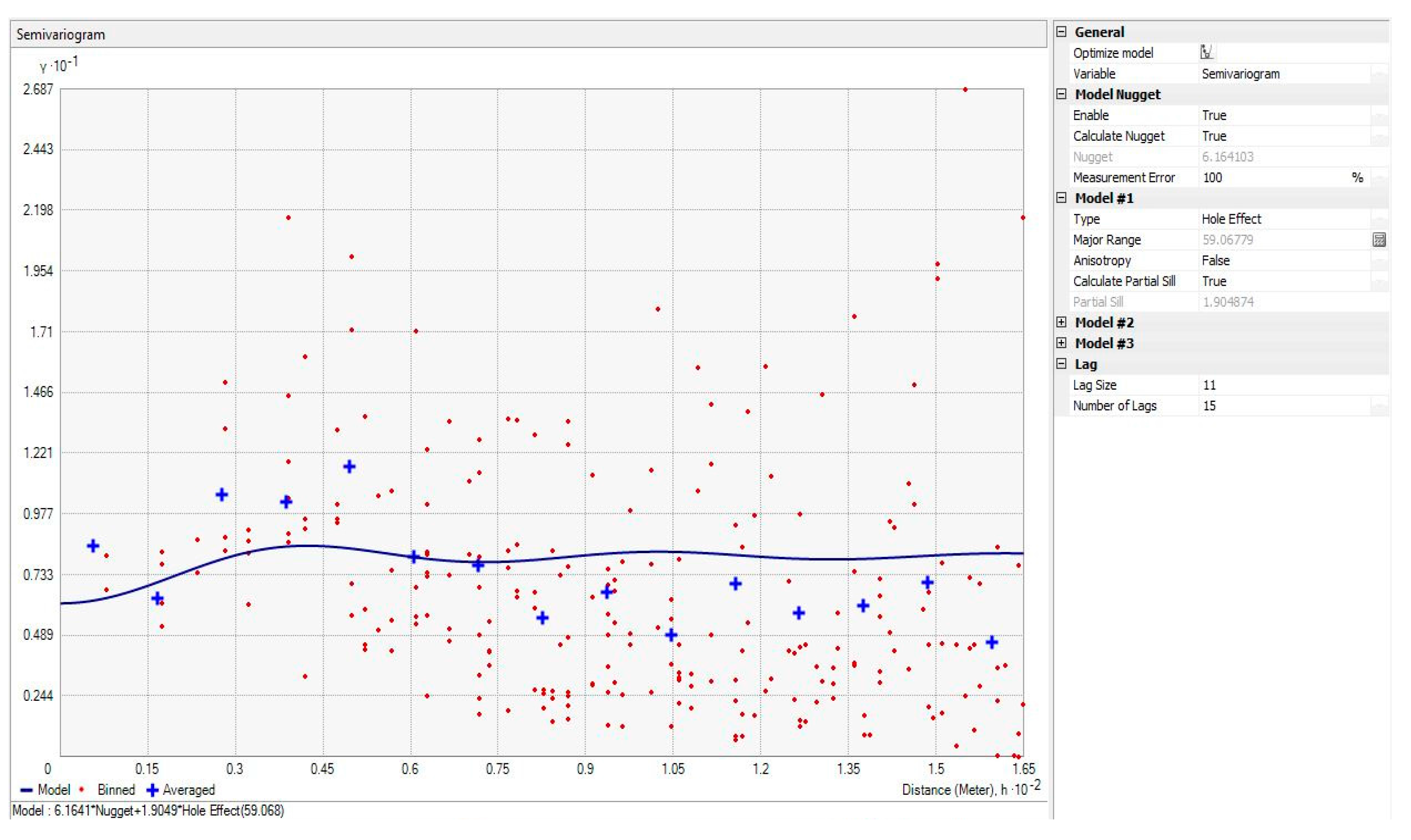
2.5. Geostatistical Analysis
3. Results
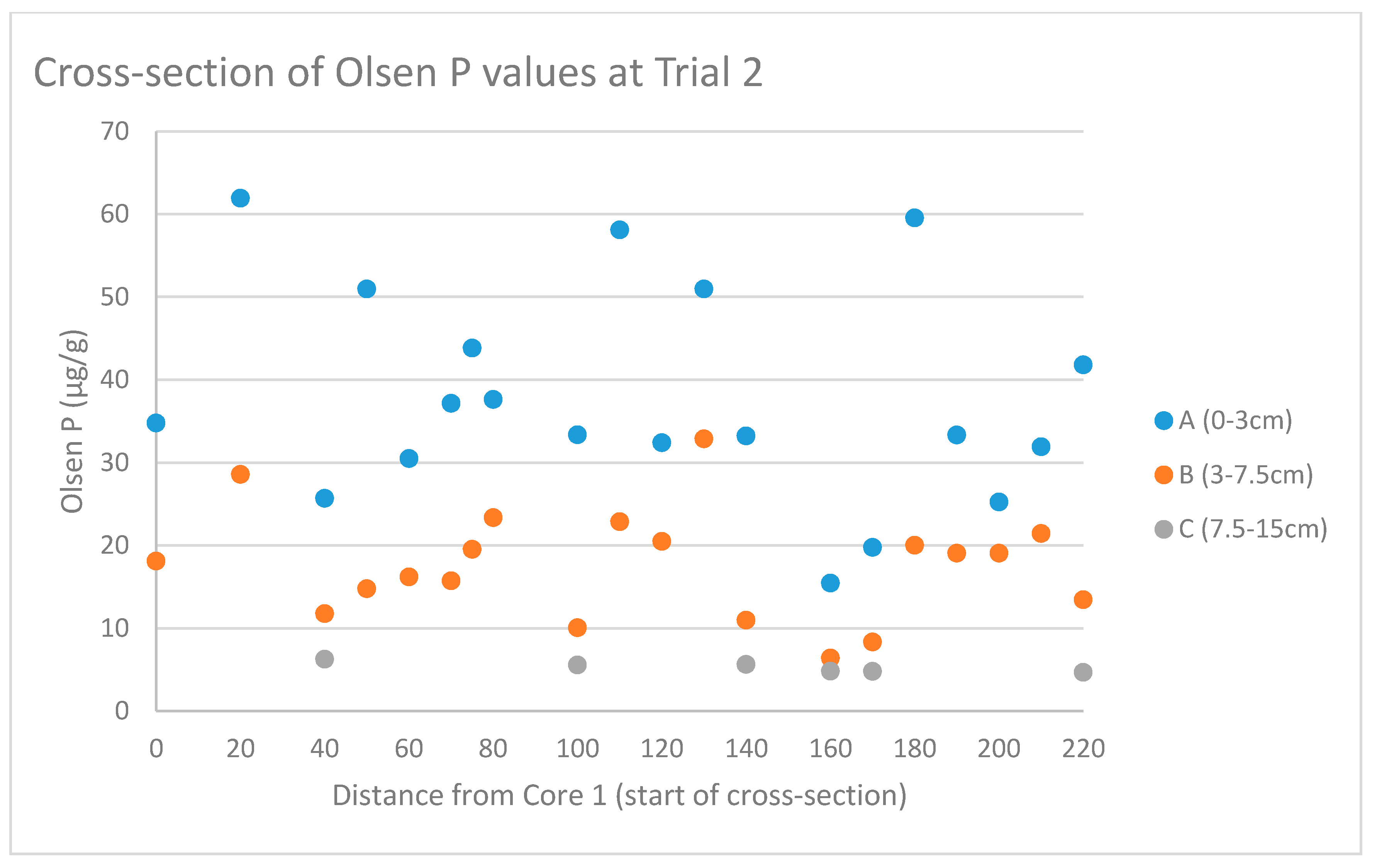
3.1. Spatial Variability of Phosphorus at Different Depth Ranges
3.2. Variogram and Kriging Summary Statistics
3.3. Analysis of Variance (ANOVA)
3.3.1. F-Test to Compare Trial Sites of Two Distinct Slope Classes
3.3.2. F-test for Comparison of Variability of Sections within Each Trial
3.3.3. F-Test for Comparison of Variability of Different Sampling Methods
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morton, J.D.; Sinclair, A.G.; Morrison, J.D.; Smith, L.C.; Dodds, K.G. Balanced and adequate nutrition of phosphorus and sulphur in pasture. N. Z. Agric. Res. 1998, 41, 487–496. [Google Scholar] [CrossRef]
- Cornforth, I.S.; Sinclair, A.G. Fertiliser and Lime Recommendations for Pastures and Crops in New Zealand; Ministry of Agriculture and Fisheries: Wellington, New Zealand, 1984; 76p.
