Removal of Neonicotinoid Residues from Beeswax Using an Eco-Friendly Oxalic Acid Treatment: A Sustainable Solution for Apicultural Decontamination
Abstract
1. Introduction
2. Methodology
2.1. Materials and Chemicals
2.2. Beeswax Sample
2.3. Standard Solutions
2.4. LC-MS System
2.5. Sample Preparation and Analysis
2.6. Cleaning Methods (New and Traditional)
2.7. Determination of Neonicotinoid Removal After Cleaning
2.8. Analysis of the Beeswax Coloration After Cleaning (Bleaching)
2.9. Evaluation of Purified Beeswax in Experimental Hives
3. Results and Discussion
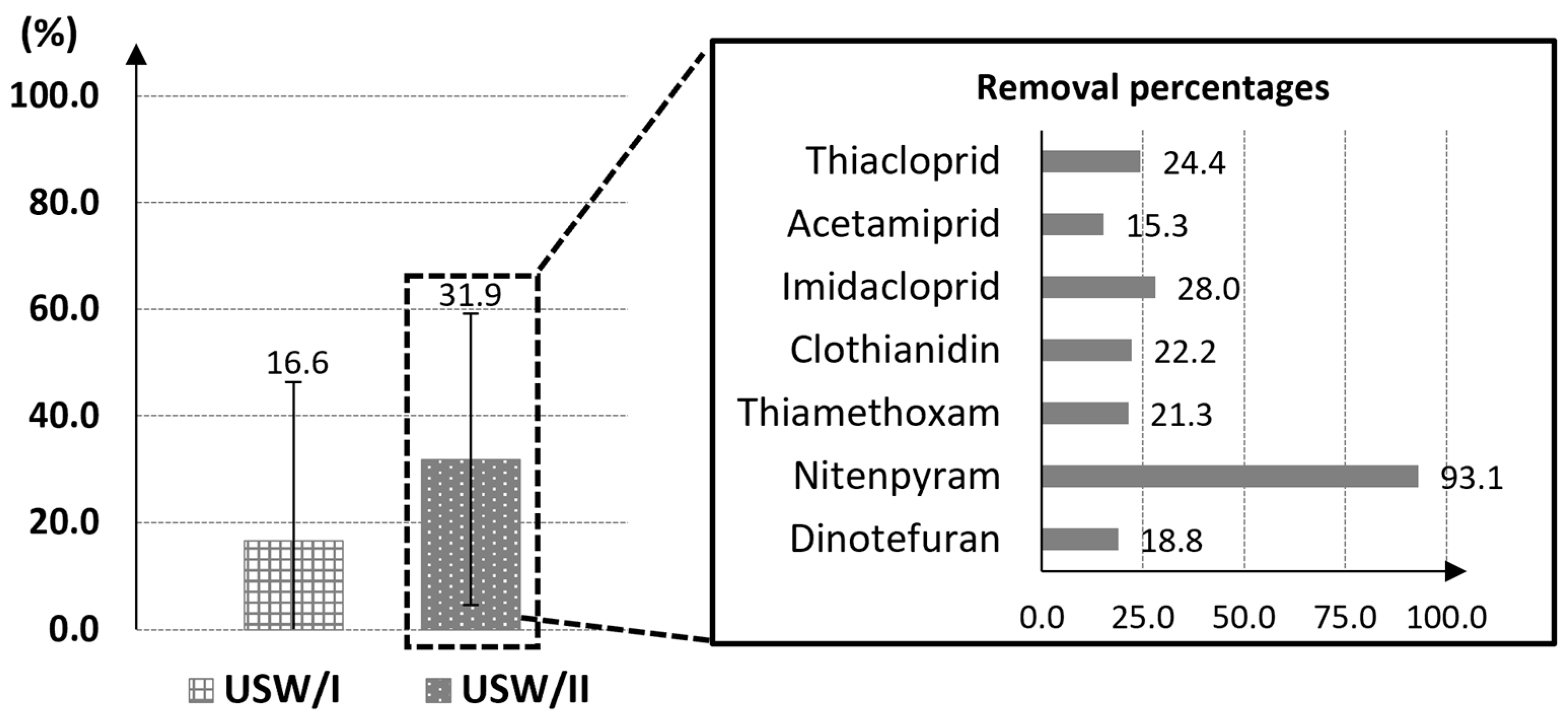
3.1. Determination of Contaminants in Cleaned Beeswax with the New Proposed Methods
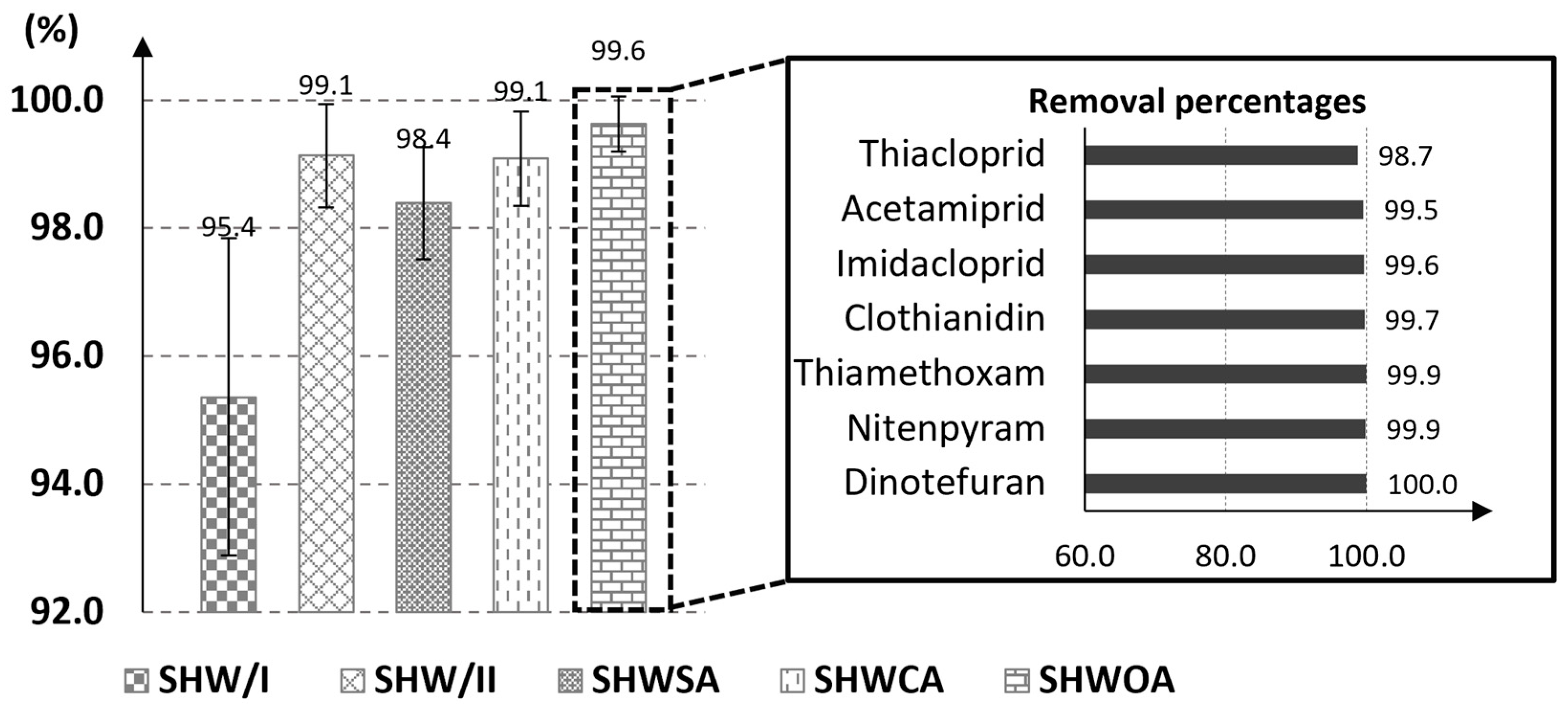
3.2. Determination of Contaminants in Clean Beeswax Using Improved Traditional Methods
3.3. Analysis of Cleaned Beeswax Coloration
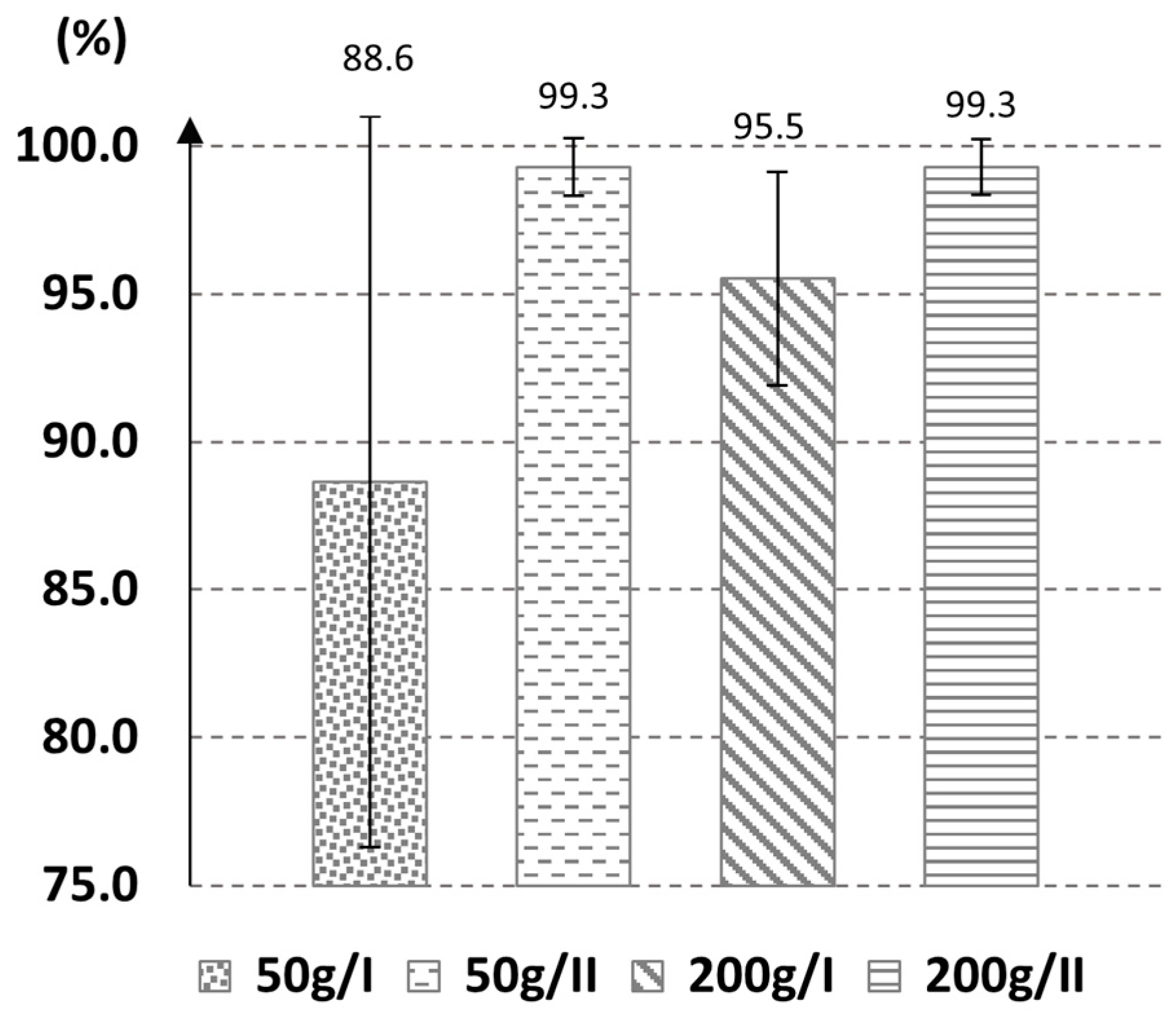
3.4. Evaluation of the Pesticide Removal Efficacy of the Best Method in Larger Quantities of Wax
3.5. Qualitative Evaluation of the Acceptance Level of Wax Cleaned by Bees in Hives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Insolia, L.; Molinari, R.; Rogers, S.R.; Williams, G.R.; Chiaromonte, F.; Calovi, M. Honey bee colony loss linked to parasites, pesticides and extreme weather across the United States. Sci. Rep. 2022, 12, 20787. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.G.; Brito, J.C.M.; Almeida, M.O.; Gomes, M.P.; Calaça, P. Unbounded Bees: A Systematic Review and Meta-analysis Investigating Pesticide Contamination in Brazilian Bees and Hive Products. J. Hazard. Mater. Adv. 2025, 17, 100632. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E. Anthropogenic influences on bee foraging. Science 2022, 375, 970–972. [Google Scholar] [CrossRef]
- Mu, G.; Yan, S.; Pan, F.; Xu, H.; Jing, X.; Xue, X. Based on theoretical design simultaneous analysis of multiple neonicotinoid pesticides in beeswax by deep eutectic solvents extraction combined with UHPLC-MS/MS. Food Chem. X 2025, 25, 102073. [Google Scholar] [CrossRef]
- Bass, C.; Hayward, A.; Troczka, B.J.; Haas, J.; Nauen, R. The molecular determinants of pesticide sensitivity in bee pollinators. Sci. Total Environ. 2024, 915, 170174. [Google Scholar] [CrossRef]
- Rhodes, C.J. Pollinator decline—An ecological calamity in the making? Sci. Prog. 2018, 101, 121–160. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Chen, Z.; Mao, D.; Luo, Y. Spatial distribution of two acaricides and five neonicotinoids in beehives and surrounding environments in China. J. Hazard. Mater. 2024, 469, 133892. [Google Scholar] [CrossRef]
