Ecuadorian Littoral Musaceae Producers’ Typification Based on Their Production Systems, Agronomic Management, Biosecurity Measures, and Risk Level Against Foc TR4
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analysis
2.4. Production System Vulnerability
3. Results
3.1. Farms and Their Managers
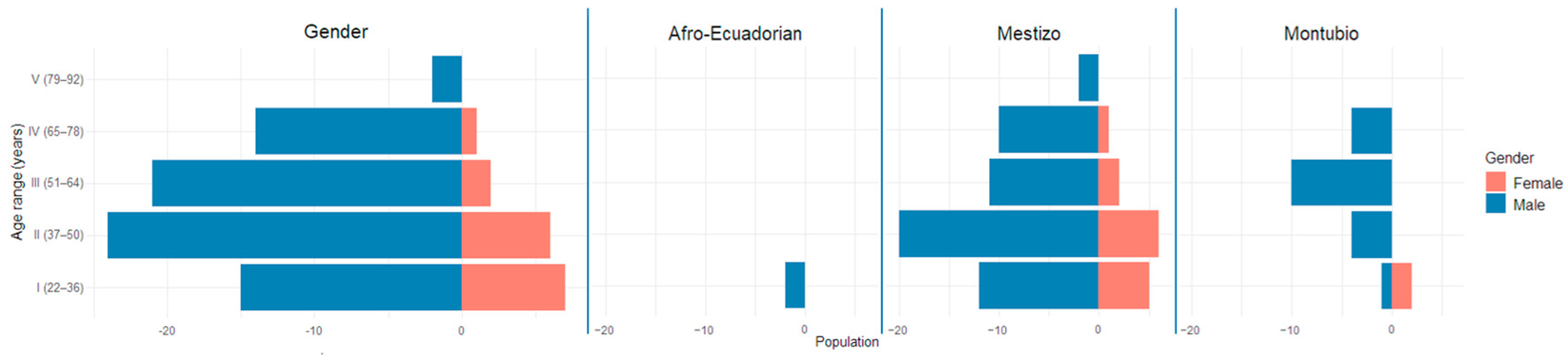
3.2. Sociological Characteristics
3.3. Cultivated Varieties
3.4. Musaceae Production Systems
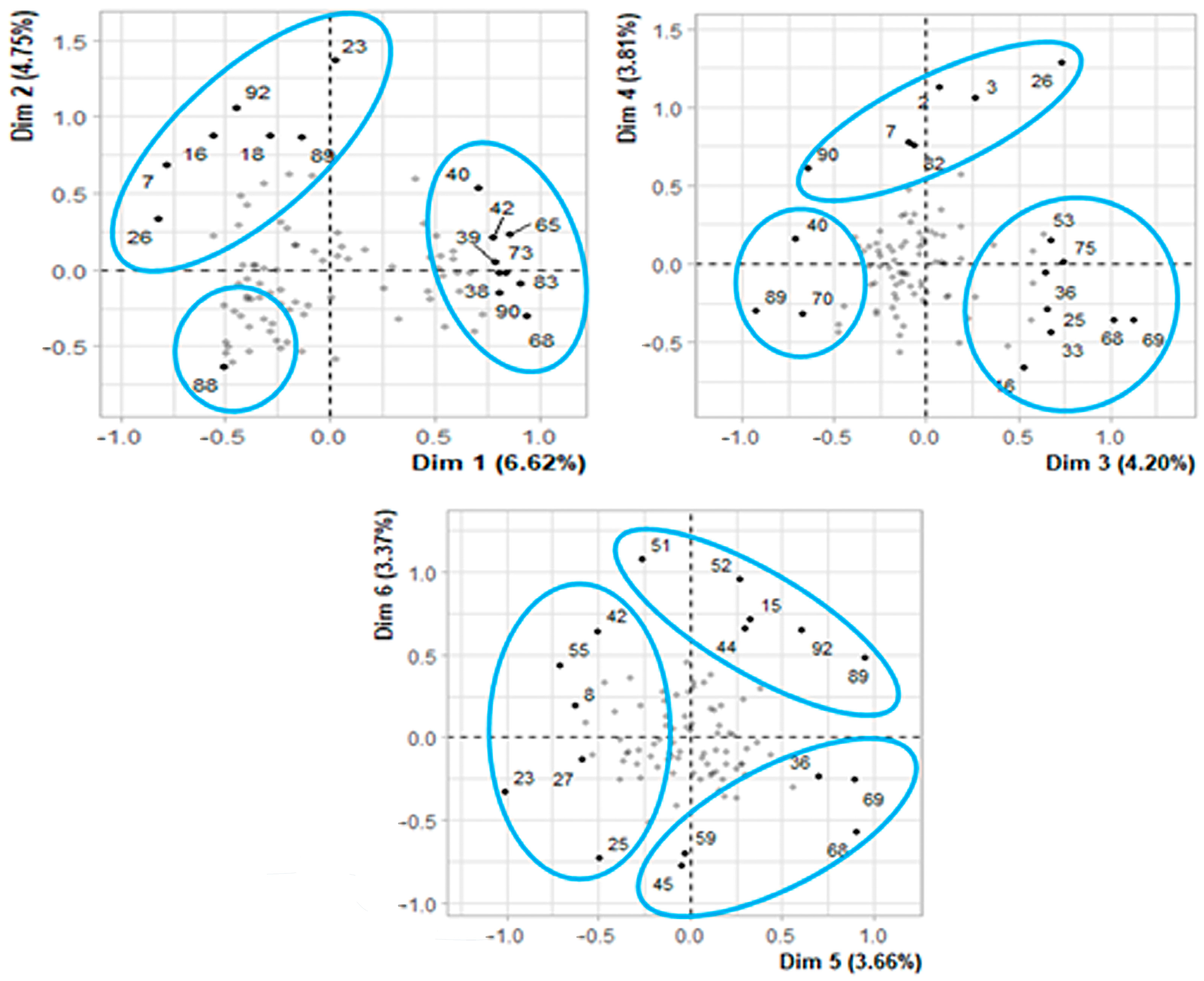
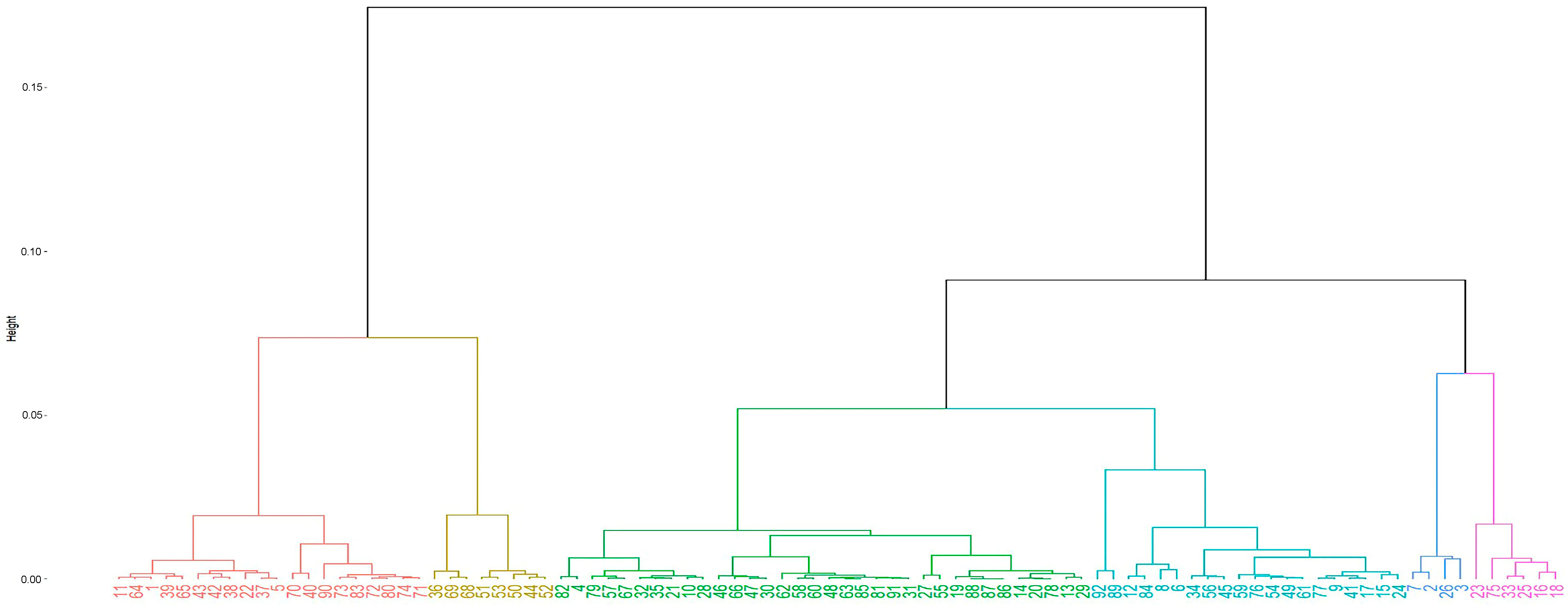
3.5. Producer Typification
3.5.1. Importance of the Considered Variables
3.5.2. Typology of Producers
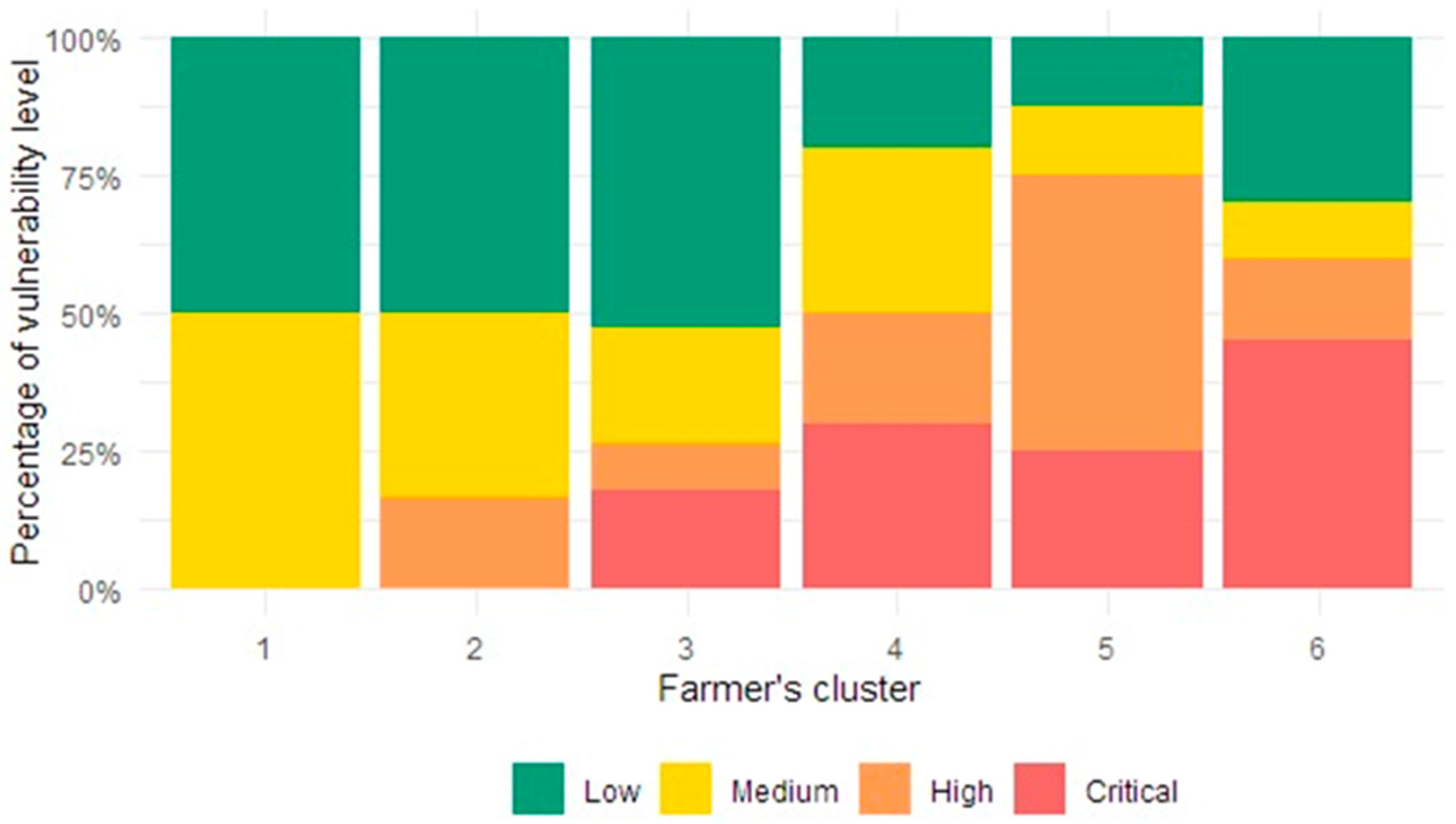
3.6. Production System Vulnerability
4. Discussion
4.1. Age, Ethnicity, Educational Level, and Associativity
4.2. Cultivars and Biosecurity Measures
4.3. Typification of Producers’ Profiles
4.4. Vulnerability Facing Foc TR4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janick, J. The origins of fruits, fruit growing, and fruit breeding. Plant Breed. Rev. 2005, 25, 255–320. [Google Scholar] [CrossRef]
- Martínez, G.; Pargas, R.; Manzanilla, E. Orden Zingiberales: Las musáceas y su relación con plantas afines. Agron. Trop. 2012, 62, 171–178. [Google Scholar]
- WFO. Zingiberales Griseb. 2025. Available online: https://www.worldfloraonline.org/taxon/wfo−9000000577 (accessed on 2 August 2025).
- WFO. Musaceae Juss. 2025. Available online: http://www.worldfloraonline.org/taxon/wfo−7000000398 (accessed on 2 August 2025).
- Sardos, J.; Cenci, A.; Martin, G.; Breton, C.; Guignon, V.; Van den Houwe, I.; Mendez, Y.; Sachter-Smith, G.L.; Chase, R.; Ruas, M. Painting the diversity of a world’s favorite fruit: A next generation catalog of cultivated bananas. Plants People Planet 2024, 7, 263–283. [Google Scholar] [CrossRef]
- Simmonds, N.; Shepherd, K. The taxonomy and origins of the cultivated bananas. Bot. J. Linn. Soc. 1955, 55, 302–312. [Google Scholar] [CrossRef]
- Vera, F. Biodiversidad Intraespecífica Varietal Para Mejorar Ambientes Degradados por Monocultivos en Musáceas, Como Medida de Control de Plagas y Enfermedades. Ph.D. Thesis, Universidad Autónoma de Barcelona, Barcelona, España, 2017. Available online: https://ddd.uab.cat/pub/tesis/2017/hdl_10803_457711/dfva1de1.pdf (accessed on 2 November 2024).
- López, J.A.; Pérez, J. Historia natural de los plátanos y las bananas. Quercus 2011, 212, 54. Available online: http://hdl.handle.net/10261/93714 (accessed on 16 January 2025).
