Effect of Different Light–Dark Cycles on the Growth and Nutritional Quality of Celery
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Environment
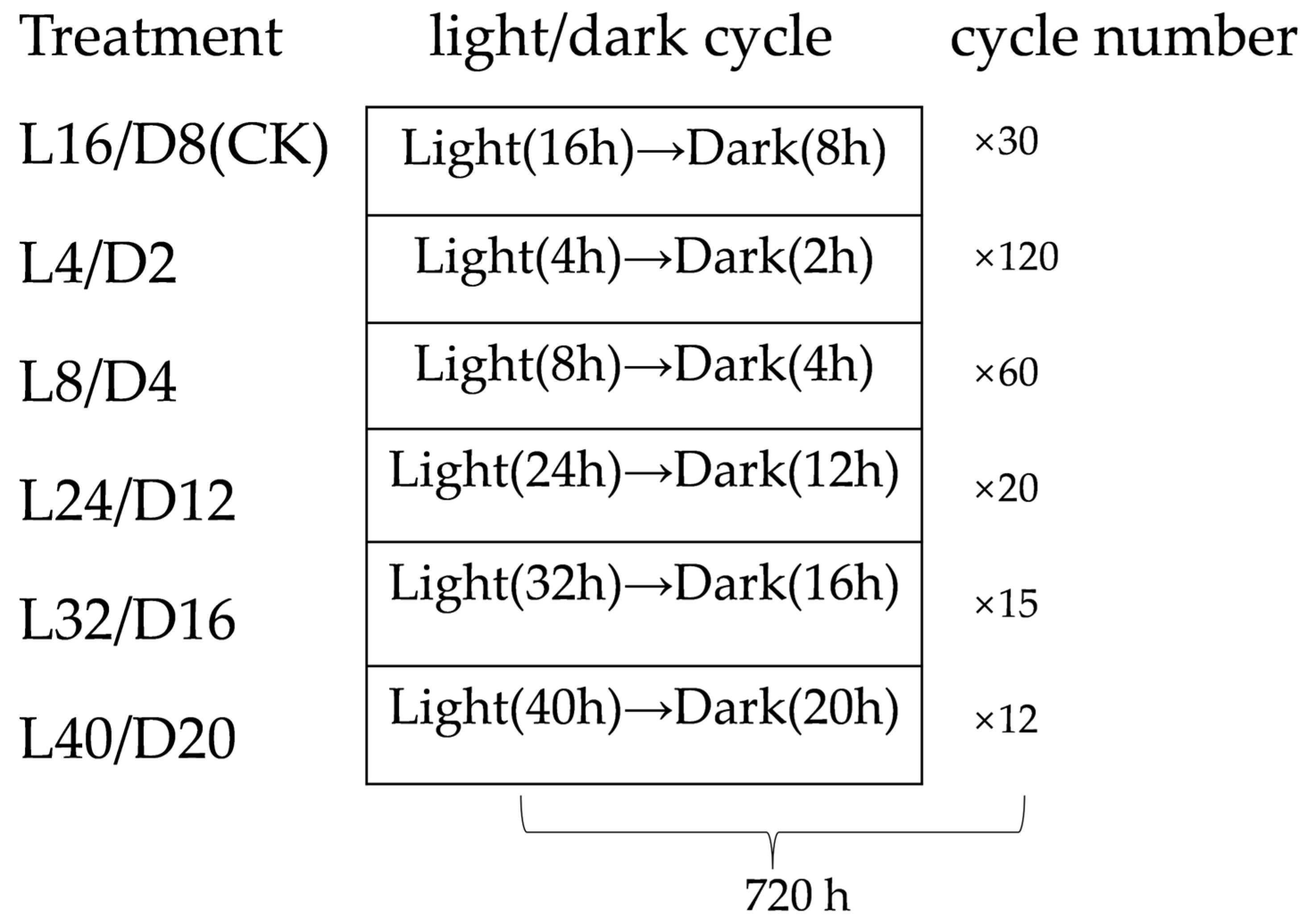
2.2. Experimental Treatment
2.3. Determination of Growth Indicators
2.4. Determination of Root Activity
2.5. Determination of Chlorophyll Fluorescence and Photosynthetic Parameters
2.6. Determination of Photosynthetic Pigments
2.7. Determination of Physiological Indexes
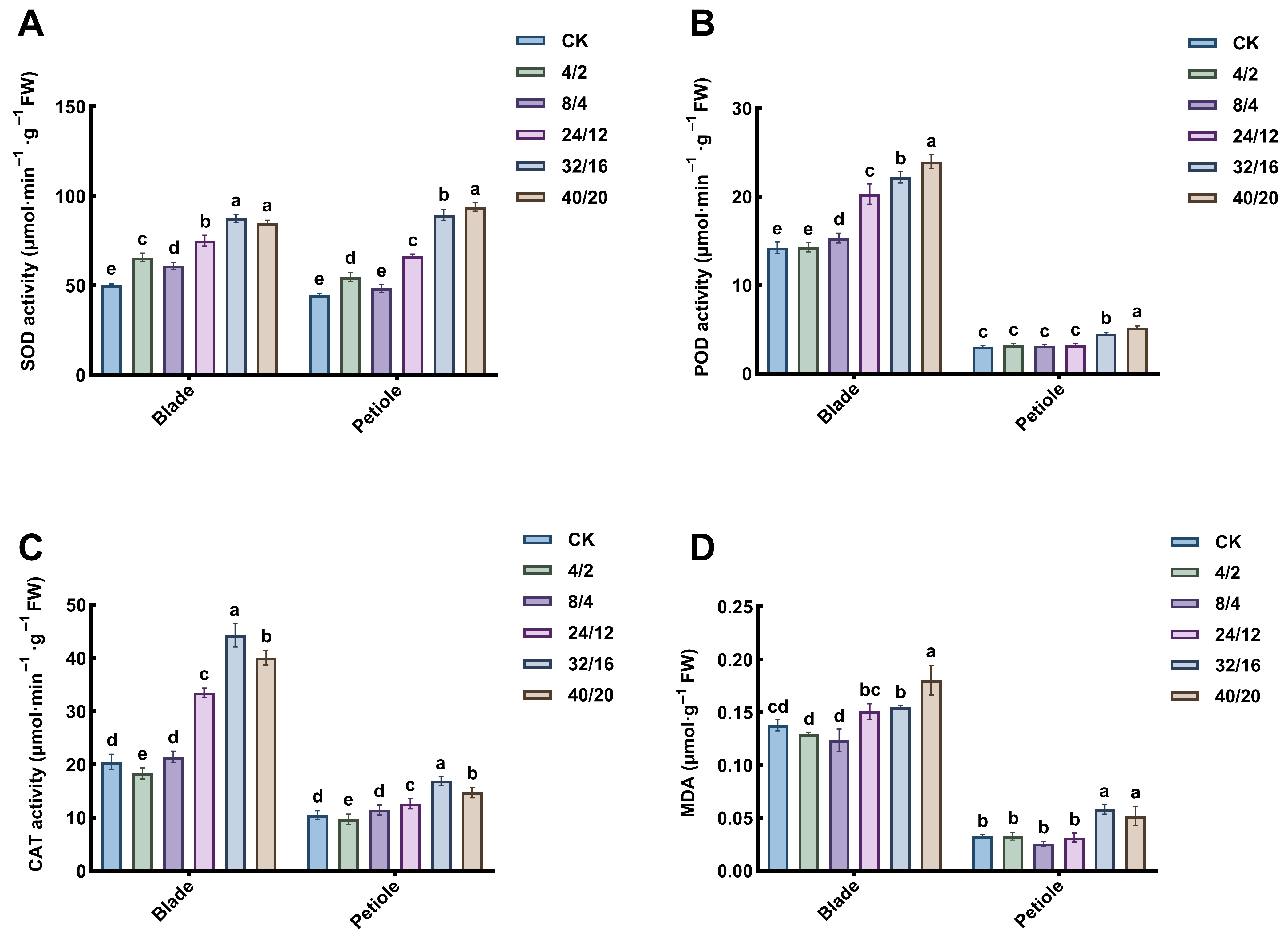
2.7.1. Determination of Antioxidant Enzymes and MDA Content
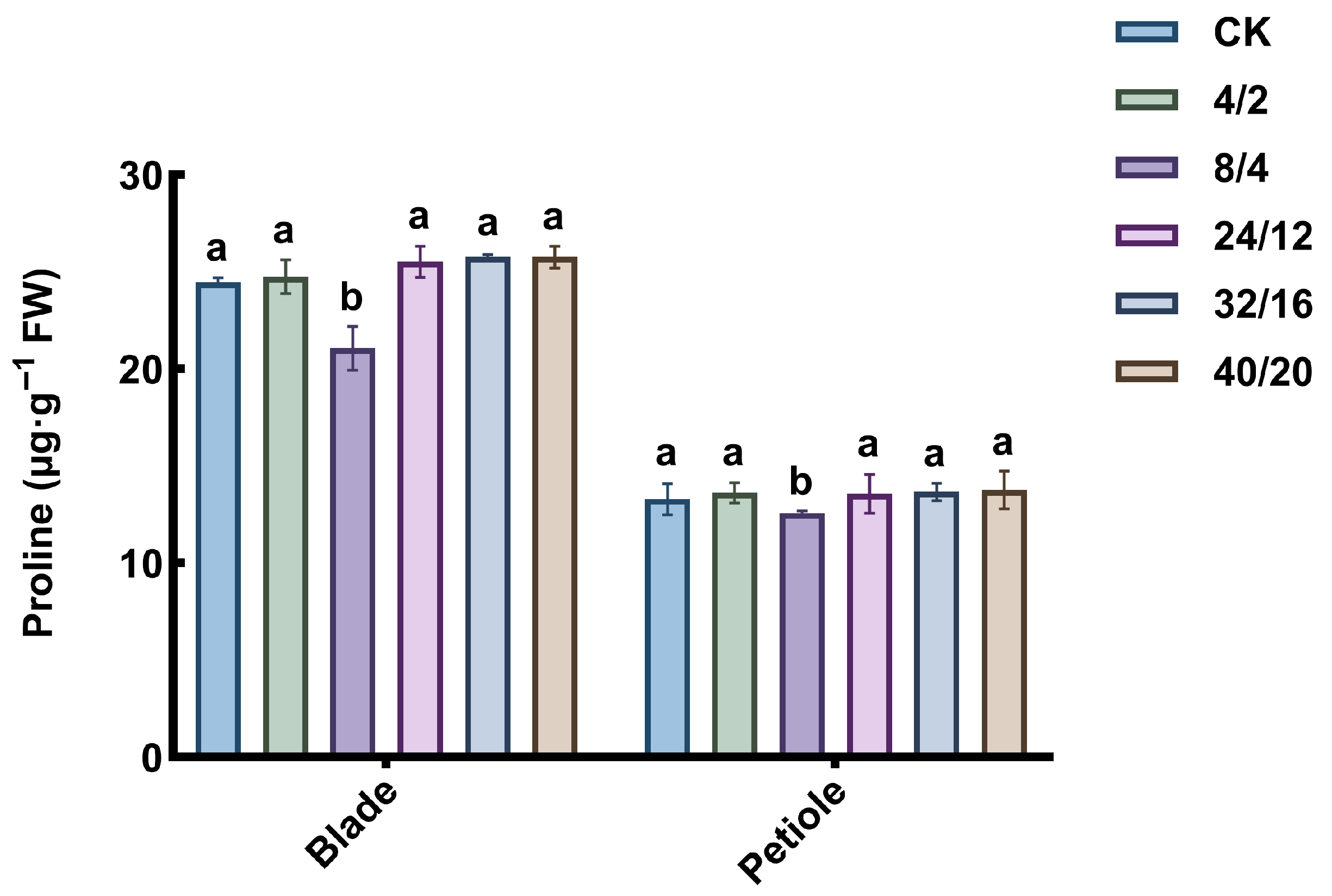
2.7.2. Determination of Proline Content
2.7.3. Determination of Antioxidant Substances
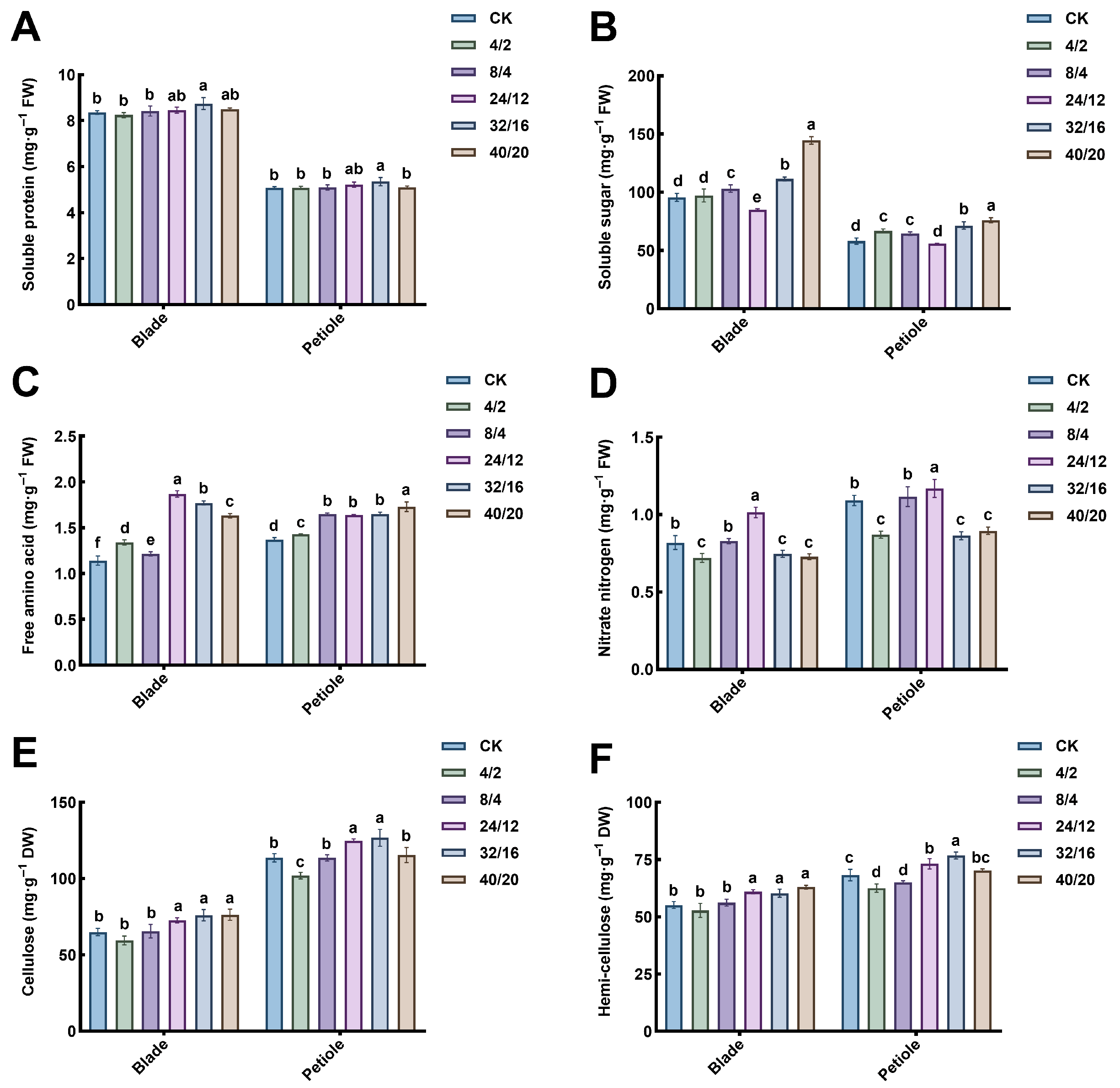
2.7.4. Determination of Nutritional Quality
2.8. Data Analyses
3. Results
3.1. Effects of Light–Dark Cycle on Growth
3.2. Effects of Light–Dark Cycle on Root Activity
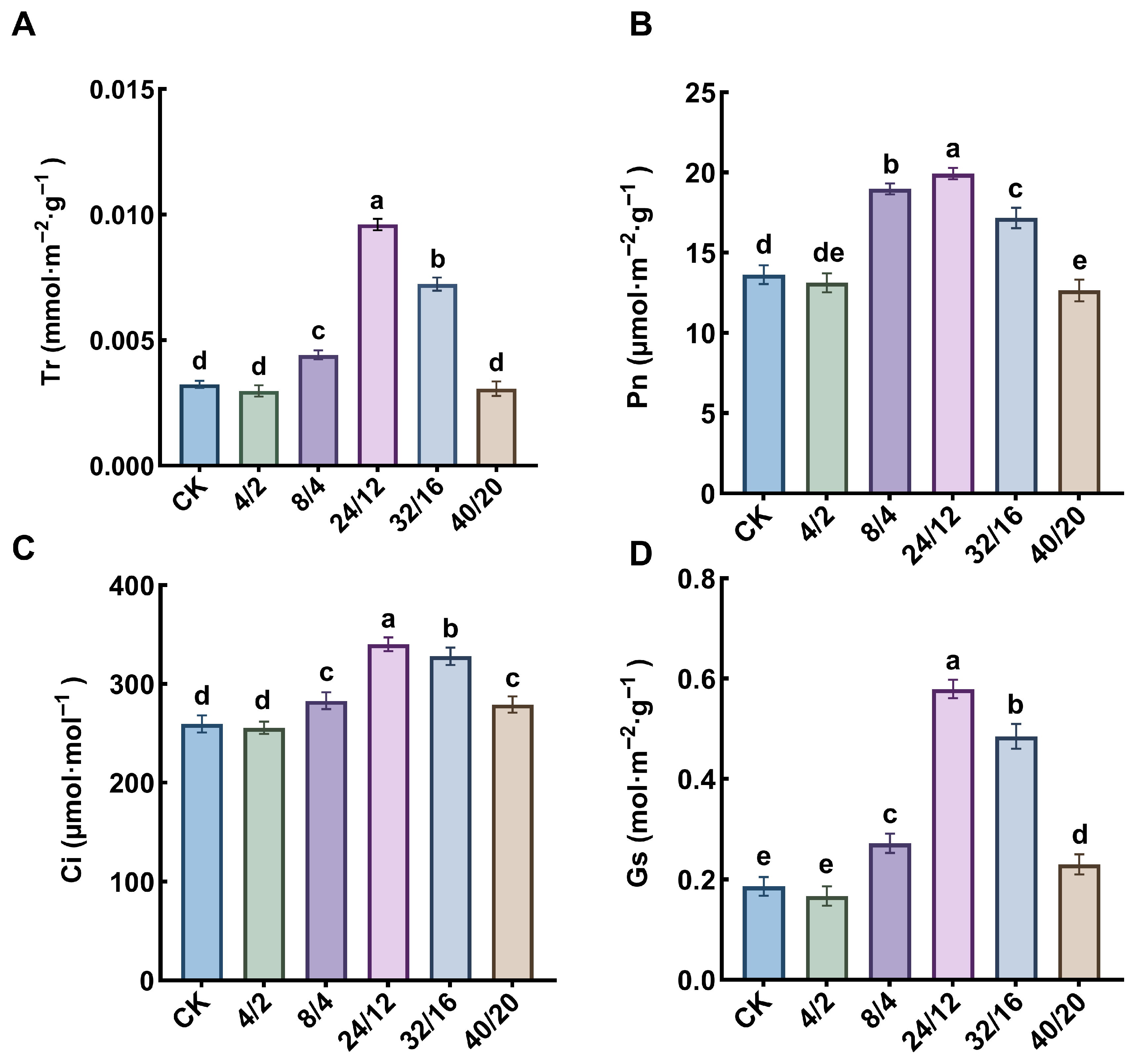
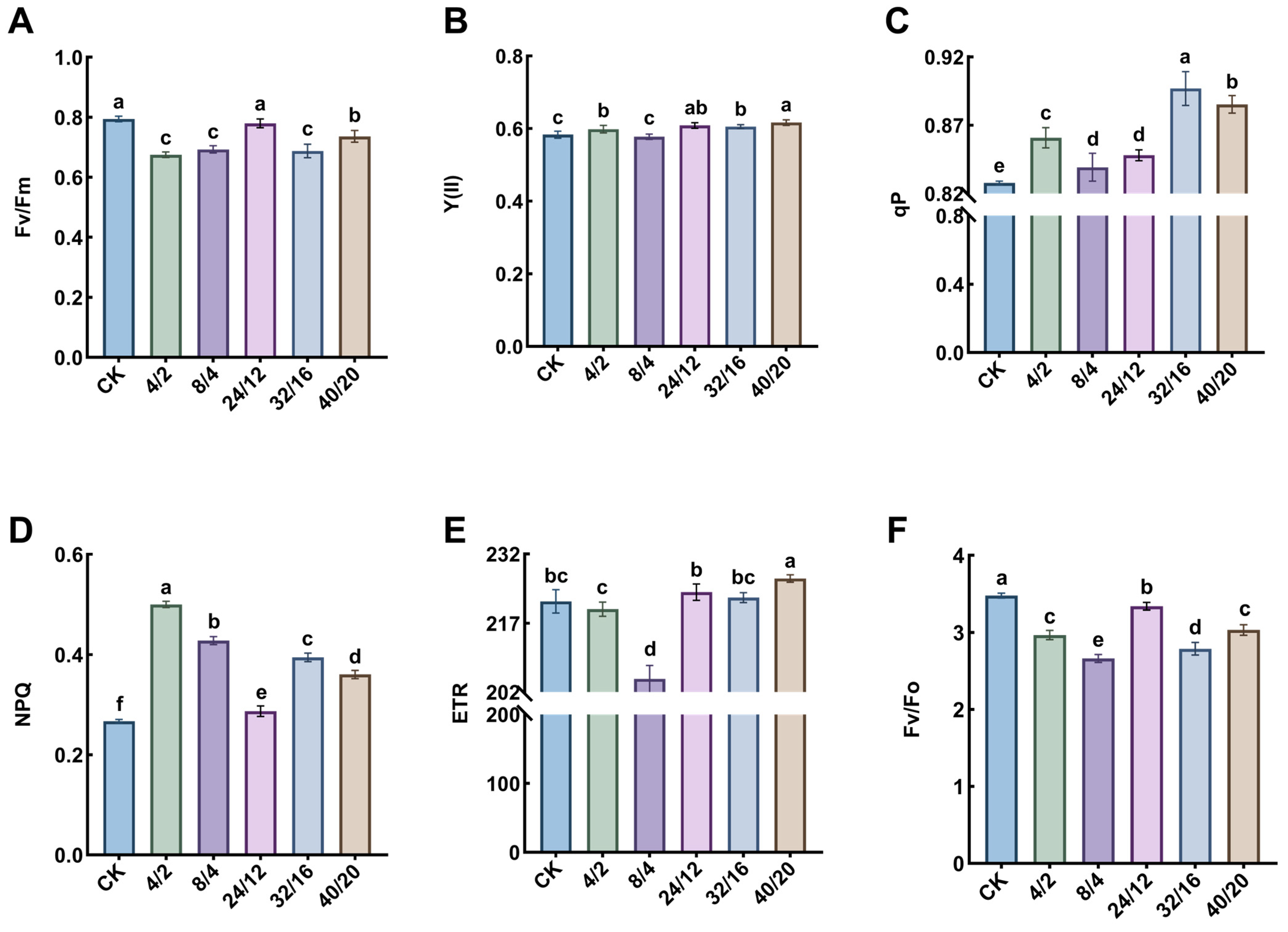
3.3. Effects of Light–Dark Cycle on Photosynthetic Parameters and Chlorophyll Fluorescence
3.4. Effects of Light–Dark Cycle on Photosynthetic Pigments
3.5. Effect of Light–Dark Cycle on Antioxidase and MDA
3.6. Effect of Light–Dark Cycle on Proline Content
3.7. Effect of Light–Dark Cycle on Antioxidant Substances
3.8. The Effect of Light–Dark Cycle on Nutrients
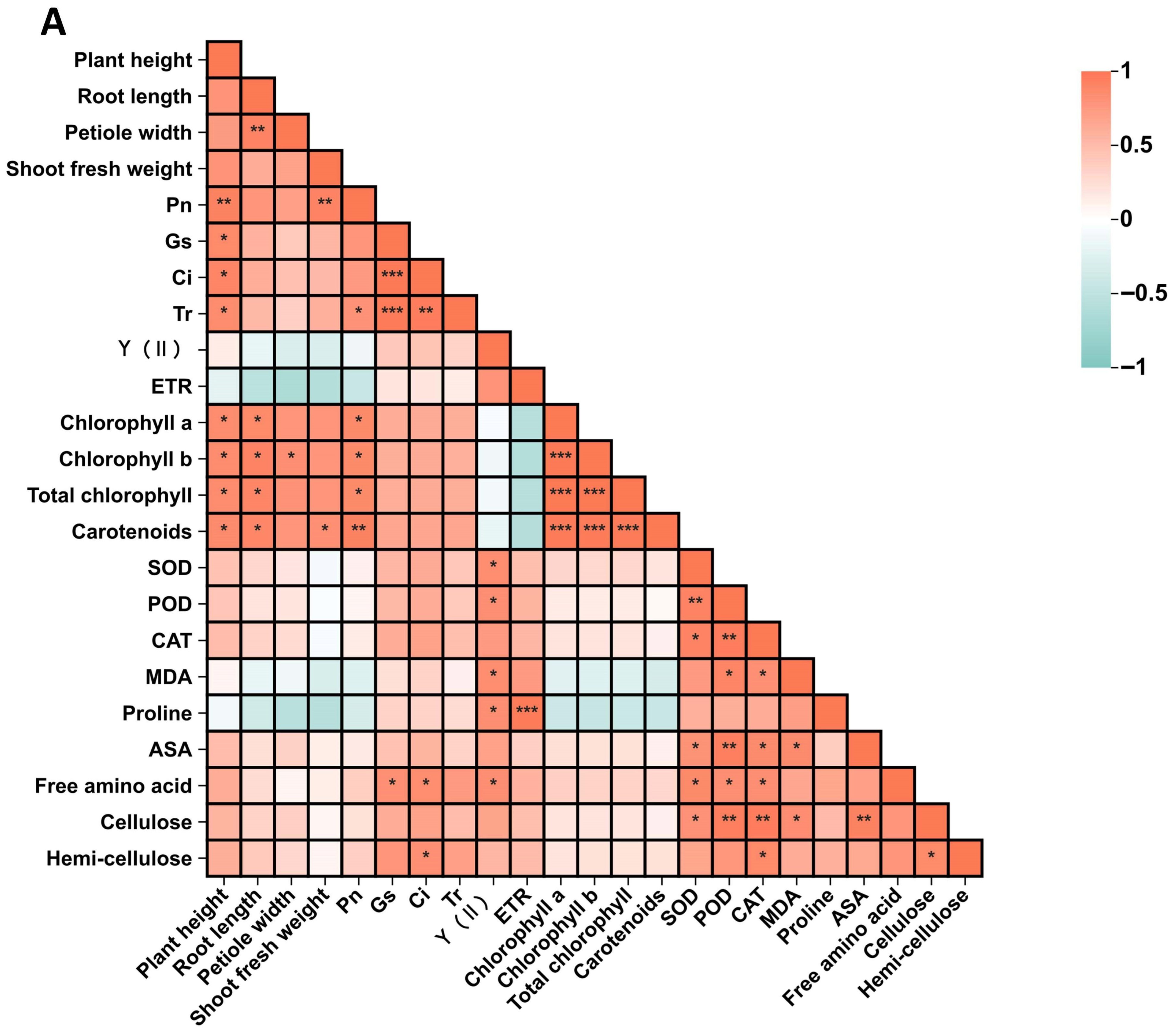
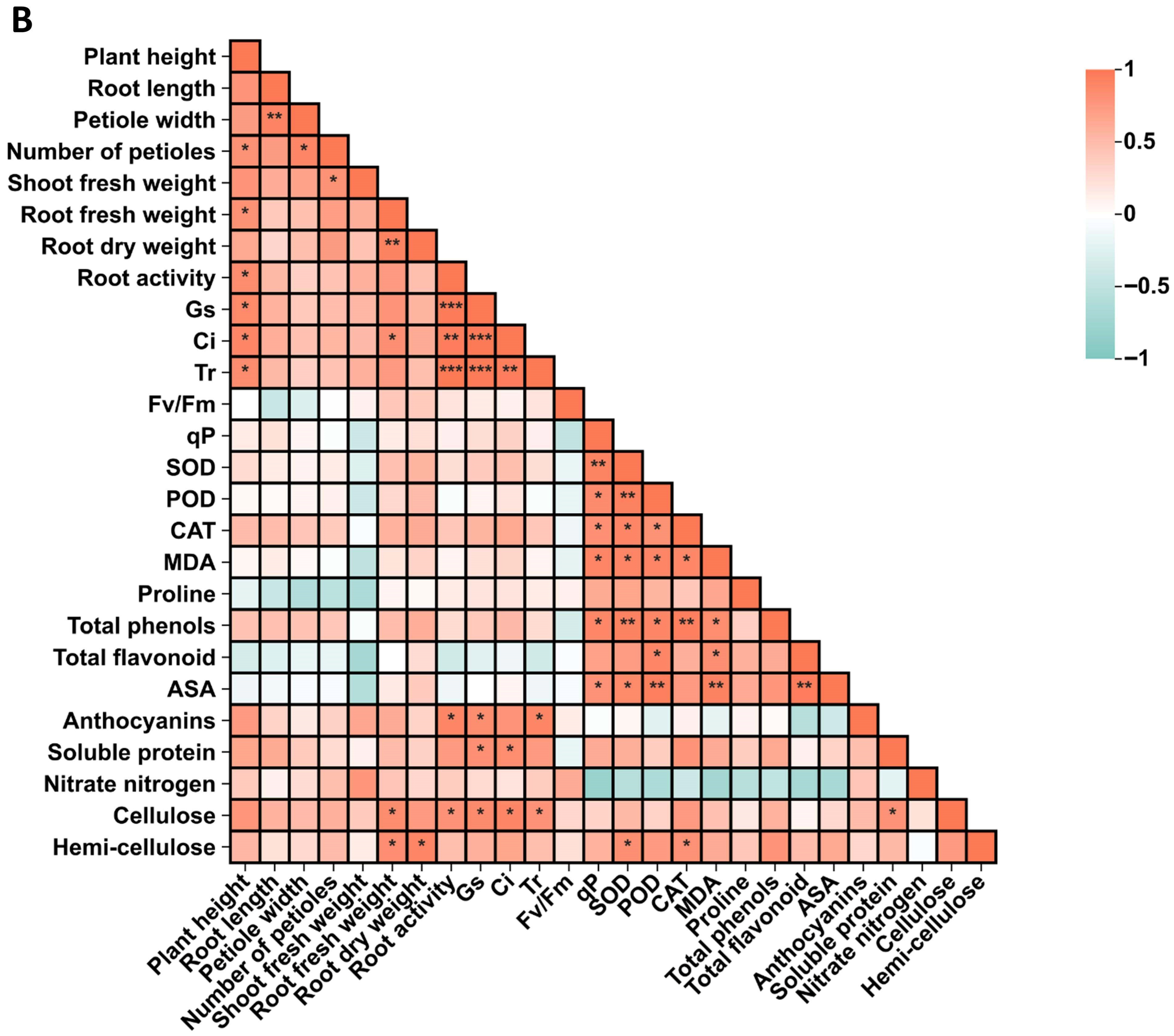
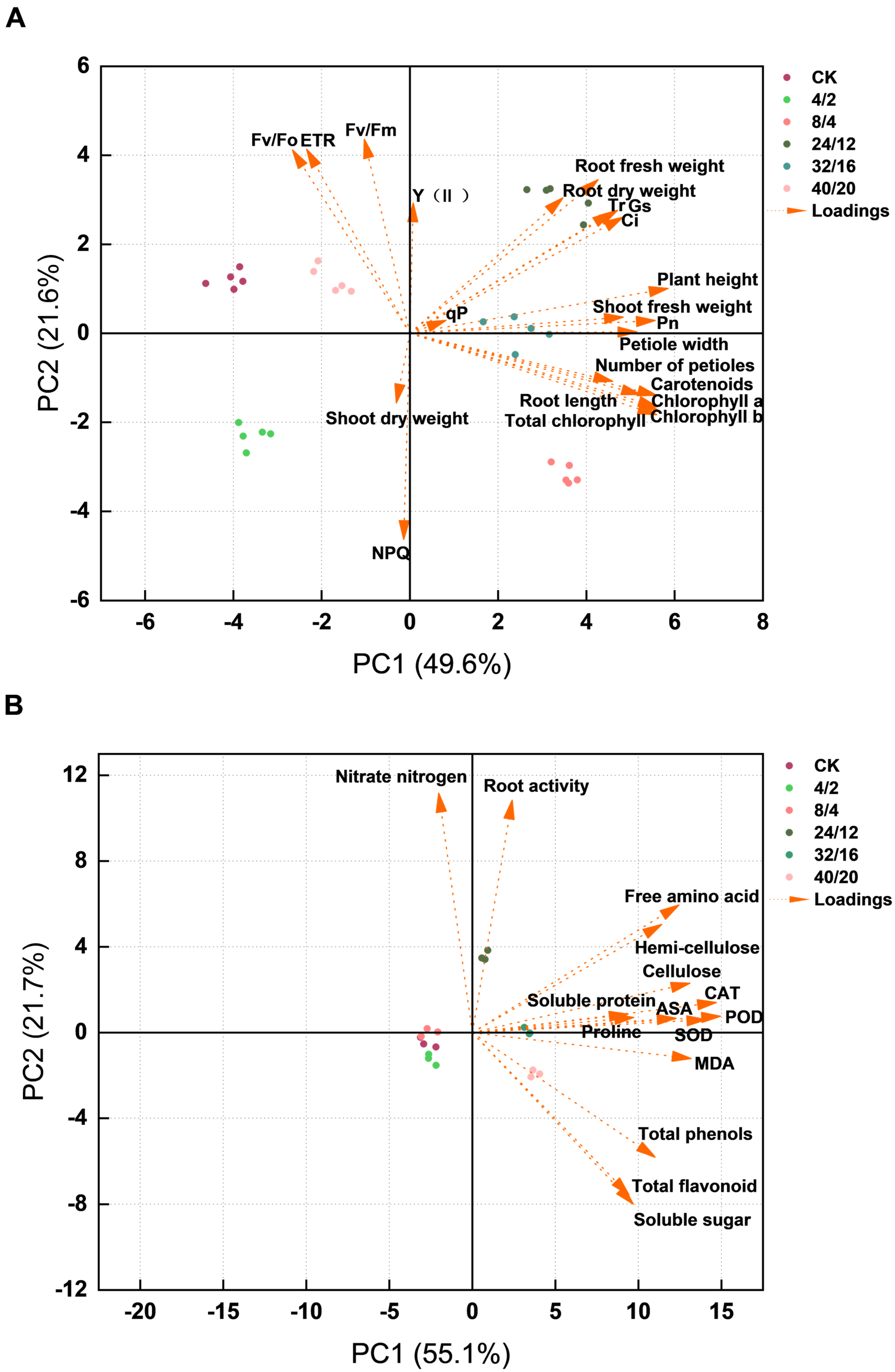
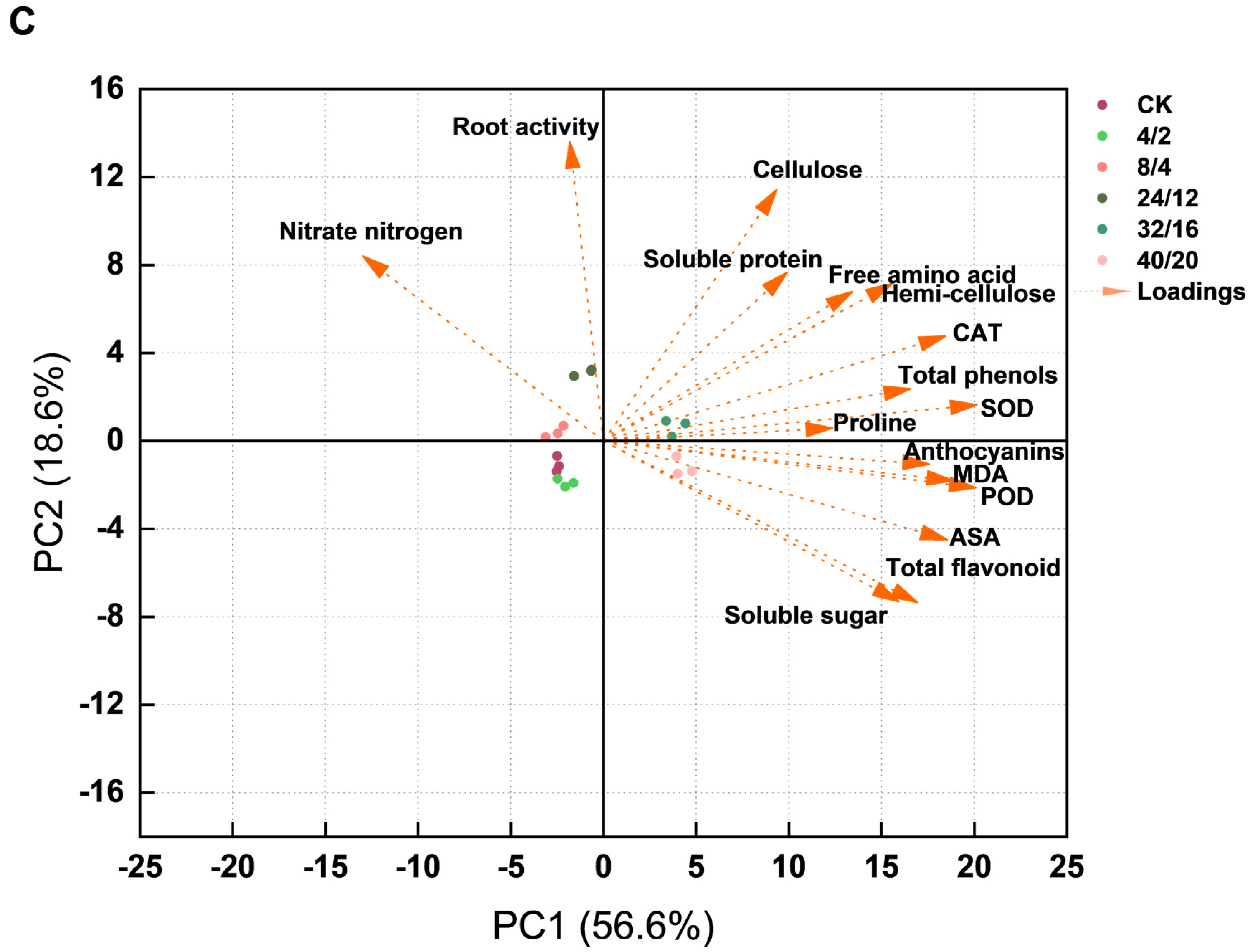
3.9. Correlation Analysis and Principal Component Analysis
4. Discussion
4.1. Effect of Different Light–Dark Cycles on Celery Growth and Photosynthetic Characteristics
4.2. Effect of Different Light Cycles on the Antioxidant Capacity of Celery
4.3. Effect of Different Light–Dark Cycles on the Nutritional Quality of Celery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.Y.; Hou, X.L.; Wang, F.; Tan, G.F.; Xu, Z.S.; Xiong, A.S. Advances in the research of celery, an important Apiaceae vegetable crop. Crit. Rev. Biotechnol. 