- Sánchez--Bayo, F.; Wyckhuys, K.A. Further evidence for a global decline of the entomofauna. Austral Entomol. 2021, 60, 9–26. [Google Scholar] [CrossRef]
- Balayiannis, G.; Balayiannis, P. Bee honey as an environmental bioindicator of pesticides’ occurrence in six agricultural areas of Greece. Arch. Environ. Contam. Toxicol. 2008, 55, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A. The honey bee Apis mellifera: An insect at the interface between human and ecosystem health. Biology 2022, 11, 233. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Sabo, R.; Kasiotis, K.M.; Machera, K.; Sabová, L.; Majchrák, T. Adult honeybees and beeswax as indicators of trace elements pollution in a vulnerable environment: Distribution among different apicultural compartments. Molecules 2022, 27, 6629. [Google Scholar] [CrossRef]
- Bogdanov, S. Beeswax: Production, Properties Composition and Control. In Beeswax Book; Bee Product Science: Muehlethurnen, Switzerland, 2016; pp. 2–10. [Google Scholar]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Swiatly-Blaszkiewicz, A.; Klupczynska-Gabryszak, A.; Matuszewska-Mach, E.; Matysiak, J.; Attard, E.; Kowalczyk, D.; Adamkiewicz, A.; Kupcewicz, B.; Matysiak, J. Pesticides in Honeybee Products—Determination of Pesticides in Bee Pollen, Propolis, and Royal Jelly from Polish Apiary. Molecules 2025, 30, 275. [Google Scholar] [CrossRef]
- Tremolada, P.; Bernardinelli, I.; Colombo, M.; Spreafico, M.; Vighi, M. Coumaphos distribution in the hive ecosystem: Case study for modeling applications. Ecotoxicology 2004, 13, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Flores, J.M.; Miguel, E.; Fernández-Alba, A.R.; Hernando, M.D. Coumaphos residue transfer to honey bee brood (Apis mellifera) in realistic scenarios. Res. Vet. Sci. 2023, 159, 106–124. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, X.; Wang, X.; Shen, J.; Ding, S. Determination of neonicotinoid insecticides residues in bovine tissues by pressurized solvent extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2011, 879, 117–122. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martinez, M.-A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef]
- Kamel, A. Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bees and bee products by liquid chromatography− tandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 2010, 58, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- Vallianatos, E. Glyphosate and Neonicotinoids are Poisoning Honeybees (and the World). CounterPunch 2024. [Google Scholar]