- Suarez, H.F. Rendimiento y Calidad de Grano de Líneas Avanzadas de Trigo Harinero (Triticum aestivum L.) del CIMMYT-México en Condiciones de la CC Tres De Diciembre-Chupaca. Bachelor’s Thesis, Universidad Nacional del Centro del Perú, Mantaro, Perú, 2019. Available online: http://hdl.handle.net/20.500.12894/6451 (accessed on 16 January 2025).
- FAOSTAT. Statistical Database. Food and Agriculture Organization of the United Nations, FAO. 2025. Available online: https://www.fao.org/faostat/es/#data (accessed on 22 January 2025).
- Trujillo-Sandoval, D.; Pereira-Ordóñez, S.; Torres-Cabrera, G. Factores que afectan la variación de los ingresos FOB por exportación de banano y plátano ecuatoriano. Econ. Negoc. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Vera Valdiviezo, N.A.; Bonilla Delgado, M.I.; Ruíz Palomino, P. Competitividad del sector bananero del Ecuador. Un análisis de su evolución. Cienc. Lat. Rev. Cienc. Multidiscip. 2025, 9, 82. [Google Scholar] [CrossRef]
- Villavicencio, J.D.H.; Loja, J.H.C.; Romero, J.M.P. Estrategia Competitiva para la Exportación de Abacá de Ecuador hacia el Mercado de Filipinas 2024. Cienc. Lat. Rev. Cienc. Multidiscip. 2024, 8, 6460–6473. [Google Scholar] [CrossRef]
- Farías, R.; Muñoz, L.; Marcillo, C.; Viteri, M.; Vinueza, J.; Galarza, C.; Cevallos Chóez, J. COVID−19: Impacto en las Exportaciones de Organizaciones de Pequeños Productores, Afectaciones, Desafíos y Oportunidades; Ministro de Producción, Comercio Exterior, Inversiones y Pesca: Quito, Ecuador, 2020. [Google Scholar]
- Mendoza Saltos, M. El Plátano Verde Mueve US$ 210 Millones al año y Ahora es la Vedette de una Guía Culinaria. Forbes Ecuador. 2024. Available online: https://www.forbes.com.ec/lifestyle/el-platano-verde-mueve-us-210-millones-ano-ahora-vedette-una-guia-culinaria-n49410 (accessed on 22 September 2025).
- Zambrano, J. El Plátano Ecuatoriano Gana Terreno en Estados Unidos y Busca Consolidarse en Europa con Productos Procesados. FreshPlaza.es. 2025. Available online: https://www.freshplaza.es/article/9726283/el-platano-ecuatoriano-gana-terreno-en-estados-unidos-y-busca-consolidarse-en-europa-con-productos-procesados/ (accessed on 22 September 2025).
- Jácome Gómez, L.; Martinez, M.C.; De La Cruz Chicaiza, M.; Chica Solórzano, H.; Valencia Enriquez, X. Rendimiento de fibra de dos variedades de Abacá (Musa textiles) en tres densidades de Siembra. Cienc. Lat. Rev. Cienc. Multidiscip. 2023, 7, 3866–3878. [Google Scholar] [CrossRef]
- SIPA. Boletín Situacional de Banano 2024. Retrieved from Sistema de Información Pública Agropecuaria. 2024. Available online: https://sipa.agricultura.gob.ec/boletines/situacionales/2023/boletin_situacional_banano_2023.pdf (accessed on 21 July 2025).