2017, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H. On the ecology of selenium accumulation in plants. Plants 2019, 8, 197. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Du, J.; Li, W.; He, R.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; Tang, H. Effects of exogenous application of the strigolactone GR24 on quality and flavor components during postharvest storage of celery. Postharvest Biol. Technol. 2024, 212, 112900. [Google Scholar] [CrossRef]
- Asif, H.M.; Akram, M.; Usmanghani, K.; Akhtar, N.; Shah, P.A.; Uzair, M.; Ramzan, M.; Ali Shah, S.M.; Rehman, R. Monograph of Apium graveolens Linn. J. Med. Plant Res. 2011, 5, 1494–1496. [Google Scholar]
- Profir, A.; Vizireanu, C. Effect of the preservation processes on the storage stability of juice made from carrot, celery and beetroot. J. Agroaliment. Process. Technol. 2013, 19, 99–104. [Google Scholar]
- Kooti, W.; Ghasemi-boroon, M.; Ghafourian, M.; Asadi-Samani, M.; Harizi, M.; Sharafi-Ahvazi, N.; Afrisham, R.l. The effects of celery leave extract on male hormones in rats. J. HerbMed Pharmacol. 2015, 4, 56–60. [Google Scholar]
- Inada, K.; Yabumoto, Y. Effect of light quality, day length and periodic temperature variation on the growth of lettuce and radish plants. Jpn. J. Crop Sci. 1989, 58, 689–694. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Booij, R.; Meurs, E. Flowering in celeriac (Apium graveolens L. var. rapaceum (Mill.) DC.): Effects of photoperiod. Sci. Hortic. 1994, 58, 271–282. [Google Scholar] [CrossRef]
- Chu, Q.; Qin, Y.; Li, C.; Cheng, S.; Su, L.; He, Z.; Zhou, X.; Shao, D.; Guo, X. Effects of different photoperiods on the growth and nutritional characteristics of two celery cultivars in plant factory. Agronomy 2023, 13, 3039. [Google Scholar] [CrossRef]
- Chen, C.; Huang, T.; Wang, L.-X.; Xiong, J.-S.; Li, M.-Y.; Tan, G.-F.; Liu, P.-Z.; Li, Y.-P.; Hu, Z.-H.; Liu, H.; et al. High-throughput transcriptomic analysis of circadian rhythm of flavonoid metabolism under different photoperiods in celery. Veg. Res. 2025, 5, e028. