Provenancing Flower Bulbs by Analytical Fingerprinting: Convallaria Majalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Material and Study Design
2.2. Stable Isotope Analysis by IRMS
2.3. Volatile Organic Compound Analysis by PTR-MS

2.4. Statistical Analysis
3. Results and Discussion
3.1. Stable Isotope Analysis by IRMS
| Provenance | δ-15N | δ-13C | δ-18O |
|---|---|---|---|
| The Netherlands | 1.5 ± 2.4 x | −26.6 ± 2.6 y | 36.0 ± 3.2 x |
| Belgium | −0.9 ± 0.1 xy | −25.5 ± 3.4 xy | 35.5 ± 2.7 xy |
| France | 0.4 ± 1.2 x | −24.2 ± 0.7 x | 34.7 ± 3.1 xy |
| Germany | 1.8 ± 0.2 x | −24.5 ± 1.3 xy | 33.0 ± 1.4 xy |
| China | −2.8 ± 2.2 y | −26.4 ± 2.0 xy | 30.4 ± 4.0 y |
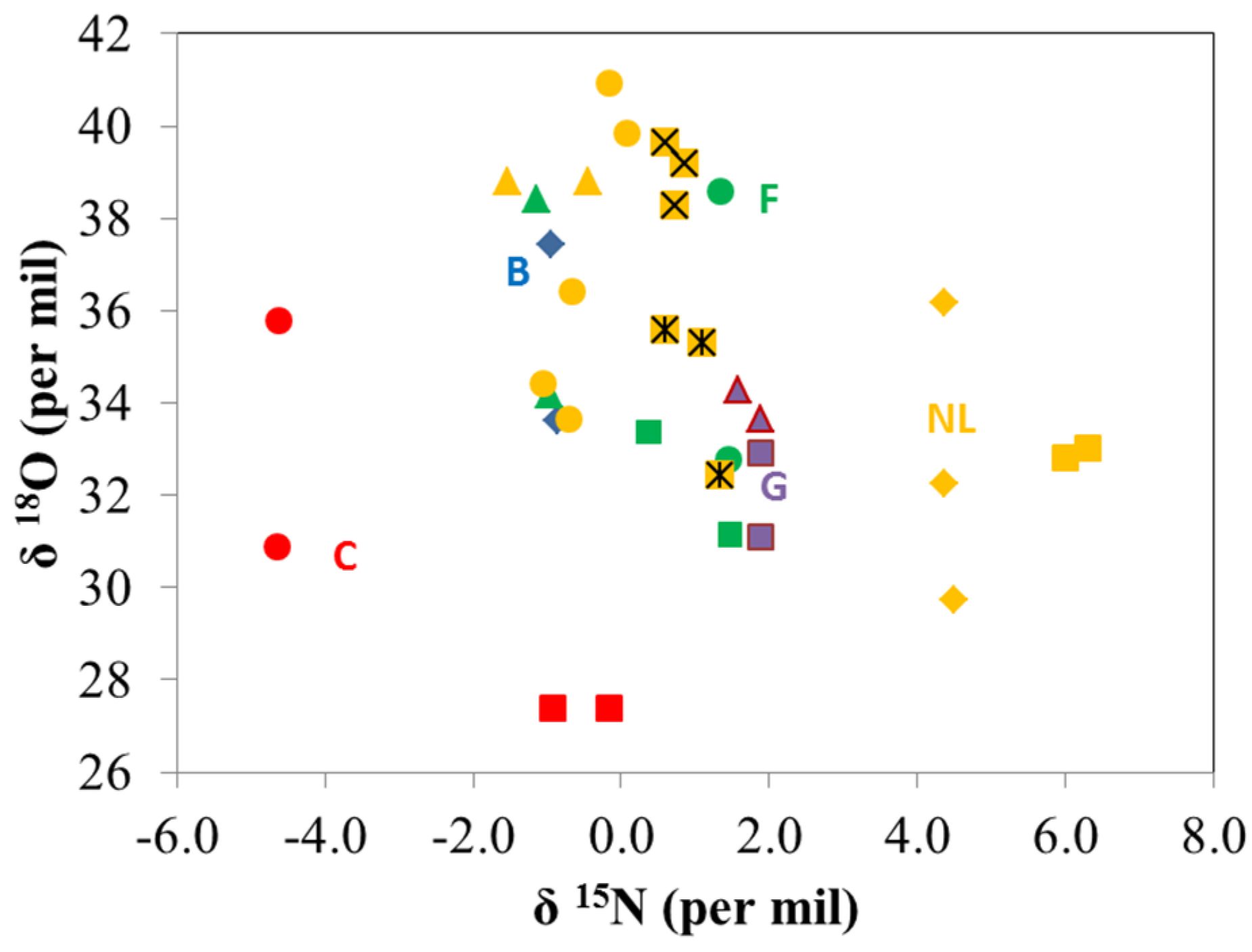
3.2. Volatile Organic Compound Analysis by PTR-MS
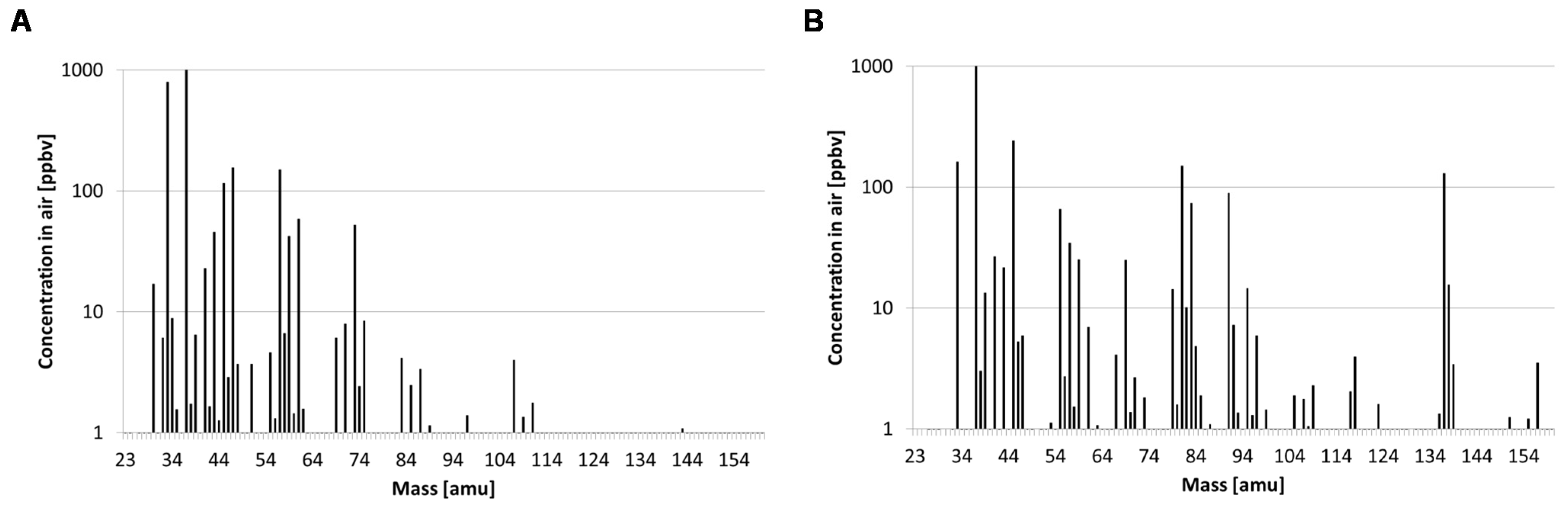
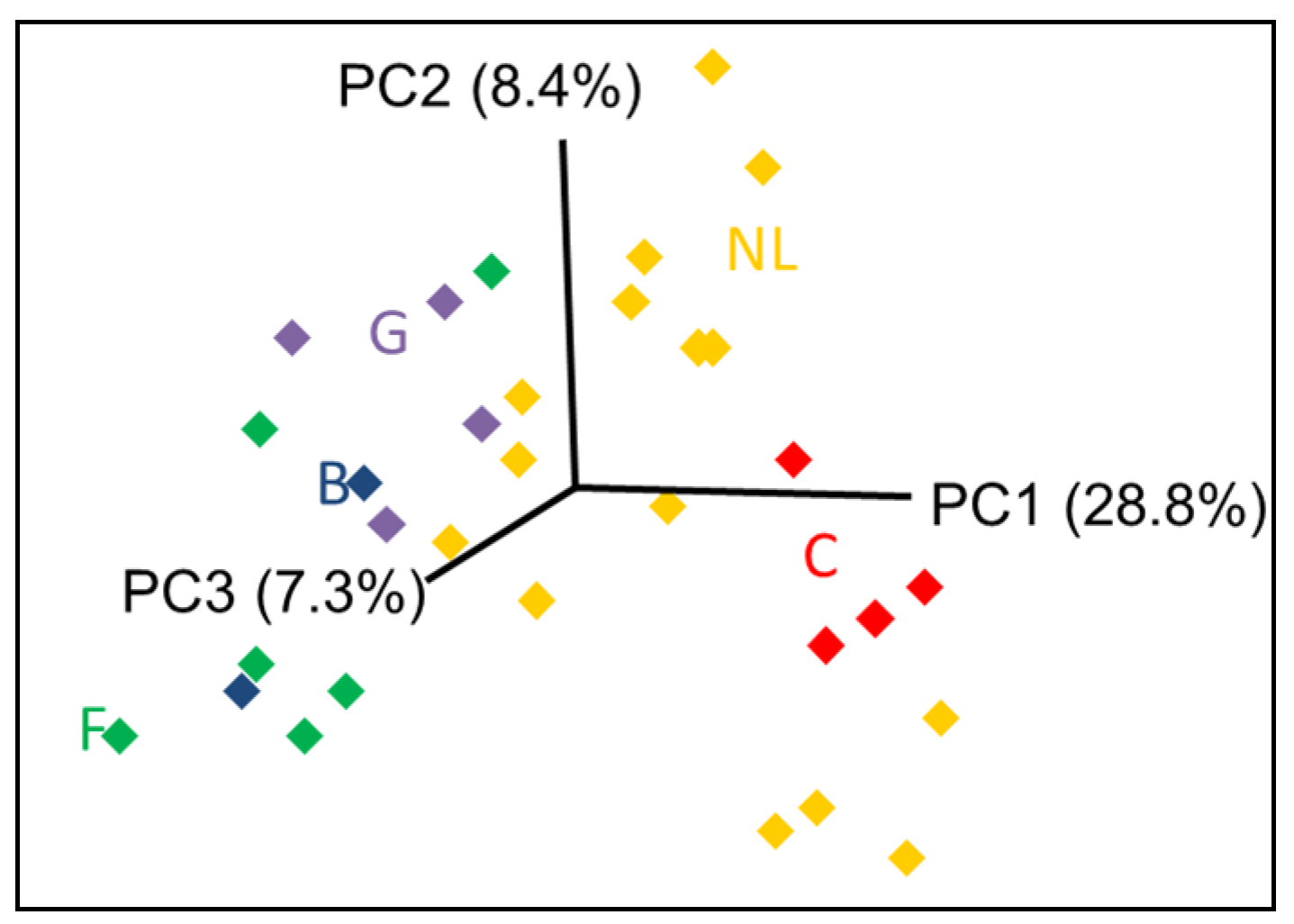
| Provenance | Prediction | ||
|---|---|---|---|
| The Netherlands | Other EU Countries a | China | |
| The Netherlands | 100% | 0% | 0% |
| Other EU countries | 0% | 100% | 0% |
| China | 0% | 0% | 100% |
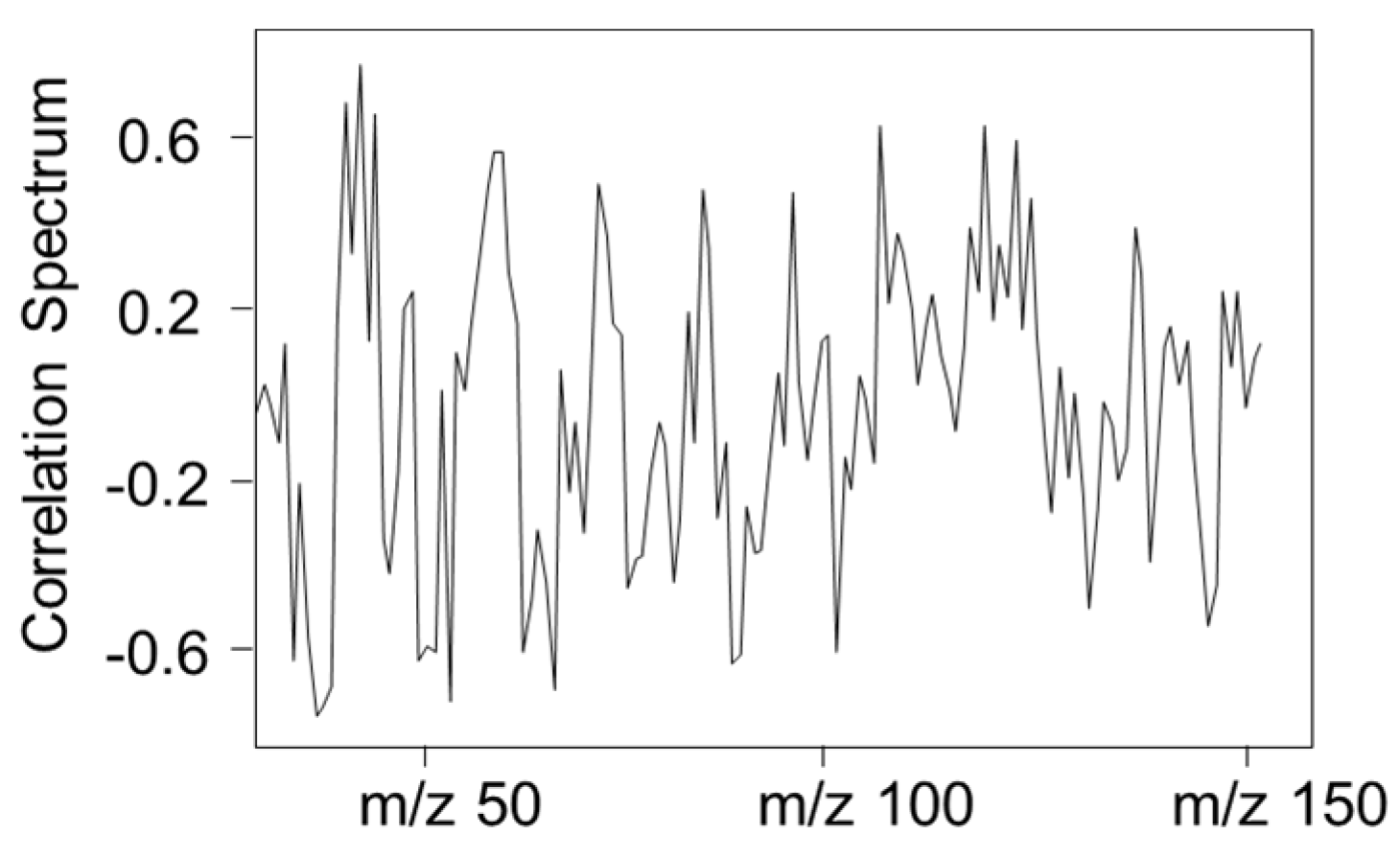
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsen, P.; Borit, M. How to define traceability. Trends Food Sci. Technol. 2012, 29, 142–150. [Google Scholar] [CrossRef]