- SIPA. Comercio Exterior del Sector Agropecuario y Agroindustrial Sistema de Información Pública Agropecuaria. 2024. Available online: https://sipa.agricultura.gob.ec/index.php/comext-productos (accessed on 28 July 2025).
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y. New geographical insights of the latest expansion of Fusarium oxysporum f. sp. cubense tropical race 4 into the greater Mekong subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- García-Bastidas, F.; Drenth, A.; Kema, G. The past, present and future of Fusarium wilt of banana caused by Tropical Race 4. In Achieving Sustainable Cultivation of Bananas Volume 3: Diseases and Pests; Drenth, A., Kema, G., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2024; pp. 113–144. [Google Scholar]
- ACORBANEC. 7 Años Aniversario 2024; Asociación de Comercialización Y Exportación de Banano: Guayaquil, Ecuador, 2023. [Google Scholar]
- INEC-ESPAC. Encuesta de Superficie y Producción Agropecuaria Continua-ESPAC Instituto Nacional de Estadísticas y Censos. 2024. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/espac/2024/Presentacion_de_resultados_ESPAC_2024.pdf (accessed on 22 December 2024).
- SIPA. Información Productiva Territorial Sistema de Información Pública Agropecuaria. 2024. Available online: https://sipa.agricultura.gob.ec/index.php/cifras-agroproductivas (accessed on 31 January 2025).
- Velasco, P. Riego en territorios comunales: Oportunidad (o amenaza) para la pequeña economía campesina. El trasvase Chongón San Vicente en la Provincia de Santa Elena. In Economía y las Oportunidades de Desarrollo: Desafíos en América-Latina; Ramos, M., García, L., Eds.; ECORFAN Tópicos Selectos de Economía; Dialnet: México City, México, 2014; Volume 1, pp. 118–142. [Google Scholar]
- Caicedo-Camposano, O.; Soplín-Villacorta, H.; Balmaseda-Espinosa, C.; Cadena-Piedrahita, L.; Leyva-Vázquez, M. Sustentabilidad de sistemas de producción de banano (Musa paradisiaca AAA) en Babahoyo, Ecuador. Rev. Investig. Oper. 2020, 41, 379–388. [Google Scholar]
- López Mejía, F.X.; Muñoz Flórez, J.E.; Vivas Cedeño, J.S.; Cedeño Zambrano, J.R.; Tacuri Troya, E.; Cruzatty Loor, N.M. Caracterización de agrosistemas productores de plátano (Musa AAB) en los cantones Santo Domingo y El Carmen, Ecuador. Idesia 2022, 40, 45–52. [Google Scholar] [CrossRef]
- Narvaez, A.I.; Espinosa, D. Análisis Comparativo Entre los Sistemas de Producción de Banano Orgánico y Convencional en El Oro, Ecuador. Grado, Zamorano: Escuela Agrícola Panamericana, 2021. San Antonio de Oriente, Honduras. 2021. Available online: https://bdigital.zamorano.edu/items/8b495e55-4cf6-4ea0-9893-bc003d2fbe11 (accessed on 22 July 2025).
- Quevedo Carranza, E.I.; Prado Carpio, E.; Valarezo Macías, C.A.; Rentería Minuche, P. Análisis de los Beneficios Económicos y Ambientales: Producción de Banano Orgánico. Cienc. Lat. Rev. Multidiscip. 2024, 8, 6696–6709. [Google Scholar] [CrossRef]
- National Research Council. Genetic Vulnerability of Major Crops; The National Academies Press: Washington, DC, USA, 1972. [Google Scholar]
- Drenth, A.; Kema, G. The vulnerability of bananas to globally emerging disease threats. Phytopathology 2021, 111, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Delgado, W.M.; Barreto, A.O. Revisión integral de los aspectos más importantes causados por Ralstonia solanacearum en musáceas. Polo Conoc. 2024, 9, 570–630. [Google Scholar]
- Betancourt, M.; Dita Rodriguez, M.A.; Saini, E. Agenda Para la Prevención y el Manejo de Brotes de la Raza 4 Tropical de Fusarium (R4T) en el Cultivo de Musáceas en América Latina y el Caribe (ALC); Perez, D., Ed.; BID Lab: Wellington, FL, USA, 2020. [Google Scholar] [CrossRef]
- Munhoz, T.; Vargas, J.; Teixeira, L.; Staver, C.; Dita, M. Fusarium Tropical Race 4 in Latin America and the Caribbean: Status and global research advances towards disease management. Front. Plant Sci. 2024, 15, 1397617. [Google Scholar] [CrossRef]
- Terrero, P.; Rodulfo, P.; Solís, K.; Borja, E. Reconocimiento de Ralstonia Solanacearum Smith Raza 2 (moko) y Medidas de Bioseguridad en Plantaciones de Musáceas Afectadas en Ecuador; Instituto Nacional de Investigaciones Agropecuarias INIAP: Mocache, Ecuador, 2024. [Google Scholar]
- Ibarra-Zapata, E.; Aguirre-Salado, C.A.; Miranda-Aragón, L.; Escoto-Rodríguez, M.; Loredo-Osh, C.; Mora Aguilera, G.; Casiano-Domínguez, M.; Aguirre-Salado, A.I.; Ramos-Méndez, C.; Villegas-Jiménez, N. Análisis geoespacial fitosanitario de la Fusariosis de las Musáceas a nivel global, con énfasis en América Pantropical. Investig. Geogr. 2021. [Google Scholar] [CrossRef]
- Fernández-Ledesma, C.M.; Garcés-Fiallos, F.R.; Rosso, F.; Cordero, N.; Ferraz, S.; Durigon, A.; Portalanza, D. Assessing the risk of Fusarium oxysporum f. sp. cubense Tropical Race 4 outbreaks in Ecuadorian banana crops using spatial climatic data. Sci. Agropecu. 2023, 14, 301–312. [Google Scholar] [CrossRef]
- Álava Murillo, A.G.; Reyes Bermeo, M.d.R.; Tapia Bolaño, R. Estudio socioeconómico de los productores de banano orgánico, Cantón Milagro, Ecuador. Rev. Tecnológica-Espol 2021, 33, 168–180. Available online: http://www.rte.espol.edu.ec/index.php/tecnologica/article/view/869 (accessed on 25 September 2025). [CrossRef]
- Álvarez, E.L.; León, S.A.; Sánchez, M.L.; Cusme, B.L. Evaluación socioeconómica de la producción de plátano en la zona norte de la Provincia de los Ríos. J. Bus. Entrep. Stud. 2020, 4, 86–95. [Google Scholar] [CrossRef]
- Cedeño-Aviles, J.; Vera-Avilés, D.F.; Cabezas-Guerrero, F.; Tubay-Vergara, J.L. Resiliencia de dos sistemas de producción de musáceas en dos zonas del trópico ecuatoriano. Rev. Cienc. Tecnol. 2021, 14, 17–26. [Google Scholar] [CrossRef]
- Lara-García, S.; Vera-Aviles, D.; Cabanilla-Lamulle, M.; González-Osorio, B. Desarrollo comunitario: Producción de Musácea en dos zonas de la costa ecuatoriana. Rev. Cienc. Soc. 2021, 27, 340–354. [Google Scholar] [CrossRef]
- García-Garizábal, I.; Romero, P.; Jiménez, S.; Jordá, L. Evolución climática en la costa de Ecuador por efecto del cambio climático. Dyna 2017, 84, 37–44. [Google Scholar] [CrossRef]
- Porrut, P.; Gómez, G.; Bermeo, A.; Toscano, G. Clima del Ecuador. In El Agua en el Ecuador Clima, Precipitaciones, Escorrentía; Porrut, P., Ed.; Corporación Editora Nacional: Quito, Ecuador, 1995; Volume 7, pp. 13–26. [Google Scholar]
- Varela, A.; Ron, S. Geografía y Clima del Ecuador. BIOWEB. Pontificia Universidad Católica del Ecuador. 2022. Available online: https://bioweb.bio/fungiweb/GeografiaClima/ (accessed on 25 July 2025).
- Winckell, A. Presentación general de los grandes rasgos del relieve del Ecuador. In Los Paisajes Naturales del Ecuador; Winckell, A., Marocco, R., Winter, T., Huttel, C., Pourrut, P., Zebrowski, C., Sourdat, M., Eds.; Centro Ecuatoriano de Investigación Geográfica CEDIG: Quito, Ecuador, 1997; Volume 1, pp. 3–15. [Google Scholar]
- Moreno, J.; Sevillano, G.; Valverde, O.; Loayza, V.; Haro, R.; Zambrano, J. Suelos de la Costa. In Suelos del Ecuador Clasificación, uso y Manejo; Espinoza, J., Moreno, J., Bernal, G., Eds.; Instituto Geográfico Militar (IGM): Quito, Ecuador, 2022; pp. 45–103. [Google Scholar]
- Morales, L.; Sinchigalo, R.; Córdova, A.; Bedoya, M. Producción de Frutas Tropicales en Ecuador: Especialización productiva y función de optimización. Rev. Cienc. UNEMI 2024, 17, 177–193. [Google Scholar] [CrossRef]
- Buendía Eisman, L.; Colás Bravo, M.P.; Hernández Pina, F. Métodos de Investigación en Psicopedagogía; McGraw-Hill: Madrid, Spain, 1998. [Google Scholar]
- Aguilar-Barojas, S. Fórmulas para el cálculo de la muestra en investigaciones de salud. Salud Tabasco 2005, 11, 333–338. [Google Scholar]
- Cochran, W.G. Sampling Techniques; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- National Institute of Statistics. VIII Censo de Población y VII de Vivienda. 2022. Available online: https://www.censoecuador.gob.ec/resultados-censo/#tabulados (accessed on 15 September 2025).