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.; Zhao, S.; Zeng, T.; Li, Z.; Theodorakis, P.E.; Hu, H.; Wang, C. LED red and blue light source fresh preservation improved the quality of celery and broccoli during cold storage. Food Sci. Biotechnol. 2025, 34, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of light intensity on celery growth and flavonoid synthesis. Front. Plant Sci. 2023, 14, 12. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, L.; Gao, X.; Elsabagh, M.; Feng, Y.; Li, Z.; Khanaki, H.; Chen, H.; Liu, F. Melatonin modulates lipopolysaccharides-induced inflammatory response and maintains circadian rhythm associated with histone H3 acetylation in bovine mammary epithelial cells. J. Funct. Foods 2024, 116, 106156. [Google Scholar] [CrossRef]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Roy, A.; Pandey, D.M.; Dwivedi, A. A review on modeling approaches for the transcriptional regulatory network intricacies of circadian clock genes in plants. Planta 2025, 262, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of light intensity and temperature on the photosynthesis characteristics and yield of lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Hassan, N.E. Light pollution and its effects on human health and the environment: A review. Asian J. Environ. Ecol. 2024, 23, 96–108. [Google Scholar] [CrossRef]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.-S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P.; Salome, P.A.; Yu, H.J.; Spencer, T.R.; Sharp, E.L.; McPeek, M.A.; Alonso, J.M.; Ecker, J.R.; McClung, C.R. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 2003, 302, 1049–1053. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Sakagami, Y. Peptide hormones in plants. Annu. Rev. Plant Biol. 2006, 57, 649–674. [Google Scholar] [CrossRef] [PubMed]
- Anton-Sales, C.; Bergh, E.S.v.D.; Thérèse-Navarro, A.; Severing, E.; Moñino-López, D.; DiPalma, J.; Proveniers, M.; McClung, C.R.; Jeuken, M.; Bonnema, G. Breeding for delayed bolting decelerated the circadian clock in cultivated lettuce. New Phytol. 2025, 248, 1892–1902. [Google Scholar] [CrossRef]