- Monahana, F.J.; Moloneyb, A.P.; Osorioa, M.T.; Röhrlea, F.T.; Schmidta, O.; Brennanc, L. Authentication of grass-fed beef using bovine muscle, hair or urine. Trends Food Sci. Technol. 2012, 28, 69–76. [Google Scholar] [CrossRef]
- Mattarucchi, E.; Stocchero, M.; Moreno-Rojas, J.M.; Giordano, G.; Reniero, F.; Guillou, C. Authentication of Trappist beers by LC-MS fingerprints and multivariate data analysis. J. Agric. Food Chem. 2010, 58, 12089–12095. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.A.; Engen, J.R.; Mazzeo, J.R.; Jones, G.B. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 2012, 11, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Schama, S. The Embarrassment of Riches: An Interpretation of Dutch Culture in the Golden Age; Alfred A. Knopf: New York, NY, USA, 1987; p. 698. [Google Scholar]
- Burrows, G.E.; Tyrl, R.I. The Liliaceae. In Toxic Plants of North America; Burrows, G.E., Tyrl, R.I., Eds.; Iowa State Press: Ames, IA, USA, 2001; pp. 271–295. [Google Scholar]
- Fitzgerald, K.T. Lily toxicity in the cat. Top. Companion Anim. Med. 2010, 25, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Ogorodnikova, A.V.; Latypova, L.R.; Mukhitova, F.K.; Mukhtarova, L.S.; Grechkin, A.N. Detection of divinyl ether synthase in Lily-of-the-Valley (Convallaria majalis) roots. Phytochemistry 2008, 69, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Delamont, S. Appetites and Identities: An Introduction to the Social Anthropology of Western Europe; Routledge: London, UK, 1994; p. 272. [Google Scholar]