- Sawicka, B.; Zahnit, W.; Barbaś, P.; Messaoudi, M.; Osmani, N.; Rebiai, A.; Hemmami, H.; Pszczółkowski, P.; Noaema, A.H. Impact of Neonicotinoids on the Bees’ Population. In Neonicotinoids in the Environment: Emerging Concerns to the Human Health and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2024; pp. 45–61. [Google Scholar]
- Jovanov, P.; Guzsvany, V.; Lazić, S.; Franko, M.; Sakač, M.; Šarić, L.; Kos, J. Development of HPLC-DAD method for determination of neonicotinoids in honey. J. Food Compos. Anal. 2015, 40, 106–113. [Google Scholar] [CrossRef]
- Bridi, R.; Larena, A.; Pizarro, P.N.; Giordano, A.; Montenegro, G. LC-MS/MS analysis of neonicotinoid insecticides: Residue findings in chilean honeys. Ciência e Agrotecnol. 2018, 42, 51–57. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, K.P.; Martín, M.T.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Trace analysis of seven neonicotinoid insecticides in bee pollen by solid–liquid extraction and liquid chromatography coupled to electrospray ionization mass spectrometry. Food Anal. Methods 2014, 7, 490–499. [Google Scholar] [CrossRef]
- Ponce-Vejar, G.; Ramos de Robles, S.L.; Macias-Macias, J.O.; Petukhova, T.; Guzman-Novoa, E. Detection and concentration of neonicotinoids and other pesticides in honey from honey bee colonies located in regions that differ in agricultural practices: Implications for human and bee health. Int. J. Environ. Res. Public Health 2022, 19, 8199. [Google Scholar] [CrossRef]
- El Agrebi, N.; Traynor, K.; Wilmart, O.; Tosi, S.; Leinartz, L.; Danneels, E.; de Graaf, D.C.; Saegerman, C. Pesticide and veterinary drug residues in Belgian beeswax: Occurrence, toxicity, and risk to honey bees. Sci. Total Environ. 2020, 745, 141036. [Google Scholar] [CrossRef] [PubMed]
- López, S.H.; Lozano, A.; Sosa, A.; Hernando, M.D.; Fernández-Alba, A.R. Screening of pesticide residues in honeybee wax comb by LC-ESI-MS/MS. A pilot study. Chemosphere 2016, 163, 44–53. [Google Scholar] [CrossRef]
- Valverde, S.; Ares, A.M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Fast determination of neonicotinoid insecticides in beeswax by ultra-high performance liquid chromatography-tandem mass spectrometry using an enhanced matrix removal-lipid sorbent for clean-up. Microchem. J. 2018, 142, 70–77. [Google Scholar] [CrossRef]
- Yáñez, K.P.; Bernal, J.L.; Nozal, M.J.; Martín, M.T.; Bernal, J. Determination of seven neonicotinoid insecticides in beeswax by liquid chromatography coupled to electrospray-mass spectrometry using a fused-core column. J. Chromatogr. A 2013, 1285, 110–117. [Google Scholar] [CrossRef]