- Martínez Valle, L. La Agricultura Familiar en El Ecuador. Serie Documentos de Trabajo N°147. Grupo de Trabajo: Desarrollo con Cohesión Territorial. Programa Cohesión Territorial para el Desarrollo; Rimisp: Santiago, Chile, 2013. [Google Scholar]
- Hyndman, R.J. The Problem with Sturges’ Rule for Constructing Histograms; Working Paper 1/95; Monash University, Dept. of Econometrics and Business Statistics: Melbourne, Australia, 1995; pp. 1–2. [Google Scholar]
- Builes Gaitan, S.; Duque Ríos, M. Socio-economic and technological typology of avocado cv. Hass farms from Antioquia (Colombia). Ciênc. Rural 2020, 50, e20190188. [Google Scholar] [CrossRef]
- Castel, J.; Mena, Y.; Delgado-Pertıñez, M.; Camúñez, J.; Basulto, J.; Caravaca, F.; Guzmán-Guerrero, J.; Alcalde, M. Characterization of semi-extensive goat production systems in southern Spain. Small Rumin. Res. 2003, 47, 133–143. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Pages, J. Principal Component Methods-Hierarchical Clustering-Partitional Clustering: Why Would We Need to Choose for Visualizing Data. 2010. Available online: http://factominer.free.fr/factomethods/hierarchical-clustering-on-principal-components.html (accessed on 11 January 2025).
- Greenacre, M. La Práctica del Análisis de Correspondencias; Fundación BBVA: Bilbao Spain, 2008. [Google Scholar]
- Foucart, T. Analyse Factorielle Des Données. Programmation sur Micro-Ordinateurs; Masson: Paris, France, 1983. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 11 January 2025).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. CRAN: Contributed Packages. 2021. Available online: https://github.com/kassambara/factoextra (accessed on 11 January 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Vaissie, P.; Monge, A.; Husson, F. Factoshiny Package for R. Rennes. 2020. Available online: https://husson.r-universe.dev/Factoshiny (accessed on 11 January 2025).
- Thuleau, S.; Husson, F. FactoInvestigate: Automatic Description of Factorial Analysis. R Package Version 1.3. 2018. Available online: https://cran.r-project.org/web/packages/FactoInvestigate/index.html (accessed on 11 January 2025).
- Pardo, C.E.; del Campo, P.C.; Pardo, M.C.E. Package ‘FactoClass’. 2018. Available online: https://cloud.r-project.org/web/packages/FactoClass/FactoClass.pdf (accessed on 11 January 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J.; Kalicinski, M.; Valery, K.; Leitienne, C.; Colbert, B.; Hoerl, D.; Miller, E.; Bryan, M.J. Package ‘Readxl’. Version 13.1. 2019. Available online: https://mirror.las.iastate.edu/CRAN/web/packages/readxl/readxl.pdf (accessed on 11 January 2025).
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 11 January 2025).
- Rodríguez-Yzquierdo, G.A.; Becerra-Campiño, J.J.; Miranda-Salas, T.C.; Alzate-Henao, S.V.; Sandoval-Contreras, H.A. Caracterización de tipologías de productores de plátano (Musa AAB) en los Llanos Orientales de Colombia. Temas Agrar. 2019, 24, 129–138. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Blomme, G.; Omondi, A.B.; Ocimati, W. Banana bunchy top disease in Africa—Predicting continent-wide disease risks by combining survey data and expert knowledge. Plant Pathol. 2023, 72, 1476–1490. [Google Scholar] [CrossRef]
- Patwardhan, A.; Semenov, S.; Schnieder, S.; Burton, I.; Magadza, C.; Oppenheimer, M.; Pittock, B.; Rahman, A.; Smith, J.; Suarez, A. Assessing key vulnerabilities and the risk from climate change. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 779–810. [Google Scholar]
- Walpole, R.; Myers, R.; Myers, S.; Ye, K. Probabilidad y Estadística Para Ingeniería y Ciencias; Pearson educación: Mexico City, Mexico, 2012. [Google Scholar]
- Ceme Vinces, R.A.; Boren-Alpizar, A.; Headrick, J.; Doerfert, D.L.; Lamiño Jaramillo, P. A case study regarding Ecuadorian farmers’ perspective on associative work in agriculture. J. Int. Agric. Ext. Educ. 2024, 31, 187–203. [Google Scholar] [CrossRef]
- Adheka, J.; Komoy, J.; Tamaru, C.; Sivirahauma, C.; Dhed’a, D.; Karamura, D.; De Langhe, E.; Swennen, R.; Blomme, G. Banana diversity in the oriental provinces, north-eastern Democratic Republic of Congo. Acta Hortic. 2018, 1196, 255–264. [Google Scholar] [CrossRef]
- Chabi, M.C.; Dassou, A.G.; Dossou-Aminon, I.; Ogouchoro, D.; Aman, B.O.; Dansi, A. Banana and plantain production systems in Benin: Ethnobotanical investigation, varietal diversity, pests, and implications for better production. J. Ethnobiol. Ethnomed. 2018, 14, 78. [Google Scholar] [CrossRef]
- Gold, C.S.; Kiggundu, A.; Abera, A.; Karamura, D. Diversity, distribution and farmer preference of Musa cultivars in Uganda. Exp. Agric. 2002, 38, 39–50. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.-P.; Jenny, C. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Dépigny, S.; Damour, G. Trait-based description of the agronomic and usage potential of a range of plantain varieties from Cameroon. Exp. Agric. 2022, 58, e53. [Google Scholar] [CrossRef]
- Ortiz, R.; Vuylsteke, D. Quantitative variation and phenotypic correlations in banana and plantain. Sci. Hortic. 1998, 72, 239–253. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits–A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Avalos Poaquiza, N.; Acurio Vásconez, R.; Lima Tandazo, L.; Monteros-Altamirano, Á.; Tapia, C.; Sigcha Morales, F.; Sørensen, M.; Paredes Andrade, N. The Morphological and Ecogeographic Characterization of the Musa L. Collection in the Gene Bank of INIAP, Ecuador. Crops 2025, 5, 34. [Google Scholar] [CrossRef]
- Cruz Arevalo, S.D. Diversidad Genética de Plantaciones de Musa acuminata y Musa balbisiana Establecidas en el Ecuador Utilizando RAPD’s. Bachelor’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2017. Available online: https://repositorio.uteq.edu.ec/items/665d8915-c80a-4da9-993a-ddafd8dc37da (accessed on 22 July 2025).