- Dimara, E.; Skuras, D. Consumer evaluations of product certification, geographic association and traceability in Greece. Eur. J. Market. 2003, 37, 690–705. [Google Scholar] [CrossRef]
- Luykx, D.M.A.M.; van Ruth, S.M. A review of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Review: Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Jasper, J.P.; Westenberger, B.J.; Spencer, J.A.; Buhse, L.F.; Nasr, M. Stable isotopic characterization of active pharmaceutical ingredients. J. Pharm. Biomed. Anal. 2004, 35, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, R.; Hatton, C.K.; Catlin, D.H. Detection of epitestosterone doping by isotope ratio mass spectrometry. Clin. Chem. 2002, 48, 629–636. [Google Scholar] [PubMed]
- Nakabayashi, R.; Sawada, Y.; Yamada, Y.; Suzuki, M.; Hirai, M.Y.; Sakurai, T.; Saito, K. Combination of liquid chromatography-Fourier transform ion cyclotron resonance-mass spectrometry with 13C-labeling for chemical assignment of sulfur-containing metabolites in onion bulbs. Anal. Chem. 2013, 85, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- De Visser, R.; Vianden, H.; Schnyder, H. Kinetics and relative significance of remobilized and current C and N incorporation in leaf and root growth zones of Lolium perenne after defoliation: Assessment by 13C and 15N steady-state labelling. Plant Cell Environ. 