- Bischoff, K.; Baert, N.; McArt, S. Pesticide contamination of beeswax from managed honey bee colonies in New York State. J. Vet. Diagn. Investig. 2023, 35, 617–624. [Google Scholar] [CrossRef]
- El Agrebi, N.; De Smet, L.; Douny, C.; Scippo, M.-L.; Svečnjak, L.; de Graaf, D.C.; Saegerman, C. A field realistic model to assess the effects of pesticides residues and adulterants on honey bee gene expression. PLoS ONE 2024, 19, e0302183. [Google Scholar] [CrossRef]
- El Agrebi, N.; Wilmart, O.; Urbain, B.; Danneels, E.L.; de Graaf, D.C.; Saegerman, C. Belgian case study on flumethrin residues in beeswax: Possible impact on honeybee and prediction of the maximum daily intake for consumers. Sci. Total Environ. 2019, 687, 712–719. [Google Scholar] [CrossRef]
- Girolami, V.; Marzaro, M.; Vivan, L.; Mazzon, L.; Greatti, M.; Giorio, C.; Marton, D.; Tapparo, A. Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J. Appl. Entomol. 2012, 136, 17–26. [Google Scholar] [CrossRef]
- Laurino, D.; Porporato, M.; Patetta, A.; Manino, A. Toxicity of neonicotinoid insecticides to honey bees: Laboratory tests. Bull. Insectology 2011, 64, 107–113. [Google Scholar]
- Maxim, L.; Van der Sluijs, J.P. Uncertainty: Cause or effect of stakeholders’ debates?: Analysis of a case study: The risk for honeybees of the insecticide Gaucho®. Sci. Total Environ. 2007, 376, 1–17. [Google Scholar] [CrossRef]
- Marzaro, M.; Vivan, L.; Targa, A.; Mazzon, L.; Mori, N.; Greatti, M.; Toffolo, E.P.; Di Bernardo, A.; Giorio, C.; Marton, D. Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bull. Insectology 2011, 64, 119–126. [Google Scholar]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of Varroa destructor and sublethal clothianidin doses during the larval stage on subsequent adult honey bee (Apis mellifera L.) health, cellular immunity, deformed wing virus levels and differential gene expression. Microorganisms 2020, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delègue, M.-H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Paoli, M.; Giurfa, M. Pesticides and pollinator brain: How do neonicotinoids affect the central nervous system of bees? Eur. J. Neurosci. 2024, 60, 5927–5948. [Google Scholar] [CrossRef] [PubMed]
- Medrzycki, P.; Montanari, R.; Bortolotti, L.; Sabatini, A.G.; Maini, S.; Porrini, C. Effects of imidacloprid administered in sub-lethal doses on honey bee behaviour. Laboratory tests. Bull. Insectology 2003, 56, 59–62. [Google Scholar]