- Higgins, J.; Osorio-Guarín, J.A.; Olave-Achury, C.; Toloza-Moreno, D.L.; Enriquez, A.; Palma, F.D.; Yockteng, R.; De Vega, J.J. Characterising genome composition and large structural variation in banana varietal groups. bioRxiv 2023. [Google Scholar] [CrossRef]
- Maseko, K.H.; Regnier, T.; Meiring, B.; Wokadala, O.C.; Anyasi, T.A. Musa species variation, production, and the application of its processed flour: A review. Sci. Hortic. 2024, 325, 112688. [Google Scholar] [CrossRef]
- Jenny, C.; Sachter-Smith, G.; Breton, C.; Rivallan, R.; Jacquemoud-Collet, J.-P.; Dubois, C.; Chabannes, M.; Lý, N.-S.; Haevermans, T.; Triệu, T.-D. Musa species in mainland Southeast Asia: From wild to domesticate. PLoS ONE 2024, 19, e0307592. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Anastasio, J.G.; Alarcón, A.; García-Ávila, C.d.J.; Ferrera-Cerrato, R.; Quezada-Salinas, A.; Almaraz-Suárez, J.J.; Espinosa-Mendoza, M.; Bocanegra-Flores, D.A.; Hernández-Ramos, L. In vitro inhibition of bacteria against Fusarium oxysporum f. sp. cubense race 2. Rev. Mex. Fitopatol. 2023, 41, 126–142. [Google Scholar] [CrossRef]
- Robinson, J.C.; Saúco, V.G. Bananas and Plantains; CABI: Wallingford, UK, 2010. [Google Scholar]
- Thangavelu, R.; Saraswathi, M.; Uma, S.; Loganathan, M.; Backiyarani, S.; Durai, P.; Raj, E.E.; Marimuthu, N.; Kannan, G.; Swennen, R. Identification of sources resistant to a virulent Fusarium wilt strain (VCG 0124) infecting Cavendish bananas. Sci. Rep. 2021, 11, 3183. [Google Scholar] [CrossRef]
- Carlier, J.; Robert, S.; Roussel, V.; Chilin-Charles, Y.; Lubin-Adjanoh, N.; Gilabert, A.; Abadie, C. Central American and Caribbean population history of the Pseudocercospora fijiensis fungus responsible for the latest worldwide pandemics on banana. Fungal Genet. Biol. 2021, 148, 103528. [Google Scholar] [CrossRef]
- Wang, M.; Kriticos, D.J.; Ota, N.; Brooks, A.; Paini, D. A general trait-based modelling framework for revealing patterns of airborne fungal dispersal threats to agriculture and native flora. New Phytol. 2021, 232, 1506–1518. [Google Scholar] [CrossRef]
- Ji, T.; Altieri, V.; Salotti, I.; Li, M.; Rossi, V. Role of rain in the spore dispersal of fungal pathogens associated with grapevine trunk diseases. Plant Dis. 2024, 108, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Palmero, D.; Rodríguez, J.; De Cara, M.; Camacho, F.; Iglesias, C.; Tello, J. Fungal microbiota from rain water and pathogenicity of Fusarium species isolated from atmospheric dust and rainfall dust. J. Ind. Microbiol. Biotechnol. 2011, 38, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, R.; Casonato, S. The infection of the fruit of ‘Cavendish’banana by Pseudocercospora fijiensis, cause of black leaf streak (black Sigatoka). Eur. J. Plant. Pathol. 2019, 155, 779–787. [Google Scholar] [CrossRef]
- Pérez-Vicente, L. A Holistic Integrated Management Approach to Control Black Sigatoka Disease of Banana Caused by Mycosphaerella Fijiensis; Food and Agriculture Organization of the United Nations: La Havana, Cuba, 2012; p. 29. [Google Scholar]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Rieux, A.; Soubeyrand, S.; Bonnot, F.; Klein, E.K.; Ngando, J.E.; Mehl, A.; Ravigne, V.; Carlier, J.; De Lapeyre de Bellaire, L. Long-distance wind-dispersal of spores in a fungal plant pathogen: Estimation of anisotropic dispersal kernels from an extensive field experiment. PLoS ONE 2014, 9, e103225. [Google Scholar] [CrossRef]
- Dissanayake, M.; Herath, H.; Jayasekara, H.; Abeywickrame, P. Efficacy of botanical mixture and fungicides to combat sigatoka disease in banana cultivation. Asian J. Mycol. 2023, 6, 26–35. [Google Scholar] [CrossRef]
- Arango Isaza, R.E.; Diaz-Trujillo, C.; Dhillon, B.; Aerts, A.; Carlier, J.; Crane, C.F.; de Jong, T.V.; De Vries, I.; Dietrich, R.; Farmer, A.D. Combating a global threat to a clonal crop: Banana black Sigatoka pathogen Pseudocercospora fijiensis (synonym Mycosphaerella fijiensis) genomes reveal clues for disease control. PLoS Genet. 2016, 12, e1005876. [Google Scholar] [CrossRef] [PubMed]
- Churchill, A.C. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: Progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Mol. Plant Pathol. 2011, 12, 307–328. [Google Scholar] [CrossRef] [PubMed]
- García Regalado, J.; Marcillo Plaza, A.; Palacios Sánchez, C. Amenazas de las manchas foliares de Sigatoka, Mycosphaerella spp., en la producción sostenible de banano en el Ecuador. Rev. Verde Agroecol. Desenv. Sustent. 2019, 14, 591–596. [Google Scholar]
- Cedeño García, G.; Suarez Capello, C.; Vera Coello, D.; Fadda, C.; Jarvis, D.; de Santis, P. Detección temprana de resistencia a Mycosphaerella fijiensis en genotipos locales de Musáceas en Ecuador. Sci. Agropecu. 2017, 8, 29–42. [Google Scholar] [CrossRef][Green Version]
- Brenes-Gamboa, S. Production and quality parameters of three banana cultivars FHIA−17, FHIA−25 and Yangambi. Agron. Mesoam. 2017, 28, 719–733. [Google Scholar] [CrossRef]
- Ramírez Céspedes, C.; Tapia Fernández, A.C.; Calvo Brenes, P. Evaluación de la calidad de fruta de banano de altura que se produce en el cantón de Turrialba, Costa Rica. InterSedes 2010, 11, 107–127. Available online: https://www.redalyc.org/articulo.oa?id=66619992007 (accessed on 11 March 2025).
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Institute: Kew, UK, 1962. [Google Scholar]
- Jiménez, B. Establecimiento de un Banco de Musáceas con Cuatro Variedades en el Centro de Investigación Sacha Wiwa-Guasaganda, Cantón La Maná. Bachelor’s Thesis, Universidad Técnica de Cotopaxi, Facultad de Ciencias Agropecuarias y Recursos Naturales, Latacunga, Ecuador, 2020. Available online: http://repositorio.utc.edu.ec/handle/27000/6933 (accessed on 5 May 2025).
- Quiroz, J. Efecto del Desbellote y Eliminación de Manos, en el Rendimiento y Calidad del Banano Orito (Musa Acuminata AA) en la Zona de Cumandá. Bachelor’s Thesis, Escuela Superior Politécnica del Litoral, Guayaquil, Ecuador, 2007. Available online: http://www.dspace.espol.edu.ec/handle/123456789/40068 (accessed on 18 August 2025).
- Díaz-González, A.M.; Ortega, J.; Seoane, C.; Morales Opazo, C. Tipología de Microrregiones en el Sector Agrícola de Ecuador: Una Herramienta Para Priorizar Inversiones en el Marco de la Iniciativa Mano de la Mano; Economía del Desarrollo Agrícola de la FAO—Estudio Técnico N.o 22: Roma, Italy, 2022. [Google Scholar] [CrossRef]
- Tamayo, C.; Cepeda, D. El dilema constante del productor bananero en tiempos de brete: ¿Asociatividad o individualismo? In Mosaico Agrario: Diversidades y Antagonismos Socio-Económicos en el Campo Ecuatoriano; Vaillant, M., Cepeda, D., Gondard, P., Zapatta, A., Meunier, A., Eds.; SIPAE-IRD-IFEA: Quito, Ecuador, 2007. [Google Scholar]
- AGROCALIDAD. Resolución 0110 Pp. 49. Ministerio de Agricultura y Ganadería. Agencia de Regulación y Control Fito y Zoosanitario. 2019. Available online: https://faolex.fao.org/docs/pdf/ecu223686.pdf (accessed on 15 May 2025).
- Soto Galarza, B.; Campos Vera, R.J.; Lopez Pincay, P.R. Gestión De Prevención Del Hongo Fusarium Raza En La Producción Bananera De Ecuador. Repique 2020, 2, 69–86. [Google Scholar] [CrossRef]
- AGROCALIDAD. Resolución 0072. Ministerio de Agricultura y Ganadería, Agencia de Regulación y Control Fito y Zoosanitario. 2022. Available online: https://faolex.fao.org/docs/pdf/ecu167368.pdf (accessed on 21 May 2025).
- Fortini, R.; Braga, M.J.; De Freitas, C.O.; Delgado, K.A.V. Prácticas agrícolas conservacionistas en Brasil:¿ cuáles son los factores asociados a su adopción? Stud. Appl. Econ. 2020, 38, 2949. [Google Scholar] [CrossRef]
- Plant Health Australia. The National Plant Biosecurity Status Report; Plant Health Australia: Canberra, Australia, 2020. [Google Scholar]
- FAO. Managing transboundary pests and diseases and their economic impacts. In The State of Food and Agriculture 2001; FAO, Ed.; FAO: Rome, Italy, 2001; pp. 250–267. [Google Scholar]
- Holligan, E.; Cook, S.; Poggio, M.; Rattray, D. Economic Assessment of Best Management Practices for Banana Growing; Department of Agriculture and Fisheries (DAF) and the Department of Natural Resources and Mines (DNRM): Queensland, Australia, 2017. Available online: https://era.dpi.qld.gov.au/id/eprint/9174/1/project-technical-report-bananas.pdf (accessed on 6 August 2025).
- Grajales-Amorocho, M.; Acosta-Minoli, C.; Muñoz-Pizza, D.; Manrique-Arias, O.; Munoz-Loaiza, A. Analysis of Moko disease propagation on plantain (Musa AAB Simmonds) through a model based on system dynamics. Eur. J. Plant Pathol. 2024, 168, 437–445. [Google Scholar] [CrossRef]
- Ritter, T.; Álvarez, D.; Mosquera, L.E.; Martey, E.; Mockshell, J. A socioeconomic and cost benefit analysis of Tropical Race 4 (TR4) prevention methods among banana producers in Colombia. PLoS ONE 2024, 19, e0311243. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vicente, L. Las mejores prácticas para la prevención de la raza 4 tropical de la marchitez por Fusarium y otras enfermedades exóticas en fincas bananeras. Fitosanidad 2015, 19, 243–250. Available online: https://fitosanidad.edicionescervantes.com/index.php/fitosanidad/article/view/153 (accessed on 6 August 2025).