- Ocaña, D.N.; Haanskorf, T.; Yang, L.; Sfondrini, E.; Reddy, A.T.; Marcelis, L.F.; Heuvelink, E. Non-natural day length does not negatively affect lettuce growth as a result of acclimated carbohydrate rhythms. Sci. Hortic. 2025, 350, 114291. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.-Q.; Tao, J.-P.; Chen, C.; Su, L.-Y.; Xiong, J.-S.; Xiong, A.-S. A telomere-to-telomere gapless genome reveals SlPRR1 control of circadian rhythm and photoperiodic flowering in tomato. GigaScience 2025, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, S.; Li, W.; Li, Z.; Xu, Z.; Zhou, W.; Meng, X.; Xie, Y.; Wang, S.; Jin, L.; et al. Identification of biological rhythms related GIGANTEA genes in tomato and functional analysis under heat stress. Plant Stress 2025, 15, 100736. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Zhang, H.; Jiang, Y.; Li, Q.; Shen, J.; Li, Z. Transcriptome analysis of CsWOX1 mutant and overexpressing lines reveals downstream genes regulating cucumber leaf morphogenesis. Veg. Res. 2025, 5, 1–12. [Google Scholar] [CrossRef]
- Simeanu, D. Melatonin: An overview on the synthesis processes and on its multiple bioactive roles played in animals and humans. Agriculture 2025, 15, 273. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Long, D.M.; Oaks, A.; Rothstein, S.J. Effect of light/dark cycles on expression of nitrate assimilatory genes in maize shoots and roots. Plant Physiol. 1991, 95, 281–285. [Google Scholar] [CrossRef]
- Cheung, C.M.; Poolman, M.G.; Fell, D.A.; Ratcliffe, R.G.; Sweetlove, L.J. A diel flux balance model captures interactions between light and dark metabolism during day-night cycles in C3 and crassulacean acid metabolism leaves. Plant Physiol. 2014, 165, 917–929. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Kamitamari, A.; Okamura, N.; Ukai, K.; Okamura, K.; Tezuka, T.; Fukuda, H. Characterization of circadian rhythms through a bioluminescence reporter assay in Lactuca sativa L. Environ. Control. Biol. 2014, 52, 21–27. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Cheng, Y.; He, D.; He, J.; Niu, G.; Gao, R. Effect of light/dark cycle on photosynthetic pathway switching and CO2 absorption in two Dendrobium species. Front. Plant Sci. 2019, 10, 659. [Google Scholar] [CrossRef]