- Crall, J.D.; Raine, N.E. How do neonicotinoids affect social bees? Linking proximate mechanisms to ecological impacts. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 64, pp. 191–253. [Google Scholar]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delègue, M.-H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef]
- Wintermantel, D.; Odoux, J.-F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-induced mortality risk for bees foraging on oilseed rape nectar persists despite EU moratorium. Sci. Total Environ. 2020, 704, 135400. [Google Scholar] [CrossRef]
- Siefert, P.; Hota, R.; Ramesh, V.; Grünewald, B. Chronic within-hive video recordings detect altered nursing behaviour and retarded larval development of neonicotinoid treated honey bees. Sci. Rep. 2020, 10, 8727. [Google Scholar] [CrossRef]
- Mommaerts, V.; Reynders, S.; Boulet, J.; Besard, L.; Sterk, G.; Smagghe, G. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 2010, 19, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bison, L.; Roffet-Salque, M.; Botto, M.; Madrigali, E.; Salis, G.; Cramp, L.J. Direct evidence of the use of beehive products in pre-roman Sardinia. Archaeometry 2023, 65, 354–369. [Google Scholar] [CrossRef]
- Fasasi, K.A. Aspects of the Biology of Apis Mellifera Adansonii (1758) (Hymenoptera: Apidae: Apoidea) with Emphasis on Honey and Beeswax Production. Ph.D Thesis, University of Lagos, Lagos, Nigeria, 2008. [Google Scholar]
- Fuente-Ballesteros, A.; Nozal, M.J.; Ares, A.M.; Bernal, J. Evaluation of the potential migration of acaricides from stamped beeswax to honey simulating beehive conditions: A pilot study. J. Food Compos. Anal. 2023, 123, 105542. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; VanEngelsdorp, D.; Picó, Y. Beeswax cleaning by solvent extraction of pesticides. MethodsX 2019, 6, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Alonso, R.; Cutillas, V.M.; Ferrer, C.M.; Gómez-Ramos, M.J.; Hernando, D.; Valverde, A.; Flores, J.M.; Fernández-Alba, A.R.; Fernández-Alba, A.R. Removal of pesticide residues from beeswax using a methanol extraction-based procedure: A pilot-scale study. Environ. Technol. Innov. 2021, 23, 101606. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Orantes-Bermejo, F.J. Discoloration and adsorption of acaricides from beeswax. J. Food Process Eng. 2017, 40, e12344. [Google Scholar] [CrossRef]
- Bogdanov, S. Beeswax: Uses and trade. In The Beeswax Book; Bee Product Science Publishing: Muehlethurnen, Switzerland, 2009; pp. 1–16. [Google Scholar]
