Use of Biofungicides for Controlling Plant Diseases to Improve Food Availability
Abstract
:1. Introduction
2. Results and Discussion
| Year | Formulation | AUncMOPC | AUncPC | AUcfuPC |
|---|---|---|---|---|
| 2004 | FOR7 | 149,902 b | 3.59 × 106 a | 43,873 a |
| FOR8 | 70,801 c | 1.55 × 107 b | 168,521 b | |
| cyproconazole | 241,333 b | - | - | |
| control | 837,524 a | - | - | |
| 2005 | FOR7 | 479,941 b | 1.37 × 107 b | 152,798 b |
| FOR8 | 49,326 c | 1.39 × 107 b | 83,866 a | |
| tebuconazole | 408,852 b | - | - | |
| control | 565,004 a | - | - | |
| MSE | 1.96 × 1011 | 1.80 × 1013 | 4.19 × 109 |
| Year | Formulation | AUncMOPC | AUncPC | AUcfuPC |
|---|---|---|---|---|
| 2004 | FOR7 | 2,663,570 a | 4.29 × 106 a | 33,753 a |
| FOR8 | 621,875 b | 2.54 × 107 b | 644,628 c | |
| cyproconazole | 2,559,080 a | - | - | |
| control | 3,121,590 a | - | - | |
| 2005 | FOR7 | 356,349 b | 8.27 × 106 a | 43,995 a |
| FOR8 | 59,868 c | 1.92 × 107 b | 138,062 b | |
| tebuconazole | 308,256 b | - | - | |
| control | 419,585 b | - | - | |
| MSE | 1.56 × 1014 | 1.71 × 1014 | 1.31 × 1010 |
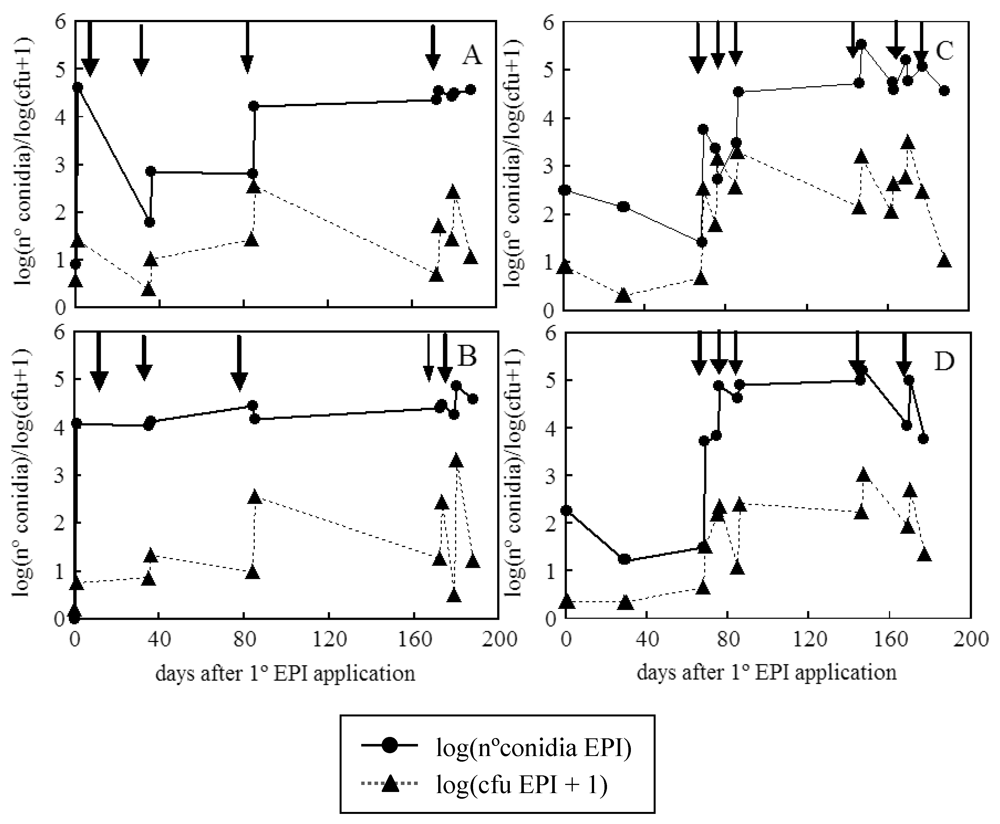
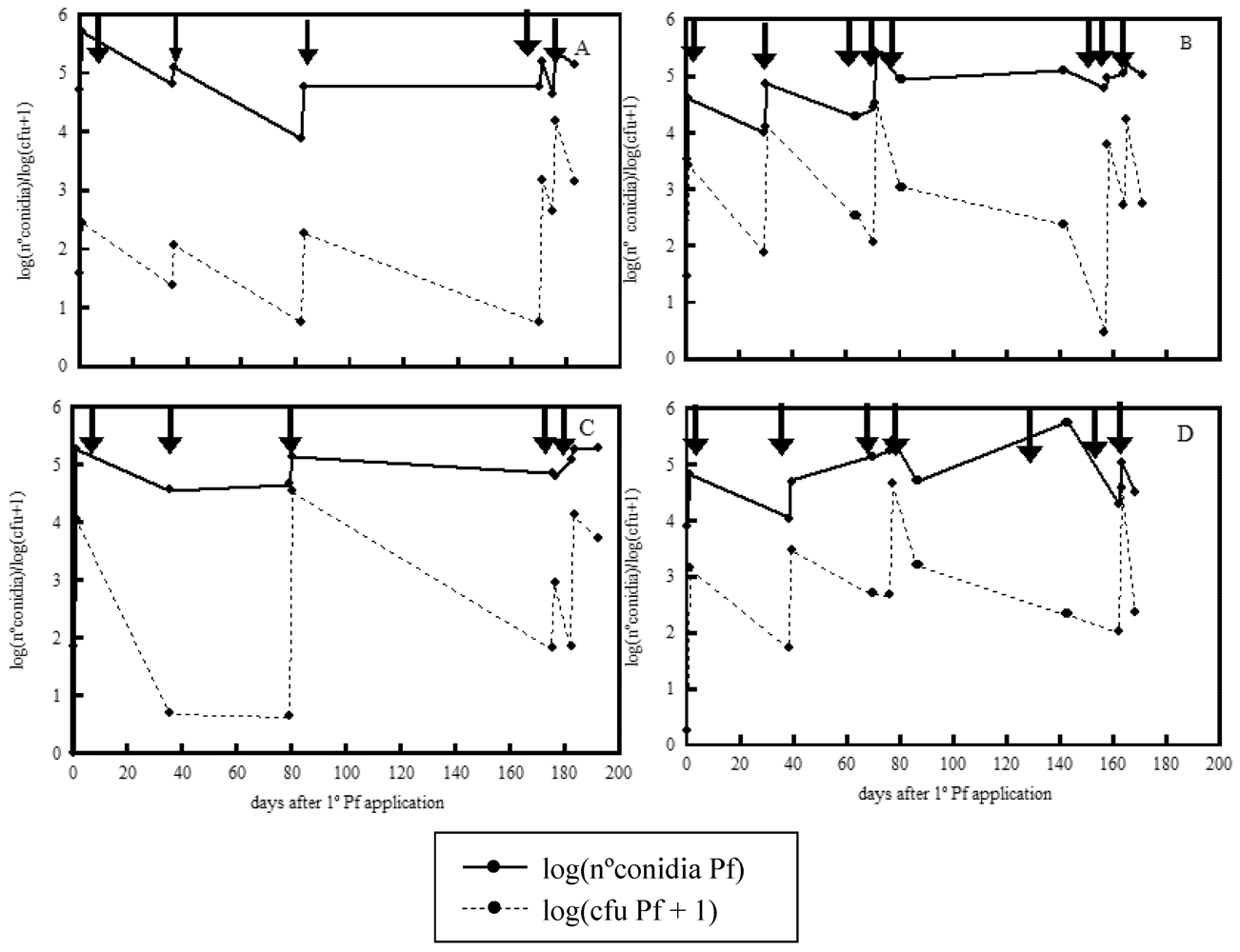
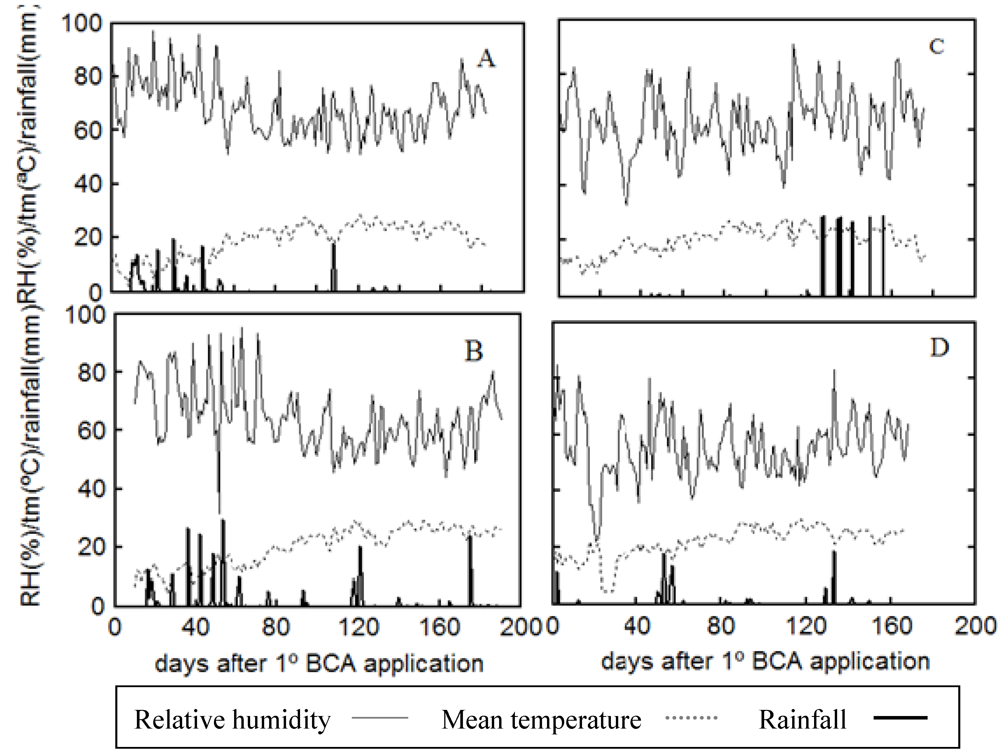
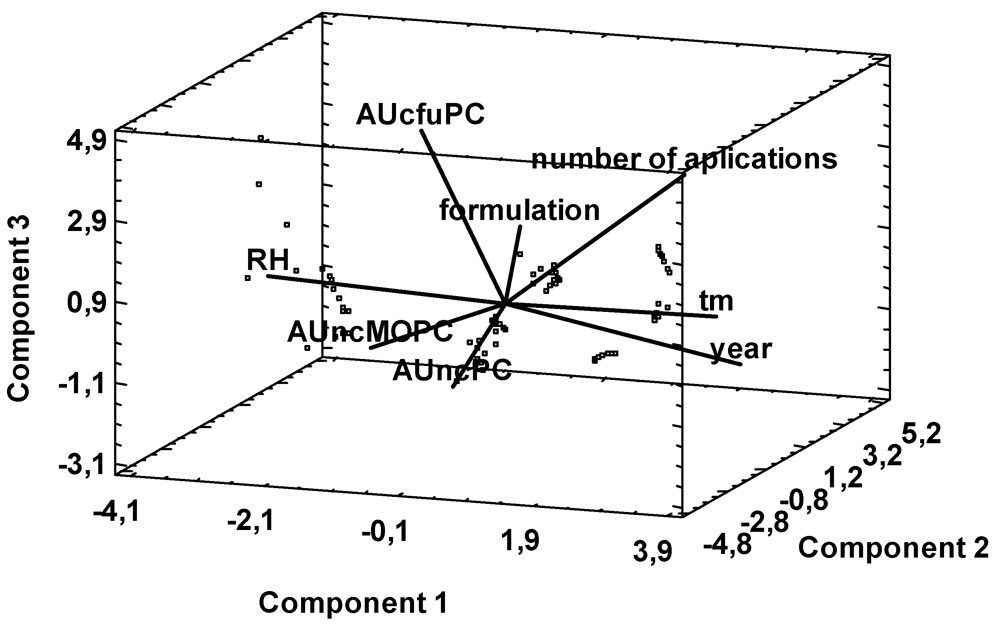
3. Experimental Section
3.1. Cultures and Formulations
3.2. Treatments and Experimental Design
3.3. Population Dynamics of E. nigrum and P. frequentans
3.4. Biocontrol Efficacy of E. nigrum and P. frequentans Conidial Formulations
3.5. Data Analysis
4. Conclusions
Acknowledgments
References
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant diseases: The European situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Nicot, P.C.; Decognet, V.; Fruit, L.; Bardin, M.; Trottin, Y. Combined effect of microclimate and dose of application on the efficacy of biocontrol agents for the protection of pruning wounds on tomatoes against Botrytis cinerea. Bulletin IOBC/SROP 2002, 25, 73–76. [Google Scholar]
- Byrde, R.J.; Willetts, H.J. The Brown Rot Fungi of Fruit. Their Biology and Control., 1st ed; Pergamon Press: Oxford, UK, 1977; p. 171. [Google Scholar]
- Ahmadi, H.; Biasi, W.V.; Mitcham, E.J. Control of brown rot decay of nectarines with 15% carbon dioxide atmosphere. J. Am. Soc. Hort. Sci. 1999, 124, 708–712. [Google Scholar]