- Chen, X.-L.; Yang, Q.-C. Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci. Hortic. 2018, 234, 220–226. [Google Scholar] [CrossRef]
- Urairi, C.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Optimization of light-dark Cycles of Lactuca sativa L. in plant factory. Environ. Control. Biol. 2017, 55, 85–91. [Google Scholar] [CrossRef]
- Shao, M.; Liu, W.; Zha, L.; Zhou, C.; Zhang, Y.; Li, B. Altering light–dark cycle at pre-harvest stage regulated growth, nutritional quality, and photosynthetic pigment content of hydroponic lettuce. Acta Physiol. Plant. 2021, 43, 1–9. [Google Scholar] [CrossRef]
- Kurata, H.; Achioku, T.; Furusaki, S. The light/dark cycle operation with an hour-scale period enhances caffeine production by Coffea arabica cells. Enzym. Microb. Technol. 1998, 23, 518–523. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Hang, T.; Li, P. Photosynthetic characteristics and growth performance of lettuce (Lactuca sativa L.) under different light/dark cycles in mini plant factories. Photosynthetica 2022, 58, 740–747. [Google Scholar] [CrossRef]
- Chen, X.-L.; Li, Y.-L.; Wang, L.-C.; Yang, Q.-C.; Guo, W.-Z. Responses of butter leaf lettuce to mixed red and blue light with extended light/dark cycle period. Sci. Rep. 2022, 12, 6924. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Sherudilo, E.G.; Ikkonen, E.; Rubaeva, A.A.; Levkin, I.A.; Titov, A.F. Effects of extended light/dark cycles on solanaceae plants. Plants 2024, 13, 244. [Google Scholar] [CrossRef]
- Ouyang, H.; Zhou, J.; Ren, J.G.; Wang, J.L. Analysis of electrode bacteria of soil microbial fuel cell (MFC) and the effects of its operation on rice growth activities. J. Anhui Agric. Univ. 2021, 48, 115–120. [Google Scholar]