- Carter, E.C. Encyclopedia of Color Science and Technology; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Griber, Y.A.; Samoilova, T.; Al-Rasheed, A.S.; Bogushevskaya, V.; Cordero-Jahr, E.; Delov, A.; Gouaich, Y.; Manteith, J.; Mefoh, P.; Odetti, J.V. “Playing” with Color: How Similar Is the “Geometry” of Color Harmony in the CIELAB Color Space across Countries? Arts 2024, 13, 53. [Google Scholar] [CrossRef]
- Liu, X.; Timar, M.C.; Varodi, A.M.; Nedelcu, R.; Torcătoru, M.-J. Colour and surface chemistry changes of wood surfaces coated with two types of waxes after seven years exposure to natural light in indoor conditions. Coatings 2022, 12, 1689. [Google Scholar] [CrossRef]
- Arredondo-Ochoa, T.; García-Almendárez, B.E.; Escamilla-García, M.; Martín-Belloso, O.; Rossi-Márquez, G.; Medina-Torres, L.; Regalado-González, C. Physicochemical and antimicrobial characterization of beeswax–starch food-grade nanoemulsions incorporating natural antimicrobials. Int. J. Mol. Sci. 2017, 18, 2712. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Sothornvit, R. Reinforcement of banana flour biocomposite film with beeswax and montmorillonite and effects on water barrier and physical properties. Int. J. Food Sci. Technol. 2018, 53, 2642–2649. [Google Scholar] [CrossRef]
- Pereira, R.; Silveira, J.; Dias, S.; Cardoso, A.; Mata, A.; Marques, D. Bleaching efficacy and quality of life of different bleaching techniques—Randomized controlled trial. Clin. Oral Investig. 2022, 26, 7167–7177. [Google Scholar] [CrossRef]
- Flores, J.M.; Luna, A.; Rodríguez Fernández-Alba, A.; Hernando, M.D. Acceptance by Honey Bees of Wax Decontaminated through an Extraction Process with Methanol. Insects 2023, 14, 593. [Google Scholar] [CrossRef]
- Ledjanac, S.; Hoxha, F.; Jasnić, N.; Tasić, A.; Jovanović, M.; Blagojević, S.; Plavša, N.; Tosti, T. The Influence of the Chemical Composition of Beeswax Foundation Sheets on Their Acceptability by the Bee’s Colony. Molecules 2024, 29, 5489. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, Y.; Jat, B.L. Honeybee: Diversity, castes and life cycle. In Industrial Entomology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 5–34. [Google Scholar]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Arvidsson, R. On the use of ordinal scoring scales in social life cycle assessment. Int. J. Life Cycle Assess. 2019, 24, 604–606. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Hrynko, I.; Kaczyński, P.; Łuniewski, S.; Łozowicka, B. Removal of triazole and pyrethroid pesticides from wheat grain by water treatment and ultrasound-supported processes. Chemosphere 2023, 333, 138890. [Google Scholar] [CrossRef]