- Sommer, N.F. Role of controlled environments in suppression of post-harvest diseases. Can. J. Plant Pathol. 1985, 7, 331–339. [Google Scholar]
- Osorio, J.M.; Adaskaveg, J.E.; Ogawa, J.W. Comparative efficacy and systemic activity of iprodione and the experimental anilide E-0858 for control of brown rot on peach fruit. Plant Dis. 1993, 77, 1140–1143. [Google Scholar] [CrossRef]
- Hong, C.X.; Michailides, T.J.; Holtz, B.A. Effects of wounding, inoculum density, and biological control agents on postharvest brown rot of stone fruit. Plant Dis. 1998, 82, 1210–1216. [Google Scholar] [CrossRef]
- Ogawa, J.M.; Zehr, E.I.; Biggs, A.R. Brown rot. In Compendium of Stone Fruit Diseases; Ogawa, J.M., Zehr, E.I., Bird, G.W., Ritchie, D.F., Uriu, K., Uyemoto, J.K., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 7–10. [Google Scholar]
- Hong, C.X.; Holtz, B.A.; Morgan, D.P.; Michailides, T.J. Significance of thinned fruit as a source of the secondary inoculum of Monilinia fructicola in California nectarine orchards. Plant Dis. 1997, 81, 519–524. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; Usall, J.; Segarra, J.; Lamarca, N.; De Cal, A. Secondary inoculum dynamics of Monilinia spp. and relationship to the incidence of postharvest brown rot in peaches and the weather conditions during the growing season. Eur. J. Plant Pathol. 2012. [Google Scholar] [CrossRef]
- Xu, X.-M.; Bertone, C.; Berrie, A. Effects of wounding, fruit age and wetness duration on the development of cherry brown rot in the UK. Plant Pathol. 2007, 56, 114–119. [Google Scholar]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008, 121, 487–498. [Google Scholar] [CrossRef]
- Villarino, M.; Sandin-España, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peach reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agr. Food Chem. 2011, 59, 3205–3213. [Google Scholar]
- Penrose, L.J.; Davis, K.C.; Koffmann, W. The distribution of benomyl-tolerant Slerotinia fructicola (Wint.) Rehm in stone fruit orchards in New South Wales and comparative studies with susceptible isolates. Aust. J. Agric. Res. 1979, 30, 307–319. [Google Scholar] [CrossRef]