- Chen, C.; Chen, P.; Wu, Z.; Liu, Q.; Zhong, Y.; Liu, T.; Zheng, Y.; Yu, F. Colchicine-induced chromosome doubling in Cinnamomum camphora and its effect on morphological and terpenoid storage structures. Ind. Crop. Prod. 2025, 233, 121338. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Ganesan, M.; Song, I.-J.; Han, Y.; Kim, J.-I.; Lee, H.-Y.; Song, P.-S. Transgenic turfgrasses expressing hyperactive Ser599Ala phytochrome A mutant exhibit abiotic stress tolerance. J. Plant Growth Regul. 2016, 35, 11–21. [Google Scholar] [CrossRef]
- An, R.; Luo, S.; Zhou, H.; Zhang, Y.; Zhang, L.; Hu, H.; Li, P. Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. Chinensis). Sci. Hortic. 2021, 289, 110469. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED supplemental lighting improves quality of tomato seedlings by increasing biomass accumulation in a controlled environment. Agronomy 2024, 14, 1888. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Chen, X.; Liu, Y.; Liu, C.; Li, L. Assessing the product quality and biological activities of barley (Hordeum vulgare L.) grasses at different harvest times. Food Biosci. 2022, 46, 101549. [Google Scholar] [CrossRef]
- Pan, L.; Sun, R.M. The Determination of the Content of Vitamin C in Medicine by Indirect Spectrophotometry. J. Huangshan Univ. 2011, 13, 47–49. [Google Scholar]
- Li, Y.-Y.; Mao, K.; Zhao, C.; Zhao, X.-Y.; Zhang, H.-L.; Shu, H.-R.; Hao, Y.-J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Qureshi, W.A.; Sheikh, S.A.; Chen, J.; Chandio, F.A.; Lakhiar, I.A.; Solangi, K.A. Effects of droplet size and spray interval on root-to-shoot ratio, photosynthesis efficiency, and nutritional quality of aeroponically grown butterhead lettuce. Int. J. Agric. Biol. Eng. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.N.; Liu, M. Quantitative determination of total free-amino acid in Nervilia fordii(Hance) Schltr. by ninhydrin colorimetric method. Chin. J. Inf. Tradit. Chin. Med. 2010, 17, 50–52. [Google Scholar]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED spectrum on the quality and nitrogen metabolism of lettuce under recycled hydroponics. Front. Plant Sci. 2021, 12, 197–203. [Google Scholar] [CrossRef]
- GB/T 5009.10-2003; Determination of Crude Fiber in Plant-Based Foods. China National Standardization Press: Beijing, China, 2018.