- Azam, S.R.; Ma, H.; Xu, B.; Devi, S.; Stanley, S.L.; Siddique, M.A.B.; Mujumdar, A.S.; Zhu, J. Multi-frequency multi-mode ultrasound treatment for removing pesticides from lettuce (Lactuca sativa L.) and effects on product quality. LWT 2021, 143, 111147. [Google Scholar] [CrossRef]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef] [PubMed]
- Cochennec, M.; Devriendt-Renault, Y.; Massat, F.; Guérin, T.; Ollivier, P.; Colombano, S.; Parinet, J. Microwave-enhanced thermal removal of organochlorine pesticide (chlordecone) from contaminated soils. Chemosphere 2024, 352, 141486. [Google Scholar] [CrossRef]
- Venturelli, A.; Brighenti, V.; Mascolo, D.; Pellati, F. A new strategy based on microwave-assisted technology for the extraction and purification of beeswax policosanols for pharmaceutical purposes and beyond. J. Pharm. Biomed. Anal. 2019, 172, 200–205. [Google Scholar] [CrossRef] [PubMed]
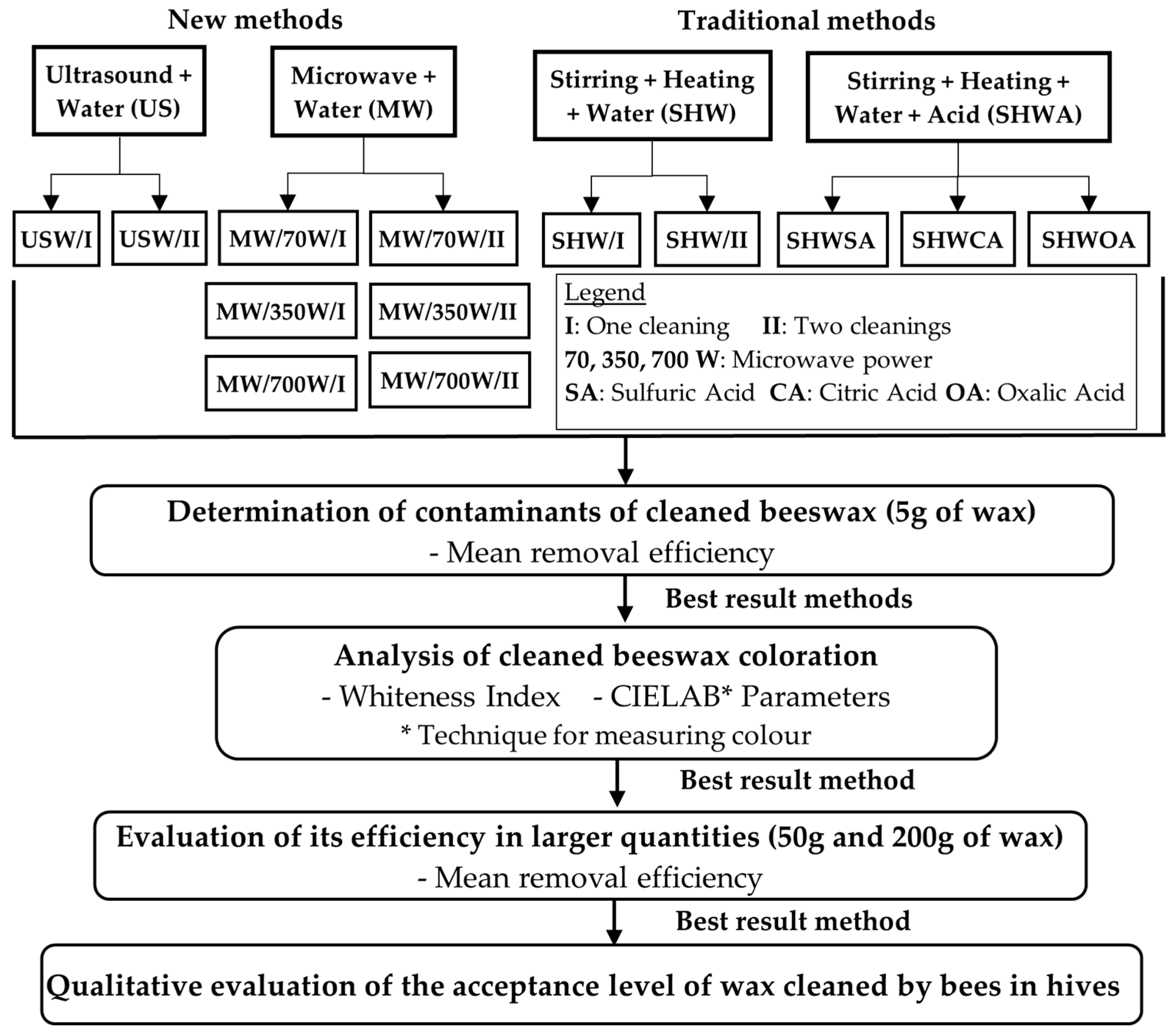


| New Methods | Solvent | Equipment | Temp. or Power | Time (min) | Cleanings | Acronyms |
|---|---|---|---|---|---|---|
| Ultrasound + Water | Water | Ultrasonic bath | 40 °C | 40 | I | USW/I |
| 80 | II | USW/II | ||||
| Microwave + Water | Water | Microwave oven | 70 W | 4 | I | MW70W/I |
| 350 W | 1 | I | MW350W/I | |||
| 700 W | 0.5 | I | MW700W/I | |||
| 70 W | 4 | II | MW70W/II | |||
| 350 W | 1 | II | MW350W/II | |||
| 700 W | 0.5 | II | MW700W/II | |||
| Traditional methods | Solvent | Equipment | Temp. or power | Time (min) | Cleanings | Acronyms |
| Stirring + Heating + Water | Water | Magnetic Stirrer (700 rpm) | 70 °C | 10 | I | SHW/I |
| II | SHW/II | |||||
| Stirring + Heating + Water + Acid | Water/Sulfuric acid (0.1% v/v) | I | SHWSA | |||
| Water/Citric acid (0.25% v/v) | SHWCA | |||||
| Water/Oxalic acid (0.25% v/v) | SHWOA |
| Activity | Day | Point | Criteria and Scale Used | |||
|---|---|---|---|---|---|---|
| Initial activity | 5 | 0 | No bees inspecting the wax. | |||
| 1 | Some bees inspecting. Less than 25% of the frame with bees. | |||||
| 2 | Moderate activity. Between 25 and 50% of the frame with bees. | |||||
| 3 | High activity. More than 50% of the frame with bees. | |||||
| Beeswax stretching | 20 | 0 | No visible change in wax stretch. | |||
| 1 | Minor changes. Less than 25% of the frame stretched. | |||||
| 2 | Moderate changes. Between 25 and 50% of the frame stretched. | |||||
| 3 | Significant progress. More than 50% of the frame stretched. | |||||