- Bernués, A.; Herrero, M. Farm intensification and drivers of technology adoption in mixed dairy-crop systems in Santa Cruz, Bolivia. Span. J. Agric. Res. 2008, 2, 279–293. [Google Scholar] [CrossRef]
- Kassa, G.; Bekele, T.; Demissew, S.; Abebe, T. Plant species diversity, plant use, and classification of agroforestry homegardens in southern and southwestern Ethiopia. Heliyon 2023, 9, e16341. [Google Scholar] [CrossRef] [PubMed]
- Running, K.; Burnham, M.; Wardropper, C.; Ma, Z.; Hawes, J.; Du Bray, M.V. Farmer adaptation to reduced groundwater availability. Environ. Res. Lett. 2019, 14, 115010. [Google Scholar] [CrossRef]
- Iradukunda, F.; Bullock, R.; Rietveld, A.; van Schagen, B. Understanding gender roles and practices in the household and on the farm: Implications for banana disease management innovation processes in Burundi. Outlook Agric. 2019, 48, 37–47. [Google Scholar] [CrossRef]
- Norgrove, L.; Hauser, S. Improving plantain (Musa spp. AAB) yields on smallholder farms in West and Central Africa. Food Secur. 2014, 6, 501–514. [Google Scholar] [CrossRef]
- Ssebulime, G.; Kagezi, H.; Nyombi, K.; Mpiira, S.; Kucel, P.; Byabagambi, S.; Tushemereirwe, K.; Kubiriba, J.; Karamura, B.; Stave, C. Farmers’ knowledge of the banana (Musa sp.) agroforestry systems in Kiboga District, Central Uganda. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12557–12573. [Google Scholar] [CrossRef]
- Papić Milojević, R.; Bogdanov, N. Typology of Farms in Areas with Natural Constraints–Diversity of Livelihood Strategies and their Determinants. Appl. Ecol. Environ. Res. 2024, 22, 1051–1073. [Google Scholar] [CrossRef]
- Nyambo, D.G.; Luhanga, E.T.; Yonah, Z.Q. A review of characterization approaches for smallholder farmers: Towards predictive farm typologies. Sci. World J. 2019, 2019, 6121467. [Google Scholar] [CrossRef]
- Alvarez, S.; Timler, C.J.; Michalscheck, M.; Paas, W.; Descheemaeker, K.; Tittonell, P.; Andersson, J.A.; Groot, J.C. Capturing farm diversity with hypothesis-based typologies: An innovative methodological framework for farming system typology development. PLoS ONE 2018, 13, e0194757. [Google Scholar] [CrossRef]
- Bellamy, A.S. Banana production systems: Identification of alternative systems for more sustainable production. Ambio 2013, 42, 334–343. [Google Scholar] [CrossRef]
- Guamán, S.E. Análisis Estadístico Multivariante Para el Estudio de los Factores que Influyen en la Producción del Plátano en el Ecuador, Periodo 2014–2016. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2018. Available online: https://core.ac.uk/reader/578388900 (accessed on 10 August 2025).
- Cepeda, D.; Gondard, P.; Gasselin, P. Mega diversidad agraria en el Ecuador: Disciplina, conceptos y herramientas metodológicas para el análisis-diagnóstico de micro-regiones. In Mosaico Agrario: Diversidades y Antagonismos Socio-Económicos en el Campo Ecuatoriano; Vaillant, M., Cepeda, D., Gondard, P., Zapatta, A., Meunier, A., Eds.; SIPAE-IRD-IFEA: Quito, Ecuador, 2007; pp. 29–54. [Google Scholar]
- Pocasangre, L.E.; Pérez-Vicente, L.; Ferris, H. Organic Banana Disease Management. In Plant Diseases and Their Management in Organic Agriculture; Finckh, M.R., Van Bruggen, A., Tamm, L., Eds.; Am Phytopath Society: St. Paul, MN, USA, 2015; pp. 351–365. [Google Scholar]
- COE. Estrategia Nacional para la Prevención, Detección y Control de la Plaga Foc R4T; Comité de Operaciones de Emergencia (COE): Quito, Ecuador, 2021. [Google Scholar]
- Numminen, E.; Laine, A.-L. The spread of a wild plant pathogen is driven by the road network. PLoS Comput. Biol. 2020, 16, e1007703. [Google Scholar] [CrossRef]
- Tamayo, C.; Gaybor, A. Sistemas agrarios bananeros en La Mana. Rumipamba 2008, 22, 1–12. [Google Scholar]
- Brown, A.H. Indicators of Genetic Diversity, Genetic Erosion and Genetic Vulnerability for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2008. [Google Scholar]
- Pattison, A.B.; Wright, C.L.; Kukulies, T.; Molina, A. Ground cover management alters development of Fusarium wilt symptoms in Ducasse bananas. Australas. Plant Pathol. 2014, 43, 465–476. [Google Scholar] [CrossRef]
- Teixeira, L.; Heck, D.; Nomura, E.; Vieira, H.; Dita, M. Soil attributes, plant nutrition, and Fusarium wilt of banana in São Paulo, Brazil. Trop. Plant Pathol. 2021, 46, 443–454. [Google Scholar] [CrossRef]
- Román, C.H. Consideraciones Epidemiológicas Para el Manejo de la Marchitez por Fusarium (Fusarium oxysporum f. sp. cubense) del Banano en la Región Central del Perú. In II Congreso Latinoamericano y del Caribe de Plátanos y Bananos—Colombia, Colombia; CATIE: Turrialba, Costa Rica, 2012; Available online: https://repositorio.catie.ac.cr/handle/11554/926 (accessed on 10 May 2025).
- Brown, A.H. Variation under domestication in plants: 1859 and today. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2523–2530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MAG. Acuerdo Ministerial N.º 142: Plan Nacional de Contingencia para la Prevención, Detección y Control de Fusarium oxysporum f. sp. cubense Raza 4 Tropical (Foc R4T). Ministerio de Agricultura y Ganadería (MAG); Agencia de Regulación y Control Fito y Zoosanitario (AGROCALIDAD): Quito, Ecuador, 2020. [Google Scholar][Green Version]
- Dita, M.; Echegoyén Ramos, P.; Pérez Vicente, L. Plan de Contingencia Ante un Brote de la Raza 4 Tropical de Fusarium oxysporum f. sp. Cubense en un País de la Región del OIRSA; OIRSA: San Salvador, El Salvador, 2017. [Google Scholar][Green Version]
- INIAP. Informe Anual; Programa de Banano, Plátano y otras Musáceas; Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 2024; Available online: https://repositorio.iniap.gob.ec/handle/41000/6410 (accessed on 24 June 2025).[Green Version]
- Gallo, M.A. Análisis del Riesgo de Introducción de Fusarium oxysporum f. sp. Cubense Raza 4 Tropical (Foc R4T) Plaga Cuarentenaria Para el Ecuador. Master’s Thesis, Universidad Técnica de Cotopaxi, Latacunga, Ecuador, 2021. Available online: http://repositorio.utc.edu.ec/handle/27000/7695 (accessed on 28 May 2025).[Green Version]
- FAO. Fibres of the Future. 2025. Available online: https://www.fao.org/economic/futurefibres/pagina-principal-de-fibras-del-futuro/es/ (accessed on 21 September 2025).[Green Version]
- El Universo. Finca Bananera en Cuarentena: Ecuador Reporta Primer Foco de ‘Fusarium’. El Universo (Guayaquil, Ecuador). 2025. Available online: https://www.eluniverso.com/noticias/economia/fusarium-raza-4-en-ecuador-primer-foco-banano-el-oro-cuarentena-nota/ (accessed on 24 September 2025).[Green Version]



| Sections | Variable | Coding | Question Type | Response Type |
|---|---|---|---|---|
| Personal aspects of the producer | Producer age | AgeRang | Open-ended | Quantitative continuous |
| Producer gender | ProdGend | Closed-ended | Qualitative nominal | |
| Property type | PropType | Open-ended | Qualitative nominal | |
| Ethnic Group | Ethnic | Open-ended | Qualitative nominal | |
| Education Level | EduLev | Open-ended | Qualitative ordinal | |
| Belongs to an association | Assoc | Open-ended | Qualitative nominal | |
| How many years have you been producing Musaceae? | ProdRang | Open-ended | Quantitative continuous | |
| Geographical location of the farm | Province | Province | Open-ended | Qualitative nominal |