- Liu, X.; Liang, L.; Chen, P.; Peng, W.; Guo, K.; Huang, X.; Qin, C.; Luo, Z.; Ouyang, K.; Jiang, C.; et al. Effects of electrostatic field and CO2 interaction on growth and physiological metabolism in asparagus. Agriculture 2025, 15, 1416. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Harmer, S.L. Wheels within wheels: The plant circadian system. Trends Plant Sci. 2014, 19, 240–249. [Google Scholar] [CrossRef]
- Wijnen, H.; Naef, F.; Boothroyd, C.; Claridge-Chang, A.; Young, M.W. Control of daily transcript oscillations in drosophila by light and the circadian clock. PLoS Genet. 2006, 2, e39. [Google Scholar] [CrossRef]
- Fukuda, H.; Nakamichi, N.; Hisatsune, M.; Murase, H.; Mizuno, T. Synchronization of plant circadian oscillators with a phase delay effect of the vein network. Phys. Rev. Lett. 2007, 99, 098102. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, Y.; Zhang, Q. Morning and evening alarm of the circadian clock for flower opening times in Hemerocallis. Plant Sci. 2021, 311, 110992. [Google Scholar] [CrossRef]
- Li, M.; Tan, S.; Tan, G.; Luo, Y.; Sun, B.; Zhang, Y.; Chen, Q.; Wang, Y.; Zhang, F.; Zhang, Y.; et al. Transcriptome analysis reveals important transcription factor families and reproductive biological processes of flower development in celery (Apium graveolens L.). Agronomy 2020, 10, 653. [Google Scholar] [CrossRef]
- Booij, R.; Meurs, E. Effect of photoperiod on flower stalk elongation in celeriac (Apium graveolens L. var. rapaceum (Mill.) DC.). Sci. Hortic. 1995, 63, 143–154. [Google Scholar] [CrossRef]
- Liu, L.; Ou, C.; Chen, S.; Shen, Q.; Liu, B.; Li, M.; Zhao, Z.; Kong, X.; Yan, X.; Zhuang, F. The response of COL and FT homologues to photoperiodic regulation in carrot (Daucus carota L.). Sci. Rep. 2020, 10, 9984. [Google Scholar] [CrossRef]
- Peterson, L.E.; Clark, R.J.; Menary, R.C. Umbel initiation and stem elongation in fennel (Foeniculum vulgare) initiated by photoperiod. J. Essent. Oil Res. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Zhao, M.-R.; Wang, J.-Q.; Hu, B.-Y.; Chen, Q.-J.; Qin, Y.; Zhang, G.-Q. Effects of microbial inoculants on agronomic characters, physicochemical properties and nutritional qualities of lettuce and celery in hydroponic cultivation. Sci. Hortic. 2023, 320, 112202. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Q.; Ji, G.; Wang, J.; Ding, Y.; Wang, S. Effects of light intensity and photoperiod on morphological development and photosynthetic characteristics of coriander. Horticulturae 2024, 10, 215. [Google Scholar] [CrossRef]
- Dantas, d.B.; Luíza, L.; Eldridge, B.M.; Dorling, J.; Dekeya, R.; Lynch, D.A.; Dodd, A.N. Circadian regulation of metabolism across photosynthetic organisms. Plant J. 2023, 116, 650–668. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Cortleven, A.; Schmülling, T. Photoperiod stress induces an oxidative burst-like response and is associated with increased apoplastic peroxidase and decreased catalase activities. J. Plant Physiol. 2020, 253, 153252. [Google Scholar] [CrossRef] [PubMed]
- Krasensky-Wrzaczek, J.; Kangasjärvi, J. The role of reactive oxygen species in the integration of temperature and light signals. J. Exp. Bot. 2018, 69, 3347–3358. [Google Scholar] [CrossRef]
- Liu, J.-X.; Feng, K.; Wang, G.-L.; Wu, X.-J.; Duan, A.-Q.; Yin, L.; Shen, D.; Xu, Z.-S.; Xiong, A.-S. Effect of elevated CO2 on ascorbate accumulation and the expression levels of genes involved in ascorbate metabolism in celery. J. Plant Growth Regul. 2020, 39, 1046–1060. [Google Scholar] [CrossRef]
- Deng, M.; Qian, H.; Chen, L.; Sun, B.; Chang, J.; Miao, H.; Cai, C.; Wang, Q. Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of Chinese kale sprouts. Food Chem. 2025, 222, 1–5. [Google Scholar] [CrossRef]
- Costa, R.; Ferreira, C.; Alves, A.; Nunes, S.; Reis, F.; Malva, J.; Viana, S. Lipid-lowering statins and polyphenol-based supplementation: A scoping review on drug-food interaction potential. Front. Pharmacol. 2025, 16, 1541871. [Google Scholar] [CrossRef]
- Li, M.-Y.; Feng, K.; Hou, X.-L.; Jiang, Q.; Xu, Z.-S.; Wang, G.-L.; Liu, J.-X.; Wang, F.; Xiong, A.-S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2025, 7, 9. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Deans, C.A.; Behmer, S.T.; Fiene, J.; Sword, G.A. Spatio-temporal, genotypic, and environmental effects on plant soluble protein and digestible carbohydrate content: Implications for insect herbivores with cotton as an exemplar. J. Chem. Ecol. 2016, 42, 1151–1163. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Li, F.; Xiao, F.; Yu, H. Effect of divalent manganese (Mn2+) concentration on the growth and nitrate nitrogen content of lettuce during aeroponic intercropping with cherry radish. Hortic. Environ. Biotechnol. 2021, 62, 243–251. [Google Scholar] [CrossRef]
- Kiani, A.; Sharafi, K.; Omer, A.K.; Matin, B.K.; Davoodi, R.; Mansouri, B.; Sharafi, H.; Soleimani, H.; Massahi, T.; Ahmadi, E. Accumulation and human health risk assessment of nitrate in vegetables irrigated with different irrigation water sources- transfer evaluation of nitrate from soil to vegetables. Environ. Res. 2022, 205, 112527. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Tan, X.; Ye, Z.; Chen, X.; Zhang, Y.; Ruan, Y.; Ma, B.; Kong, D. Analysis of lettuce transcriptome reveals the mechanism of different light/dark cycle in promoting the growth and quality. Front. Plant Sci. 2024, 15, 1394434. [Google Scholar] [CrossRef]


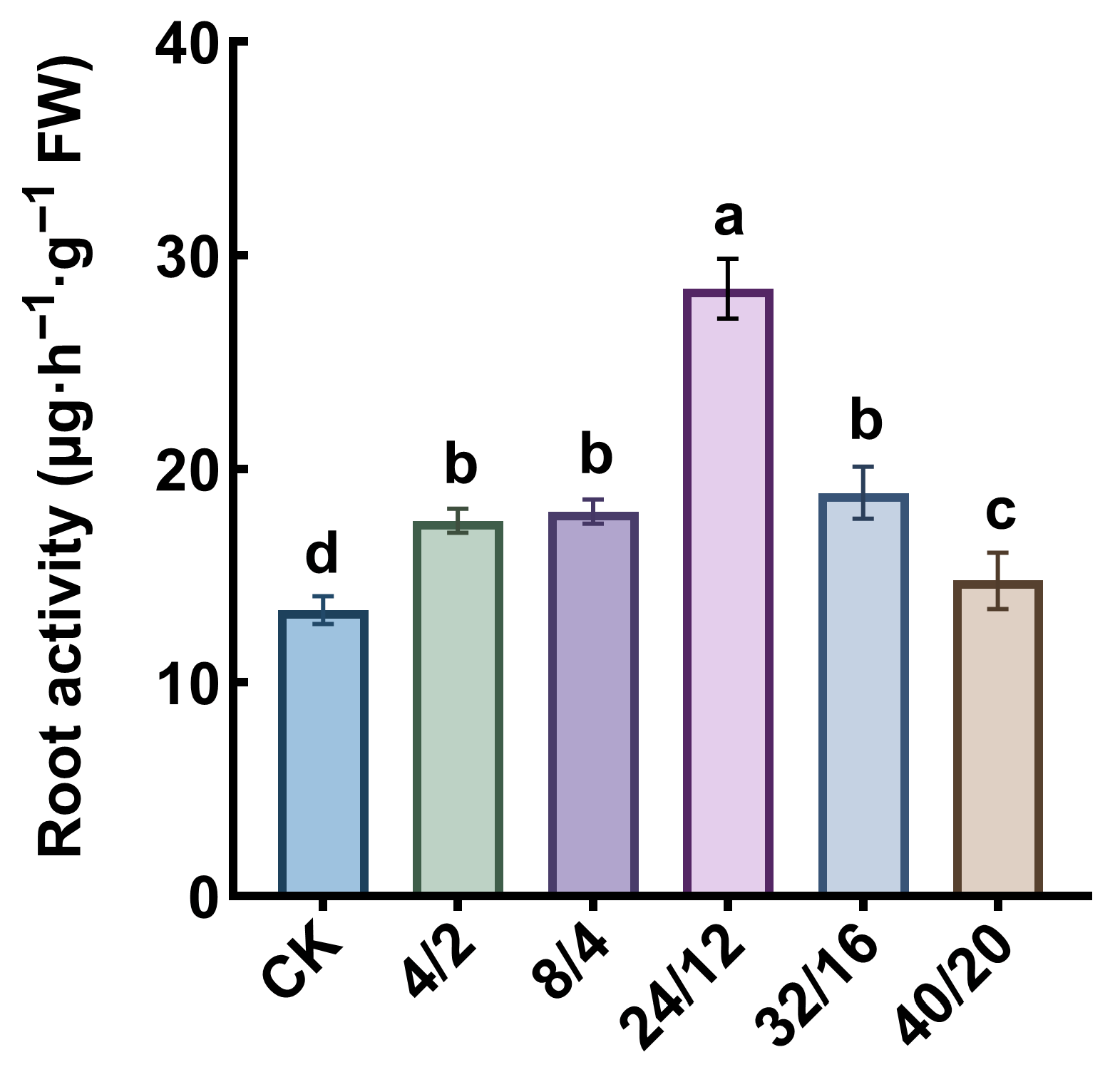