- Penrose, L.J. Prolonged field persistence of resistance to benomyl in Monilinia fructicola. Crop Prot. 1990, 9, 190–192. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Gaunt, R.E. Effect of frequency of dicarboximide applications on resistant populations of Monilinia fructicola and brown rot in New Zealand orchards. Crop Prot. 1993, 12, 83–88. [Google Scholar] [CrossRef]
- Falconi, C.J.; Mendgen, K. Epiphytic fungi on apple leaves and their value for control of the postharvest pathogens Botrytis cinerea, Monilinia fructigena and Penicillium expansum. J. Plant Dis. Prot. 1994, 101, 38–47. [Google Scholar]
- Sanoamuang, N.; Gaunt, R.E. Persistence and fitness of carbendazim- and dicarboximide-resistant isolates of Monilinia fructicola (Wint.) Honey in flowers, shoots and fruit of stone fruit. Plant Pathol. 1995, 44, 448–457. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H.; Gubler, W.D.; Teviotdale, B.L.; Thompson, D.F. Reduced risk fungicides: A new way of managing brown rot and other fungal diseases of stone fruit crops. Cal. Agric. 2004, 59, 109–114. [Google Scholar]
- Malavolta, C.; Cross, J.V.; Cravedi, P.; Jörg, E. Guidelines for Integrated Production of Stone Fruits. In Technical Guideline III, IOBC wprs Bulletin; International Organization for Biological and integrated Control of Noxious Animals and Plants, West Palaearctic Regional Section: Dijon, France, 2003; Volume 26. [Google Scholar]
- Melgarejo, P.; Carrillo, R.; Sagasta, E.M. Mycoflora of peach twigs and flowers and its possible significance in biological control of Monilinia laxa. Trans. Br. Mycol. Soc. 1985, 85, 313–317. [Google Scholar] [CrossRef]
- Melgarejo, P.; Carrillo, R.; Sagasta, E.M. Potential for biological control of Monilinia laxa in peach twigs. Crop Prot. 1986, 5, 422–426. [Google Scholar] [CrossRef]
- De Cal, A.; Sagasta, E.M.; Melgarejo, P. Biological control of peach twig blight (Monilinia laxa) with Penicillium frequentans. Plant Pathol. 1990, 39, 612–618. [Google Scholar] [CrossRef]
- Madrigal, C.; Pascual, S.; Melgarejo, P. Biological control of peach twig blight (Monilinia laxa) with Epicoccum nigrum. Plant Pathol. 1994, 43, 554–561. [Google Scholar] [CrossRef]
- Guijarro, B.; Larena, I.; Melgarejo, P.; De Cal, A. Effect of drying on conidial viability of Penicillium frequentans, a biological control agent against peach brown rot disease caused by Monilinia spp. Biocontr. Sci. Technol. 2006, 16, 257–269. [Google Scholar] [CrossRef]