1997, 20, 37–46. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 29. [Google Scholar] [CrossRef]
- Tannock, G.W.; Lawley, B.; Munro, K.; Sims, I.M.; Lee, J.; Butts, C.A.; Roy, N. RNA-stable isotope probing (RNA-SIP) shows carbon utilization from inulin by specific bacterial populations in the large bowel of rats. Appl. Environ. Microbiol. 2014, 80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Horn, M.A.; Kolb, S.; Drake, H.L. Temperature impacts differentially on the methanogenic food web of cellulose-supplemented peatland soil. Environ. Microbiol. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, G.; Peterson, D. Principles of food flavor analysis. In Instrumental Assessment of Food Sensory Quality; Kilkast, D., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 53–102. [Google Scholar]
- Heenan, S.; van Ruth, S.M. Emerging flavour analysis methods in food authentication. In Instrumental Assessment of Food Sensory Quality; Kilkast, D., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 284–312. [Google Scholar]
- Sharp, Z.D. Principles of Stable Isotope Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 2006; p. 344. [Google Scholar]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the PPB level. Int. J. Mass Spectrom. Ion Process. 1995, 149, 609–619. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Fenoll, J.; Hellín, P. The feasibility of using δ 15N and δ 13C values for discriminating between conventionally and organically fertilized pepper (Capsicum annuum L.). J. Agric. Food Chem. 2007, 55, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- De Visser, S.N.; Freymann, B.P.; Schnyder, H. Trophic interactions among invertebrates in Termitaria in the African savanna: A stable isotope approach. Ecol. Entomol. 2008, 33, 758–764. [Google Scholar]
- Miller, D.L.; Mora, C.I.; Grissino-Mayer, H.D.; Mock, C.J.; Uhle, M.E.; Sharp, Z. Tree-ring isotope records of tropical cyclone activity. Proc. Natl. Acad. Sci. USA 2006, 103, 14294–14297. [Google Scholar] [CrossRef] [PubMed]
- Helliker, B.R. On the controls of leaf-water oxygen isotope ratios in the atmospheric crassulacean acid metabolism epiphyte Tillandsia usneoides. Plant Physiol. 2011, 155, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Fráter, G.; Bajgrowicz, J.A.; Kraft, P. Fragrance chemistry. Tetrahedron 1998, 54, 7633–7703. [Google Scholar] [CrossRef]
- Takahiro, I.; Tsuyoshi, K.; Kazuhiko, T.; Fumio, M. Volatile components of Lily of the Valley (Convallaria majalis)-CHIRAROMA analysis. Koryo, Terupen oyobi Seiyu Kagaku ni kansuru Toronkai Koen Yoshishu 2006, 50, 79–81. [Google Scholar]