- Gillingham, A.G.; Morton, J.D.; Gray, A.H. Pasture responses to phosphorus and nitrogen fertilisers on East Coast hill country; total production from easy slopes. N. Z. Agric. Res. 2007, 50, 307–320. [Google Scholar] [CrossRef]
- Grafton, M.C.E.; Yule, I.J.; Lockhart, J.C. An Economic Analysis of the Topdressing Industry. In Farming’s Future: Minimizing Footprints and Maximizing Margins; Occasional Report No. 23; Currie, L.D., Christensen, C.L., Eds.; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2010; pp. 405–412. ISSN 0112-9902. [Google Scholar]
- Lucci, G.M.; McDowell, R.W.; Condron, L.M. Potential phosphorus and sediment loads from sources within a dairy farmed catchment. Soil Use Manag. 2010, 26, 44–52. [Google Scholar] [CrossRef]
- Chok, S.E.; Grafton, M.C.E.; Yule, I.J.; White, M. Accuracy of Differential Rate Application Technology for Aerial Spreading of Granular Fertilizer within New Zealand. In Proceedings of the 13th International Conference on Precision Agriculture, St. Louis, MO, USA, 31 July–3 August 2016. [Google Scholar]
- Morton, J.D.; Baird, D.B.; Manning, M.J. A soil sampling protocol to minimise the spatial variability in soil test values in New Zealand hill country. N. Z. Agric. Res. 2000, 43, 367–375. [Google Scholar] [CrossRef]
- Kaul, T.M.C.; Grafton, M.C.E.; Hedley, M.J.; Yule, I.J. Understanding soil phosphorus variability with depth for the improvement of current soil sampling methods. In Science and Policy: Nutrient Management Challenges for the Next Generation; Occasional Report No. 30; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2017; 11p, ISSN 0112-9902. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Iowa City, IA, USA, 1989; ISBN 978-0-8138-1561-9. [Google Scholar]



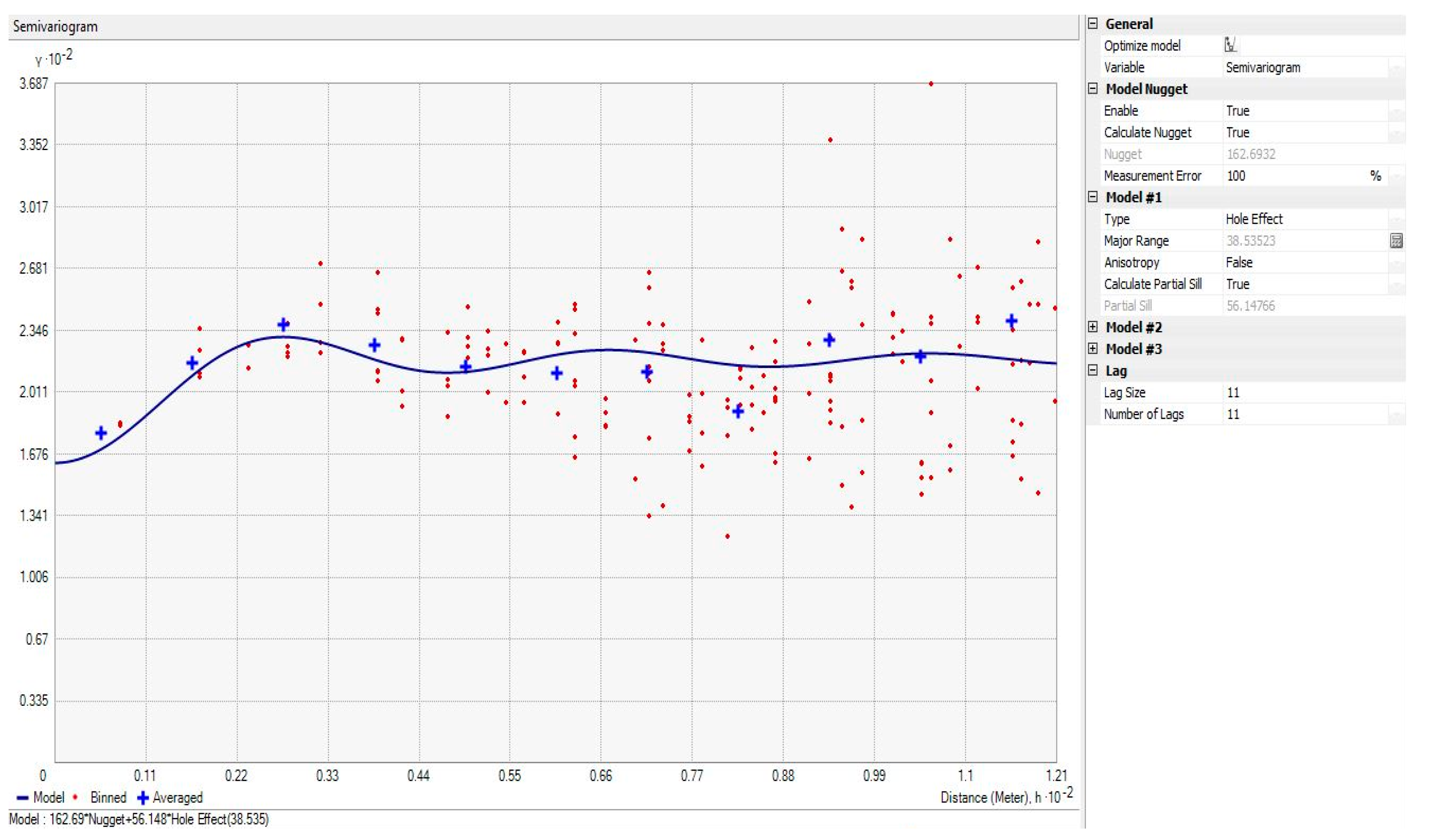