- Guijarro, B.; Melgarejo, P.; De Cal, A. Effect of stabilizers on the shelf-life of Penicillium frequentans conidia and their efficacy as a biological agent against peach brown rot. Int. J. Food Microbiol. 2007, 113, 117–124. [Google Scholar] [CrossRef]
- Larena, I.; De Cal, A.; Melgarejo, P. Effects of stabilizers on shelf-life of Epicoccum nigrum formulations and their relationship with biocontrol of postharvest brown rot by Monilinia of peaches. J. Appl. Microbiol. 2007, 102, 570–582. [Google Scholar]
- De Cal, A.; Sagasta, E.M.; Melgarejo, P. Antifungal substances produced by Penicillium frequentans and their relationship to the biocontrol of Monilinia laxa. Phytopathol. 1988, 78, 888–893. [Google Scholar] [CrossRef]
- De Cal, A.; Melgarejo, P. Interactions of and mycoflora of peach twigs. Mycol. Res. 1992, 96, 1105–1113. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Jeffers, S.N. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in cold storage. Crop Prot. 1997, 16, 629–633. [Google Scholar] [CrossRef]
- Guijarro, B.; Melgarejo, P.; De Cal, A. Effects of different biological formulations of Penicillium frequentans on brown rot of peaches. Biol. Control 2007, 42, 86–96. [Google Scholar] [CrossRef]
- Burges, H.D.; Jones, K.A. Trends in formulation of microorganisms and future research requirements. In Formulation of Microbial Biopesticides, 1st; Burges, H.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 311–332. [Google Scholar]
- Collins, D.P.; Jacobsen, B.J.; Maxwell, B. Spatial and temporal population dynamics of a phyllosphere colonizing Bacillus subtilis biological control agent of sugar beet Cercospora leaf spot. Biol. Control 2003, 26, 224–232. [Google Scholar] [CrossRef]
- Jones, E.E.; Mead, A.; Whipps, J.M. Effect of inoculum type and timing of application of Coniothyrium minitans on Sclerotinia sclerotiorum: Control of sclerotinia disease in glasshouse lettuce. Plant Pathol. 2004, 53, 611–620. [Google Scholar] [CrossRef]
- Jones, E.E.; Clarkson, J.P.; Mead, A.; Whipps, J.M. Effect of inoculum type and timing of application of Coniothyrium minitans on Sclerotinia sclerotiorum: Influence on apothecial production. Plant Pathol. 2004, 53, 621–628. [Google Scholar] [CrossRef]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Conidial density of Monilinia app. on peach fruit surfaces in relation to the incidences of latent infections and brown rot. Eur. J. Plant Pathol. 2009, 123, 415–424. [Google Scholar] [CrossRef]