| Treatments | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|
| CK | 153 ± 8 d | 52.6 ± 2.7 d | 19.5 ± 1.0 b | 4.04 ± 0.11 c |
| 4/2 | 142 ± 6 e | 42.3 ± 0.9 e | 19.0 ± 0.9 b | 2.91 ± 0.32 d |
| 8/4 | 225 ± 7 a | 59.2 ± 3.1 c | 21.2 ± 0.5 a | 4.60 ± 0.28 b |
| 24/12 | 218 ± 5 a | 73.2 ± 1.8 a | 18.5 ± 1.2 b | 5.20 ± 0.26 a |
| 32/16 | 162 ± 6 c | 61.8 ± 2.4 bc | 17.0 ± 0.9 c | 4.61 ± 0.24 b |
| 40/20 | 178 ± 4 b | 63.2 ± 1.7 b | 19.6 ± 0.4 b | 5.35 ± 0.26 a |
| Treatments | Chlorophyll a (mg·100 g−1) | Chlorophyll b (mg·100 g−1) | Total Chlorophyll (mg·100 g−1) | Carotenoids (mg·100 g−1) |
|---|---|---|---|---|
| CK | 27.0 ± 0.2 f | 12.8 ± 0.4 e | 39.7 ± 0.2 e | 9.4 ± 0.3 f |
| 4/2 | 31.3 ± 0.2 d | 14.9 ± 0.4 c | 46.2 ± 0.5 c | 10.6 ± 0.1 d |
| 8/4 | 38.1 ± 0.2 a | 19.4 ± 0.3 a | 57.5 ± 0.5 a | 13.3 ± 0.1 a |
| 24/12 | 35.7 ± 0.2 b | 17.5 ± 0.4 b | 53.2 ± 0.4 b | 12.6 ± 0.1 b |
| 32/16 | 35.4 ± 0.2 c | 17.9 ± 0.3 b | 53.3 ± 0.2 b | 12.4 ± 0.1 c |
| 40/20 | 30.0 ± 0.2 e | 14.3 ± 0.4 d | 44.3 ± 0.4 d | 9.6 ± 0.1 e |
| Classification of Indicators | |||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Plant height | 0.29 | 0.08 | 0.00 |
| Root length | 0.26 | −0.12 | 0.06 |
| Petiole width | 0.26 | 0.00 | −0.18 |
| Number of petioles | 0.23 | −0.09 | −0.06 |
| Shoot fresh weight | 0.24 | 0.03 | −0.26 |
| Root fresh weight | 0.21 | 0.29 | −0.05 |
| Shoot dry weight | −0.02 | −0.13 | −0.45 |
| Root dry weight | 0.17 | 0.25 | −0.07 |
| Pn | 0.28 | 0.02 | −0.11 |
| Gs | 0.24 | 0.23 | 0.14 |
| Ci | 0.24 | 0.22 | 0.16 |
| Tr | 0.23 | 0.23 | 0.09 |
| Fv/Fm | −0.05 | 0.36 | −0.30 |
| Y(II) | 0.00 | 0.24 | 0.35 |
| qP | 0.04 | 0.02 | 0.48 |
| NPQ | −0.01 | −0.39 | 0.25 |
| ETR | −0.12 | 0.35 | 0.27 |
| Fv/Fo | −0.13 | 0.34 | −0.20 |
| Chlorophyll a | 0.28 | −0.14 | 0.04 |
| Chlorophyll b | 0.28 | −0.15 | 0.03 |
| Total chlorophyll | 0.28 | −0.14 | 0.04 |
| Carotenoids | 0.28 | −0.12 | −0.01 |
| Eigenvalue | 10.91 | 4.76 | 3.21 |
| Percentage of variance (%) | 49.6 | 21.6 | 14.6 |
| Cumulative (%) | 49.6 | 71.2 | 85.8 |
| Blade Physiology | Petiole Physiology | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| AsA | 0.28 | 0.03 | −0.19 | 0.30 | −0.17 | 0.07 |
| Soluble protein | 0.21 | 0.04 | −0.44 | 0.16 | 0.29 | 0.26 |
| Soluble sugar | 0.22 | −0.37 | −0.12 | 0.26 | −0.27 | −0.29 |
| Nitrate-nitrogen | −0.05 | 0.52 | 0.01 | −0.21 | 0.32 | −0.10 |
| SOD | 0.32 | 0.03 | −0.05 | 0.32 | 0.06 | −0.01 |
| POD | 0.34 | 0.04 | −0.04 | 0.32 | −0.08 | −0.12 |
| CAT | 0.34 | 0.07 | −0.06 | 0.30 | 0.18 | −0.06 |
| Free amino acid | 0.28 | 0.28 | 0.11 | 0.22 | 0.26 | −0.51 |
| MDA | 0.30 | −0.06 | 0.26 | 0.30 | −0.07 | 0.08 |
| Proline | 0.22 | 0.03 | 0.66 | 0.20 | 0.02 | 0.63 |
| Total phenols | 0.25 | −0.27 | −0.30 | 0.27 | 0.09 | −0.25 |
| Total flavonoids | 0.22 | −0.35 | 0.37 | 0.27 | −0.28 | 0.04 |
| Cellulose | 0.30 | 0.11 | −0.10 | 0.15 | 0.43 | 0.06 |
| Hemi-cellulose | 0.26 | 0.23 | −0.01 | 0.25 | 0.27 | −0.06 |
| Root activity | 0.06 | 0.50 | 0.01 | −0.03 | 0.51 | 0.09 |
| Anthocyanins | 0.28 | −0.04 | 0.28 | |||
| Eigenvalue | 8.27 | 3.25 | 1.14 | 9.06 | 2.98 | 1.29 |
| Percentage of variance (%) | 55.1 | 21.7 | 7.63 | 56.6 | 18.6 | 8.06 |
| Cumulative (%) | 55.1 | 76.8 | 84.4 | 56.6 | 75.3 | 83.3 |
| Classification of Indicators | Treatment | U1 | U2 | U3 | D Value | Rank |
|---|---|---|---|---|---|---|
| Growth and photosynthetic properties | CK | 0.00 | 0.71 | 0.00 | 0.18 | 5 |
| 4/2 | 0.07 | 0.14 | 0.73 | 0.20 | 6 | |
| 8/4 | 1.00 | 0.00 | 0.02 | 0.58 | 3 | |
| 24/12 | 0.98 | 1.00 | 0.33 | 0.87 | 1 | |
| 32/16 | 0.86 | 0.52 | 1.00 | 0.80 | 2 | |
| 40/20 | 0.30 | 0.71 | 0.58 | 0.45 | 4 | |
| Wight (Wi) | 0.58 | 0.25 | 0.17 | |||
| Blade physiology | CK | 0.00 | 0.26 | 0.89 | 0.15 | 5 |
| 4/2 | 0.04 | 0.12 | 1.00 | 0.06 | 6 | |
| 8/4 | 0.02 | 0.35 | 0.00 | 0.10 | 4 | |
| 24/12 | 0.54 | 1.00 | 0.81 | 0.61 | 3 | |
| 32/16 | 0.94 | 0.36 | 0.49 | 0.70 | 1 | |
| 40/20 | 1.00 | 0.00 | 0.72 | 0.65 | 2 | |
| Weight (Wi) | 0.65 | 0.26 | 0.09 | |||
| Petiole physiology | CK | 0.02 | 0.17 | 1.00 | 0.14 | 5 |
| 4/2 | 0.08 | 0.00 | 0.86 | 0.14 | 6 | |
| 8/4 | 0.00 | 0.46 | 0.00 | 0.10 | 4 | |
| 24/12 | 0.24 | 1.00 | 0.75 | 0.46 | 3 | |
| 32/16 | 0.94 | 0.51 | 0.60 | 0.81 | 1 | |
| 40/20 | 1.00 | 0.14 | 0.00 | 0.71 | 2 | |
| Weight (Wi) | 0.68 | 0.22 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Guo, Z.; Ge, S.; Wang, S.; Liang, L.; Peng, W.; Liu, X.; Huang, X.; Qin, C.; Luo, Z.; et al. Effect of Different Light–Dark Cycles on the Growth and Nutritional Quality of Celery. Agriculture 2025, 15, 2228. https://doi.org/10.3390/agriculture15212228
Guo K, Guo Z, Ge S, Wang S, Liang L, Peng W, Liu X, Huang X, Qin C, Luo Z, et al. Effect of Different Light–Dark Cycles on the Growth and Nutritional Quality of Celery. Agriculture. 2025; 15(21):2228. https://doi.org/10.3390/agriculture15212228
Chicago/Turabian StyleGuo, Kexin, Zheng Guo, Sang Ge, Song Wang, Lirui Liang, Wenjun Peng, Xinyuan Liu, Xiaole Huang, Chi Qin, Zijing Luo, and et al. 2025. "Effect of Different Light–Dark Cycles on the Growth and Nutritional Quality of Celery" Agriculture 15, no. 21: 2228. https://doi.org/10.3390/agriculture15212228
APA StyleGuo, K., Guo, Z., Ge, S., Wang, S., Liang, L., Peng, W., Liu, X., Huang, X., Qin, C., Luo, Z., Ouyang, K., Pan, T., Jiang, C., Li, M., Zheng, Y., Wang, S., & Lu, W. (2025). Effect of Different Light–Dark Cycles on the Growth and Nutritional Quality of Celery. Agriculture, 15(21), 2228. https://doi.org/10.3390/agriculture15212228