| Trial 1 | ||||||||||
| Root Mean Square | Average Standard Error | Nugget | Major Range | Partial Sill | Sill | Lag Size | Number of Lags | Model Type | No. of Samples | |
| aA | 10.4 | 6.6 | 89 | 45 | 49 | 138 | 9 | 11 | Hole Effect | 60 |
| bB | 6.1 | 4.1 | 23 | 43 | 22 | 45 | 8 | 10 | Hole Effect | 60 |
| cC | 6.0 | 4.1 | 11 | 43 | 33 | 44 | 7 | 10 | Hole Effect | 60 |
| Trial 2 | ||||||||||
| Root Mean Square | Average Standard Error | Nugget | Major Range | Partial Sill | Sill | Lag Size | Number of Lags | Model Type | No. of Samples | |
| aA | 14.6 | 5.9 | 163 | 39 | 56 | 219 | 11 | 11 | Hole Effect | 200 |
| bB | 9.4 | 3.9 | 56 | 34 | 25 | 81 | 8 | 11 | Hole Effect | 198 |
| cC | 2.9 | 1.4 | 6 | 59 | 2 | 8 | 11 | 15 | Hole Effect | 59 |
| Soil Parameter | Trial 1 (Flat) | Trial 2 (Steep) |
|---|---|---|
| Sulphur (S) | 30 mg/kg | 24 mg/kg |
| Potassium (K) | 20 mg/kg | 14 mg/kg |
| Phosphorus (P) | 25 mg/kg | 18 mg/kg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaul, T.; Grafton, M. Geostatistical Determination of Soil Noise and Soil Phosphorus Spatial Variability. Agriculture 2017, 7, 83. https://doi.org/10.3390/agriculture7100083
Kaul T, Grafton M. Geostatistical Determination of Soil Noise and Soil Phosphorus Spatial Variability. Agriculture. 2017; 7(10):83. https://doi.org/10.3390/agriculture7100083
Chicago/Turabian StyleKaul, Therese, and Miles Grafton. 2017. "Geostatistical Determination of Soil Noise and Soil Phosphorus Spatial Variability" Agriculture 7, no. 10: 83. https://doi.org/10.3390/agriculture7100083
APA StyleKaul, T., & Grafton, M. (2017). Geostatistical Determination of Soil Noise and Soil Phosphorus Spatial Variability. Agriculture, 7(10), 83. https://doi.org/10.3390/agriculture7100083