- Larena, I.; Torres, R.; De Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandrin, J.F.; Lichou, J.; Ochoa de Eribe, X.; Usall, J. Biological control of post-harvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol. Control 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Kurtzman, C.; Droby, S. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 2001, 24, 395–399. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Dennis-Arue, R.; Bosch, J.R.; Gonzales, A.R.; Henson, D.; Janisiewicz, W.J. Control postharvest rot of nectarines and peaches by Pseudomonas species. Crop Prot. 1993, 12, 513–520. [Google Scholar] [CrossRef]
- Kinkel, L.L. Microbial population dynamics on leaves. Annu. Rev. Phytopathol. 1997, 35, 327–347. [Google Scholar] [CrossRef]
- Cartwright, K.D.; Benson, D.M. Optimization of biological control of Rhizoctonia stem rot on poinsettia by Paecilomyces lilacinus and Pseudomonas cepacia. Plant Dis. 1995, 79, 301–308. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 1st ed; Academic Press: New York, NY, USA, 1980; p. 1264. [Google Scholar]
- Teixidó, N.; Viñas, I.; Usall, J.; Magan, N. Control of blue mold of apples by preharvest application of Candida sake grown in media with different water activity. Phytopathol. 1998, 88, 960–964. [Google Scholar] [CrossRef]
- Shaw, D.A.; Adaskaveg, J.E.; Ogawa, J.M. Influence of wetness period and temperature on infection and development of shot-hole disease of almond caused by Wilsonomyces carpophilus. Phytopathol. 1990, 80, 749–756. [Google Scholar] [CrossRef]
- De Cal, A.; Larena, I.; Liñán, M.; Torres, R.; Lamarca, N.; Usall, J.; Domenichini, P.; Bellini, A.; Eribe, X.O.; Melgarejo, P. Population dynamics of Epicoccum nigrum, a biocontrol agent against brown rot in stone fruit. J. Appl. Microbiol. 2009, 106, 592–605. [Google Scholar] [CrossRef]
- Guijarro, B.; Melgarejo, P.; Torres, R.; Lamarca, N.; Usall, J.; De Cal, A. Penicillium frequentans population dynamic on peach surfaces after its application against brown rot in orchards. J. Appl. Microbiol. 2008, 104, 659–671. [Google Scholar] [CrossRef]