| Generalities of cultivation | Cultivation system? | PlanSys | Open-ended | Qualitative nominal |
| Types of plantation? | Plant | Open-ended | Qualitative nominal | |
| Types of cultivars | CultType | Open-ended | Qualitative nominal | |
| What method of propagation do you perform? | Propag | Closed-ended | Qualitative nominal | |
| Cultivated area? | CropAreaRang | Open-ended | Qualitative ordinal | |
| What irrigation system do you use? | IrrigSys | Closed-ended | Qualitative nominal | |
| Phytosanitary problems and plantation management | Do you have problems with weevil? | Picudo | Closed-ended | Qualitative ordinal |
| Do you have problems with cochineal? | Cochinilla | Closed-ended | Qualitative ordinal | |
| Having problems with caterpillar? | Caterpillar | Closed-ended | Qualitative ordinal | |
| Do you have problems with trips? | Trips | Closed-ended | Qualitative ordinal | |
| Do you have problems with whiteflies? | Whitefly | Closed-ended | Qualitative ordinal | |
| Do you have problems with nematodes? | Nemat | Closed-ended | Qualitative ordinal | |
| Do you have problems with Fusarium? | Fusarium | Closed-ended | Qualitative ordinal | |
| Do you have problems with sigatoka? | Sigatoka | Closed-ended | Qualitative ordinal | |
| Do you have problems with moko? | Moko | Closed-ended | Qualitative ordinal | |
| Do you have problems with viral diseases? | Viruses | Closed-ended | Qualitative ordinal | |
| What kind of pest and disease control do you use? | PhytoCon | Closed-ended | Qualitative nominal | |
| What soil additives do you use? | Edaph | Open-ended | Qualitative nominal | |
| Biosecurity measures | What biosecurity measures do you use? | BioSafe | Open-ended | Qualitative nominal |
| Classes | Producers’ Age | Years to Musaceae Production | Plantation Area (ha) |
|---|---|---|---|
| I | 22–36 | 1–13 | 1–46 |
| II | 36–50 | 13–25 | 46–91 |
| II | 50–64 | 25–37 | 91–136 |
| IV | 64–78 | 37–49 | 136–181 |
| V | 78–92 | 49–61 | 181–226 |
| Factors | Dimension | Indicator | Operational definition | Label |
|---|---|---|---|---|
| Exposure (risk level) | Proximity to the entry points of the country | B1. Distance to seaports | Distance in kilometres in a straight line from the farm to the nearest seaport | 1. >400 km 2. 400–250 km 3. 250–104 km 4. 100–0 km |
| B2. Distance to the Colombian land border (north) | Distance in kilometres in a straight line from the farm to the Rumichaca International Bridge | 1. >400 km 2. 400–250 km 3. 250–104 km 4. 100–0 km | ||
| B3. Distance to the Peru land border (south) | Distance in kilometres in a straight line from the farm to the Huaquillas International Bridge | 1. >400 km 2. 400–250 km 3. 250–104 km 4. 100–0 km | ||
| Biosecurity | B4. Biosecurity measures | The presence of biosecurity measures on the farm | 1. Biosecurity measures are not applied 0. Apply biosecurity measures | |
| Sensitivity (crop susceptibility) | Genetic vulnerability | A1. Cultivar resistance or susceptibility | The predominant variety grown on the farm is susceptible or not to Foc TR4 | 0. Resistant 1. Susceptible |
| A2. Genetic diversity | The farm has a monoculture or is associated with other varieties or species | 1. Monoculture 2. Associated | ||
| Environmental vulnerability | A3. Production type | Chemical or organic synthesis products are used on the farm | 1. Conventional 2. Organic | |
| A4. Sanitary product access | Use or non-use of external products for disease and pest control | 1. Chemicals 2. Biological | ||
| A5. Planting material origin | The crop is established with certified planting material | 0. Corms or seedlings 1. Vitroplants | ||
| Socioeconomics | Management capacity | C1. Cultivated area | Number of hectares used for the main crop | 1. Little 2. Medium 3. Big |
| C2. Associativity | The farm owner belongs to an organization that can provide support or financing | 1. Belongs to an organization 2. Does not belong to any organization | ||
| Education | C3. Formal instruction | The farm owner completed higher education (university degree) | 0. Does not have higher education 1. Has higher education |
| Province | Persons Interviewed | Area | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | Sampling % | Men | Women | Total | Sampling % | |||||
| Count | % | Count | % | (ha) | % | (ha) | % | |||||
| Littoral | 69 | 71.88 | 14 | 14.58 | 83 | 86.46 | 2020 | 91.49 | 128 | 5.80 | 2148 | 97.28 |
| El Oro | 4 | 4.17 | 3 | 3.13 | 7 | 7.3 | 49 | 2.22 | 62 | 2.81 | 111 | 5.03 |
| Esmeraldas | 9 | 9.38 | 1 | 1.04 | 10 | 10.42 | 53 | 2.40 | 1 | 0.05 | 54 | 2.45 |
| Guayas | 11 | 11.46 | 4 | 4.17 | 15 | 15.63 | 523 | 23.69 | 42 | 1.90 | 565 | 25.59 |
| Los Ríos | 16 | 16.67 | 1 | 1.04 | 17 | 17.71 | 745 | 33.74 | 4 | 0.18 | 749 | 33.92 |
| Manabí | 14 | 14.58 | 4 | 4.17 | 18 | 18.75 | 43 | 1.95 | 17 | 0.77 | 60 | 2.72 |
| Santa Elena | 3 | 3.13 | 0 | 0 | 3 | 3.13 | 341 | 15.44 | 341 | 15.44 | ||
| Santo Domingo | 12 | 12.5 | 1 | 1.04 | 13 | 13.54 | 266 | 12.05 | 2 | 0.09 | 268 | 12.14 |
| Andean | 11 | 11.46 | 2 | 2.08 | 13 | 13.54 | 49 | 2.22 | 11 | 0.50 | 60 | 2.72 |
| Bolívar | 4 | 4.17 | 2 | 2.08 | 6 | 6.25 | 31 | 1.40 | 11 | 0.50 | 42 | 1.90 |
| Chimborazo | 1 | 1.04 | 0 | 0 | 1 | 1.04 | 2 | 0.09 | 0 | 0 | 2 | 0.09 |
| Cotopaxi | 3 | 3.13 | 0 | 0 | 3 | 3.13 | 7 | 0.32 | 0 | 0 | 7 | 0.32 |
| Imbabura | 1 | 1.04 | 0 | 0 | 1 | 1.04 | 6 | 0.27 | 0 | 0 | 6 | 0.27 |
| Pichincha | 2 | 2.08 | 0 | 0 | 2 | 2.08 | 3 | 0.14 | 0 | 0 | 3 | 0.14 |
| Total | 80 | 83.33 | 16 | 16.67 | 96 | 100 | 2069 | 93.70 | 139 | 6.30 | 2208 | 100.00 |
| Education Level | Gender | Ethnicity | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | Total | Afro-Ecuadorian | Mestizo | Montubio | Total | |
| Incomplete primary | 3.8 | 0 | 3.1 | 0 | 2.9 | 4.2 | 3.1 |
| Complete primary | 22.5 | 31.3 | 24 | 50 | 24.3 | 20.8 | 24 |
| Incomplete secondary | 11.3 | 12.5 | 11.5 | 0 | 7.1 | 25 | 11.5 |
| Complete secondary | 18.8 | 18.8 | 18.8 | 0 | 18.6 | 20.8 | 18.8 |
| Incomplete university | 3.8 | 18.8 | 6.3 | 0 | 8.6 | 0 | 6.3 |
| Complete university | 32.5 | 12.5 | 29.2 | 50 | 31.4 | 20.8 | 29.2 |
| Fourth level | 7.5 | 6.3 | 7.3 | 0 | 7.1 | 8.3 | 7.3 |
| Total | 83.3 | 16.7 | 100.0 | 2.1 | 72.9 | 25.0 | 100.0 |
| Associated | 18.8 | 0 | 15.6 | 0 | 12.9 | 25 | 15.6 |
| Non-associated | 81.2 | 100 | 84.4 | 100 | 87.1 | 75 | 84.4 |
| Total | 83.3 | 16.7 | 100.0 | 2.1 | 72.9 | 25.0 | 100.0 |
| Province | Abacá | Barraganete | Dominico | Dominico Hartón | Hartón | Maqueño | Cavendish | Gros Michel | Orito | Valery | Williams | Overall Total | Number of Farms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bolívar | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 31 | 4 | 0 | 0 | 42 | 6 |
| Chimborazo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 |
| Cotopaxi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 7 | 3 |
| El Oro | 0 | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 84 | 111 | 7 |
| Esmeraldas | 39 | 6 | 2 | 0 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 54 | 10 |
| Guayas | 0 | 0 | 9 | 3 | 1 | 30 | 2 | 8 | 5 | 0 | 507 | 565 | 1 |
| Imbabura | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 17 |
| Los Ríos | 15 | 22 | 0 | 0 | 5 | 0 | 0 | 149 | 4 | 45 | 509 | 749 | 18 |