- Brunke, E.J.; Ritter, F.; Schmaus, G. New data on trace components with sensory relevance in flower scents. Dragoco Rep. 1996, 1, 5–21. [Google Scholar]
- Forsyth, S.A.; Gunaratne, H.Q.N.; Hardacre, C.; McKeown, A.; Rooney, D.W.; Seddon, K.R. Utilisation of ionic liquid solvents for the synthesis of Lily-of-the-Valley fragrance {β-Lilial®; 3-(4-t-butylphenyl)-2-methylpropanal}. J. Mol. Cat. A Chem. 2005, 231, 61–66. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Ruth, S.M.; De Visser, R. Provenancing Flower Bulbs by Analytical Fingerprinting: Convallaria Majalis. Agriculture 2015, 5, 17-29. https://doi.org/10.3390/agriculture5010017
Van Ruth SM, De Visser R. Provenancing Flower Bulbs by Analytical Fingerprinting: Convallaria Majalis. Agriculture. 2015; 5(1):17-29. https://doi.org/10.3390/agriculture5010017
Chicago/Turabian StyleVan Ruth, Saskia M., and Ries De Visser. 2015. "Provenancing Flower Bulbs by Analytical Fingerprinting: Convallaria Majalis" Agriculture 5, no. 1: 17-29. https://doi.org/10.3390/agriculture5010017
APA StyleVan Ruth, S. M., & De Visser, R. (2015). Provenancing Flower Bulbs by Analytical Fingerprinting: Convallaria Majalis. Agriculture, 5(1), 17-29. https://doi.org/10.3390/agriculture5010017