| Final use | 45 | 0 | No cells occupied (brood, pollen, and/or nectar) | |||
| 1 | Less than 25% of cells occupied (brood, pollen, and/or nectar) | |||||
| 2 | Between 25 and 50% of cells occupied (brood, pollen, and/or nectar) | |||||
| 3 | More than 50% of cells occupied (brood, pollen, and/or nectar) | |||||
| Total Score in points | 0 | 1–3 | 4–6 | 7–9 | ||
| Level Acceptance | Reject | Low | Medium | High | ||
| CONTROL | SHW/I | SHWSA | SHWCA | SHWOA | |
 |  |  |  |  | |
| WI | 19. 1 ± 0.64 a | 21.79 ± 0.51 b | 20.91 ± 0.54 b | 21.29 ± 0.62 b | 21.58 ± 0.93 b |
| L* | 30.91 ± 1.14 a | 37.07 ± 1.38 b | 35.45 ± 1.22 b | 34.27 ± 1.7 b | 37.42 ± 2.39 b |
| a* | 11.49 ± 1.52 a | 8.38 ± 1.34 b | 10.53 ± 1.33 ab | 4.66 ± 1.41 c | 10.54 ± 1.61 ab |
| b* | 40.43 ± 1.01 a | 45.62 ± 1.16 b | 44.42 ± 0.99 b | 42.95 ± 1.46 b | 45.93 ± 1.96 b |
| ΔE | - | 8.64 | 6.12 | 8.01 | 8.58 |
| Indicators | CONTROL | SWHOA | ||||
|---|---|---|---|---|---|---|
| Hives | Hives | |||||
| H1 | H2 | H3 | H1 | H2 | H3 | |
| Initial activity (0–3) | 2 | 1 | 1 | 1 | 1 | 1 |
| Beeswax stretching (0–3) | 1 | 1 | 1 | 3 | 2 | 3 |
| Final use (0–3) | 2 | 2 | 2 | 3 | 2 | 3 |
| Total Score per hive (H1 + H2 + H3) | 5 | 4 | 3 | 7 | 5 | 7 |
| Mean total score | 4.33 | 6.33 | ||||
| Standard deviation | 0.58 | 1.15 | ||||
| Level Acceptance | Medium | Medium | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yáñez, K.; Arias, R.; Ramírez, D.; Guerrero, F.; Toledo, M. Removal of Neonicotinoid Residues from Beeswax Using an Eco-Friendly Oxalic Acid Treatment: A Sustainable Solution for Apicultural Decontamination. Agriculture 2025, 15, 2409. https://doi.org/10.3390/agriculture15232409
Yáñez K, Arias R, Ramírez D, Guerrero F, Toledo M. Removal of Neonicotinoid Residues from Beeswax Using an Eco-Friendly Oxalic Acid Treatment: A Sustainable Solution for Apicultural Decontamination. Agriculture. 2025; 15(23):2409. https://doi.org/10.3390/agriculture15232409
Chicago/Turabian StyleYáñez, Karen, Ramón Arias, Daniel Ramírez, Fabián Guerrero, and Mario Toledo. 2025. "Removal of Neonicotinoid Residues from Beeswax Using an Eco-Friendly Oxalic Acid Treatment: A Sustainable Solution for Apicultural Decontamination" Agriculture 15, no. 23: 2409. https://doi.org/10.3390/agriculture15232409
APA StyleYáñez, K., Arias, R., Ramírez, D., Guerrero, F., & Toledo, M. (2025). Removal of Neonicotinoid Residues from Beeswax Using an Eco-Friendly Oxalic Acid Treatment: A Sustainable Solution for Apicultural Decontamination. Agriculture, 15(23), 2409. https://doi.org/10.3390/agriculture15232409