| Manabí | 0 | 40 | 1 | 0 | 18 | 0 | 0 | 1 | 0 | 0 | 0 | 60 | 2 |
| Pichincha | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Santa Elena | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 341 | 341 | 13 |
| Santo Domingo | 50 | 30 | 17 | 0 | 8 | 0 | 0 | 2 | 16 | 75 | 70 | 268 | 6 |
| Overall total | 104 | 105 | 32 | 3 | 38 | 30 | 29 | 206 | 34 | 120 | 1511 | 2212 | |
| Number farms | 7 | 17 | 7 | 1 | 13 | 1 | 4 | 15 | 8 | 2 | 21 | 96 |
| Category/Cultivar | Cultivars | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abacá | Banana | Plantain | |||||||||
| Cavendish | Gros Michel | Valery | Williams | Orito | Barraganete | Dominico | Dominico Hartón | Hartón | Maqueño | ||
| GENDER | |||||||||||
| Man | 7.29 | 3.13 | 11.46 | 2.08 | 17.71 | 7.29 | 14.53 | 6.25 | 1.04 | 11.46 | 1.04 |
| Woman | 0 | 1.04 | 4.17 | 0 | 4.17 | 1.04 | 3.13 | 1.04 | 0 | 2.08 | 0 |
| AGE CLASS (YEARS) | |||||||||||
| I (22–36) | 0 | 2.08 | 2.08 | 0 | 4.17 | 2.08 | 5.21 | 1.04 | 0 | 1.04 | 0 |
| II (37–50) | 2.08 | 2.08 | 2.08 | 2.08 | 11.46 | 3.13 | 3.13 | 4.17 | 0 | 4.17 | 1.04 |
| III (51–64) | 2.08 | 0 | 5.21 | 0 | 3.13 | 2.08 | 5.21 | 2.08 | 1.04 | 4.17 | 0 |
| IV (65–78) | 3.13 | 0 | 4.17 | 0 | 3.13 | 1.04 | 4.17 | 0 | 0 | 4.17 | 0 |
| V (79–92) | 0 | 0 | 2.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ETHNICITY | |||||||||||
| Afro-Ecuadorian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.08 | 0 | 0 | 0 |
| Mestizo | 5.21 | 4.17 | 13.54 | 1.04 | 16.67 | 8.33 | 10.42 | 3.13 | 0 | 10.42 | 0 |
| Montubio | 2.08 | 0 | 2.08 | 1.04 | 5.21 | 0 | 7.29 | 2.08 | 1.04 | 3.17 | 1.04 |
| EDUCATION LEVEL | |||||||||||
| Complete primary | 4.17 | 0 | 6.25 | 0 | 0 | 2.08 | 3.13 | 3.13 | 1.04 | 3.13 | 1.04 |
| Incomplete primary | 1.04 | 0 | 1.04 | 0 | 0 | 0 | 1.04 | 0 | 0 | 0 | 0 |
| Complete secondary | 2.8 | 1.04 | 0 | 1.04 | 4.17 | 1.04 | 4.17 | 0 | 0 | 5.21 | 0 |
| Incomplete secondary | 0 | 0 | 3.13 | 1.04 | 2.08 | 1.04 | 2.08 | 2.08 | 0 | 0 | 0 |
| Complete university | 0 | 2.08 | 4.17 | 0 | 12.5 | 3.13 | 2.08 | 2.08 | 0 | 3.13 | 0 |
| Incomplete university | 0 | 1.04 | 1.04 | 0 | 0 | 1.04 | 2.08 | 0 | 0 | 1.04 | 0 |
| Fourth level | 0 | 0 | 0 | 0 | 3.13 | 0 | 3.13 | 0 | 0 | 1.04 | 0 |
| BIOSECURITY MEASURES | |||||||||||
| Disinfection arch | 0 | 0 | 0 | 1.04 | 0 | 0 | 0 | 0 | 0 | 1.04 | 0 |
| Tool disinfection | 0 | 0 | 1.04 | 0 | 0 | 0 | 4.17 | 0 | 0 | 1.04 | 0 |
| Footbath | 0 | 2.08 | 0 | 0 | 4.17 | 2.08 | 1.04 | 0 | 0 | 1.04 | 1.04 |
| Wheelbath | 0 | 0 | 0 | 0 | 1.04 | 0 | 0 | 0 | 0 | 0 | 0 |
| All possible | 0 | 1.04 | 2.08 | 1.04 | 10.42 | 2.08 | 0 | 0 | 0 | 1.04 | 0 |
| None | 7.29 | 1.04 | 12.5 | 0 | 6.25 | 4.17 | 12.5 | 7.29 | 1.04 | 9.38 | 0 |
| Category | Biosecurity Measures | ||||||
|---|---|---|---|---|---|---|---|
| None | Tool Disinfection | Footbath | Wheelbath | Disinfection Arch | All Possible | Overall Total | |
| GENDER | |||||||
| Man | 51.04 | 5.21 | 9.38 | 1.04 | 2.08 | 14.58 | 83.33 |
| Woman | 10.42 | 1.04 | 2.08 | 0 | 0 | 3.13 | 16.67 |
| AGE CLASS (YEARS) | |||||||
| I (22–36) | 10.42 | 1.04 | 1.04 | 0 | 1.04 | 4.17 | 17.71 |
| II (37–50) | 16.67 | 2.08 | 7.29 | 1.04 | 1.04 | 7.29 | 35.41 |
| III (51–64) | 18.75 | 2.08 | 2.08 | 0 | 0 | 2.08 | 24.99 |
| IV (65–78) | 13.54 | 1.04 | 1.04 | 0 | 0 | 4.17 | 19.79 |
| V (79–92) | 2.08 | 0 | 0 | 0 | 0 | 0 | 2.08 |
| ETHNICITY | |||||||
| Afro-Ecuadorian | 2.08 | 0 | 0 | 0 | 0 | 0 | 2.08 |
| Mestizo | 42.71 | 5.21 | 8.33 | 1.04 | 1.04 | 14.58 | 72.91 |
| Montubio | 16.67 | 1.04 | 3.13 | 0 | 1.04 | 3.13 | 25.01 |
| EDUCATION LEVEL | |||||||
| Complete primary | 19.79 | 2.08 | 2.08 | 0 | 0 | 0 | 23.95 |
| Incomplete primary | 3.13 | 0 | 0 | 0 | 0 | 0 | 3.13 |
| Complete secondary | 12.5 | 2.08 | 1.04 | 0 | 0 | 3.13 | 18.75 |
| Incomplete secondary | 8.33 | 0 | 1.04 | 1.04 | 1.04 | 0 | 11.45 |
| Complete university | 10.42 | 1.04 | 6.25 | 0 | 0 | 11.46 | 29.17 |
| Incomplete university | 3.13 | 0 | 1.04 | 0 | 1.04 | 1.04 | 6.25 |
| Fourth level | 4.17 | 1.04 | 0 | 0 | 0 | 2.08 | 7.29 |
| CULTIVATED AREA CLASS | |||||||
| I (1–46) | 58.33 | 6.25 | 10.42 | 0 | 1.04 | 9.38 | 85.42 |
| II (46–91) | 3.13 | 0 | 0 | 1.04 | 1.04 | 4.17 | 9.38 |
| III (91–136) | 0 | 0 | 0 | 0 | 0 | 1.04 | 1.04 |
| IV (136–181) | 0 | 0 | 0 | 0 | 0 | 2.08 | 2.08 |
| V (181–226) | 0 | 0 | 1.04 | 0 | 0 | 1.04 | 2.08 |
| Organic (%) | Conventional (%) | Producers (%) | |
|---|---|---|---|
| Associated crop | 12 (12.5) | 15 (15.63) | 27 (28.13) |
| Monoculture | 34 (35.42) | 35 (36.45) | 69 (71.87) |
| Producers | 46 (47.92) | 50 (52.08) | 96 (100) |
| Dimension | Variance | Percentage | |
|---|---|---|---|
| Contribution | Accumulated | ||
| 1 | 0.211 | 6.625 | 6.625 |
| 2 | 0.151 | 4.749 | 11.365 |
| 3 | 0.134 | 4.202 | 15.567 |
| 4 | 0.121 | 3.810 | 19.377 |
| 5 | 0.117 | 3.659 | 23.035 |
| 6 | 0.107 | 3.372 | 26.408 |
| Typology | Cluster | Producers | Percentage |
|---|---|---|---|
| Small Gros Michel banana producers for the local market in the Andean foothills. | 1 | 4 | 4.4 |
| Small plantain producers for the local market in the northern Littoral region. | 2 | 6 | 6.5 |
| Small Musaceae producers (including abaca) for the international market via exporters. | 3 | 34 | 37.0 |
| Plantain producers, mostly for export. | 4 | 20 | 21.7 |
| Organic banana producers for export. | 5 | 8 | 8.7 |
| Conventional banana producers for export. | 6 | 20 | 21.7 |
| 92 | 100.0 |
| Vulnerability Level | Low | Medium | High | Critical |
|---|---|---|---|---|
| Case number | 35 | 20 | 20 | 21 |
| Farm percentage | 36.46 | 20.83 | 20.83 | 21.88 |
| Range of Iv | −0.40–−0.20 | −0.20–0.00 | 0.00–1.60 | 1.60–3.20 |
| Average Iv | −0.22 | −0.02 | 1.14 | 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borja, E.; Guara-Requena, M.; Hoyos, M.; Terrero, P.; Rodulfo, P.; Carvajal, L.; Camacho, W.; Mayorga, R.; Molina, C.; Caicedo, M. Ecuadorian Littoral Musaceae Producers’ Typification Based on Their Production Systems, Agronomic Management, Biosecurity Measures, and Risk Level Against Foc TR4. Agriculture 2025, 15, 2208. https://doi.org/10.3390/agriculture15212208
Borja E, Guara-Requena M, Hoyos M, Terrero P, Rodulfo P, Carvajal L, Camacho W, Mayorga R, Molina C, Caicedo M. Ecuadorian Littoral Musaceae Producers’ Typification Based on Their Production Systems, Agronomic Management, Biosecurity Measures, and Risk Level Against Foc TR4. Agriculture. 2025; 15(21):2208. https://doi.org/10.3390/agriculture15212208
Chicago/Turabian StyleBorja, Edwin, Miguel Guara-Requena, Miguel Hoyos, Pedro Terrero, Paola Rodulfo, Liseth Carvajal, Willian Camacho, Rafaela Mayorga, Carlos Molina, and Marlon Caicedo. 2025. "Ecuadorian Littoral Musaceae Producers’ Typification Based on Their Production Systems, Agronomic Management, Biosecurity Measures, and Risk Level Against Foc TR4" Agriculture 15, no. 21: 2208. https://doi.org/10.3390/agriculture15212208
APA StyleBorja, E., Guara-Requena, M., Hoyos, M., Terrero, P., Rodulfo, P., Carvajal, L., Camacho, W., Mayorga, R., Molina, C., & Caicedo, M. (2025). Ecuadorian Littoral Musaceae Producers’ Typification Based on Their Production Systems, Agronomic Management, Biosecurity Measures, and Risk Level Against Foc TR4. Agriculture, 15(21), 2208. https://doi.org/10.3390/agriculture15212208