- Andrews, J.H. Biological control in the phyllosphere. Annu. Rev. Phytopathol. 1992, 30, 603–635. [Google Scholar] [CrossRef]
- De Cal, A.; Larena, I.; Guijarro, B.; Melgarejo, P. Mass production of conidia of Penicillium frequentans, a biocontrol agent against brown rot of stone fruits. Biocontrol Sc. Technol. 2002, 12, 715–725. [Google Scholar] [CrossRef]
- Larena, I.; De Cal, A.; Melgarejo, P. Solid substrate production of Epicoccum nigrum conidia for biological control of brown rot on stone fruits. Int. J. Food Microbiol. 2004, 94, 161–167. [Google Scholar] [CrossRef]
- Larena, I.; De Cal, A.; Liñán, M.; Melgarejo, P. Drying of Epicoccum nigrum conidia for obtaining a shelf-stable biological product against brown rot disease. J. Appl. Microbiol. 2003, 94, 508–514. [Google Scholar] [CrossRef]
- Lacey, J.; Hill, S.T.; Edwards, M.A. Microorganisms in stored grains, their enumeration and significance. Trop. Stored Prod. Inf. 1980, 39, 19–33. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology, 1st ed; Wiley-Interscience: New York, NY, USA, 1990; p. 523. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Madden, L.; Pennypacker, S.P. Principal Component analysis of tomato early blight epidemics. Phytopathol. Z. J. Phytopathol. 1979, 95, 364–369. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Cal, A.; Larena, I.; Guijarro, B.; Melgarejo, P. Use of Biofungicides for Controlling Plant Diseases to Improve Food Availability. Agriculture 2012, 2, 109-124. https://doi.org/10.3390/agriculture2020109
De Cal A, Larena I, Guijarro B, Melgarejo P. Use of Biofungicides for Controlling Plant Diseases to Improve Food Availability. Agriculture. 2012; 2(2):109-124. https://doi.org/10.3390/agriculture2020109
Chicago/Turabian StyleDe Cal, Antonieta, Inmaculada Larena, Belén Guijarro, and Paloma Melgarejo. 2012. "Use of Biofungicides for Controlling Plant Diseases to Improve Food Availability" Agriculture 2, no. 2: 109-124. https://doi.org/10.3390/agriculture2020109
APA StyleDe Cal, A., Larena, I., Guijarro, B., & Melgarejo, P. (2012). Use of Biofungicides for Controlling Plant Diseases to Improve Food Availability. Agriculture, 2(2), 109-124. https://doi.org/10.3390/agriculture2020109







