Biodegradable Plastic Film Residues Impede Soil Organic Carbon Sequestration and Macroaggregate-Associated Carbon Storage in Agricultural Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, Straw, and Plastic Film Preparations
2.2. Experimental Design and Soil Incubation
2.3. CO2 Efflux Measurement and Soil Sampling
2.4. Aggregate Separation and Organic C Determination
2.5. Data Calculations
2.6. Statistical Analysis
3. Results
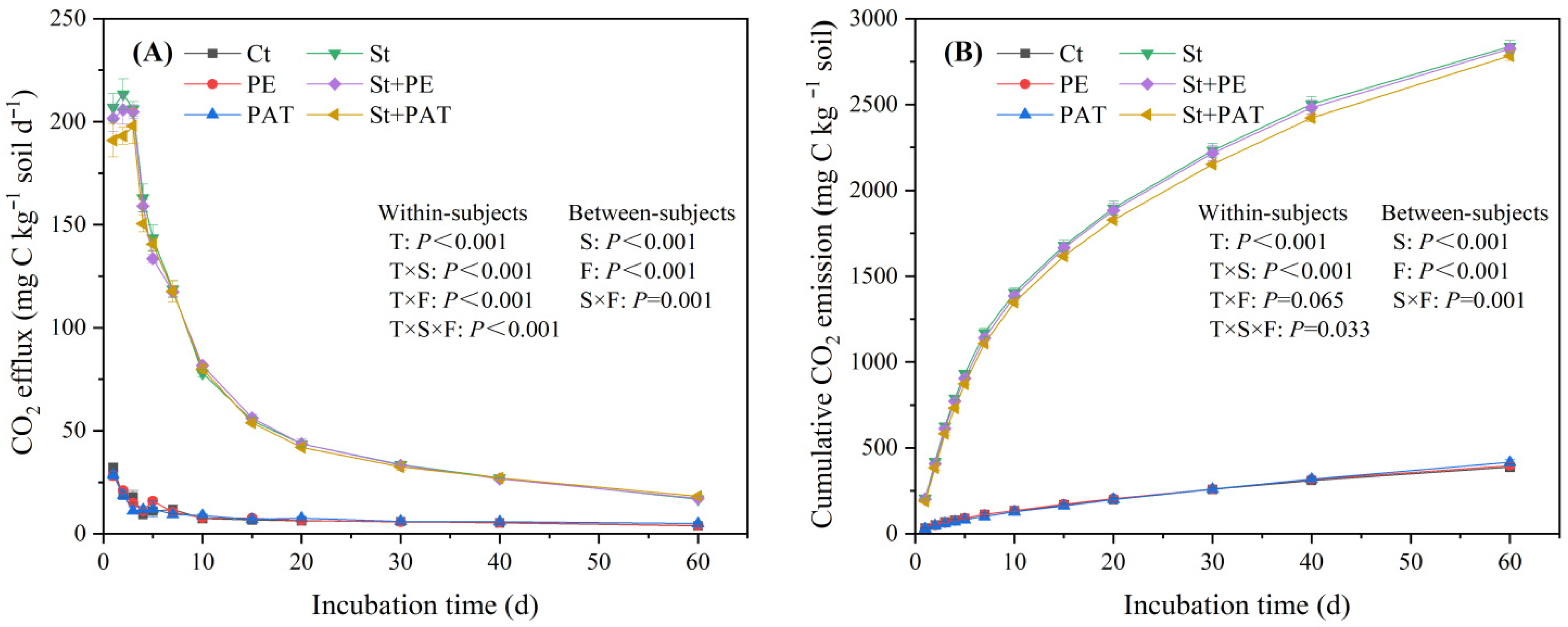
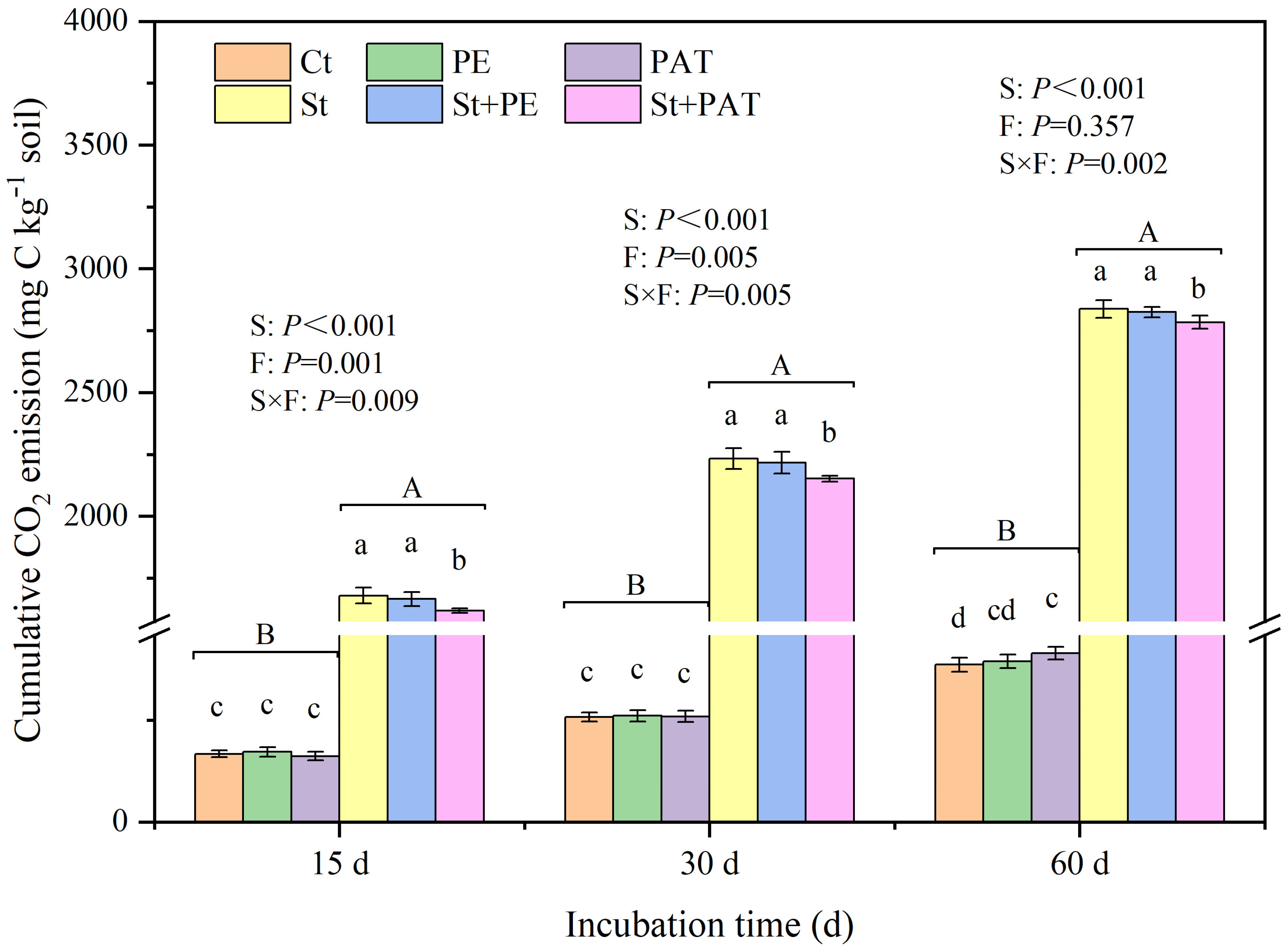
3.1. Soil CO2 Release
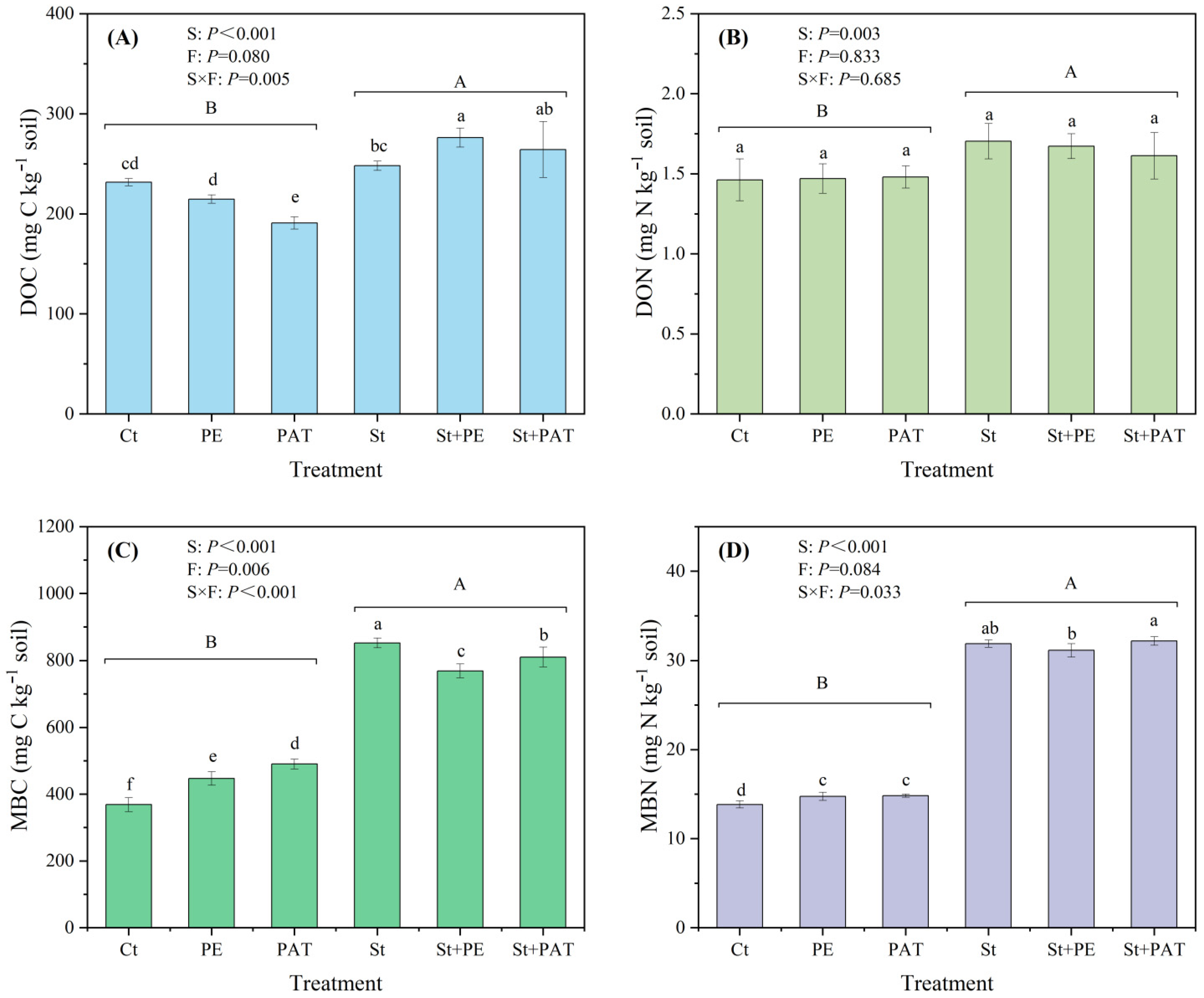
3.2. SOC Content and Related Indicators
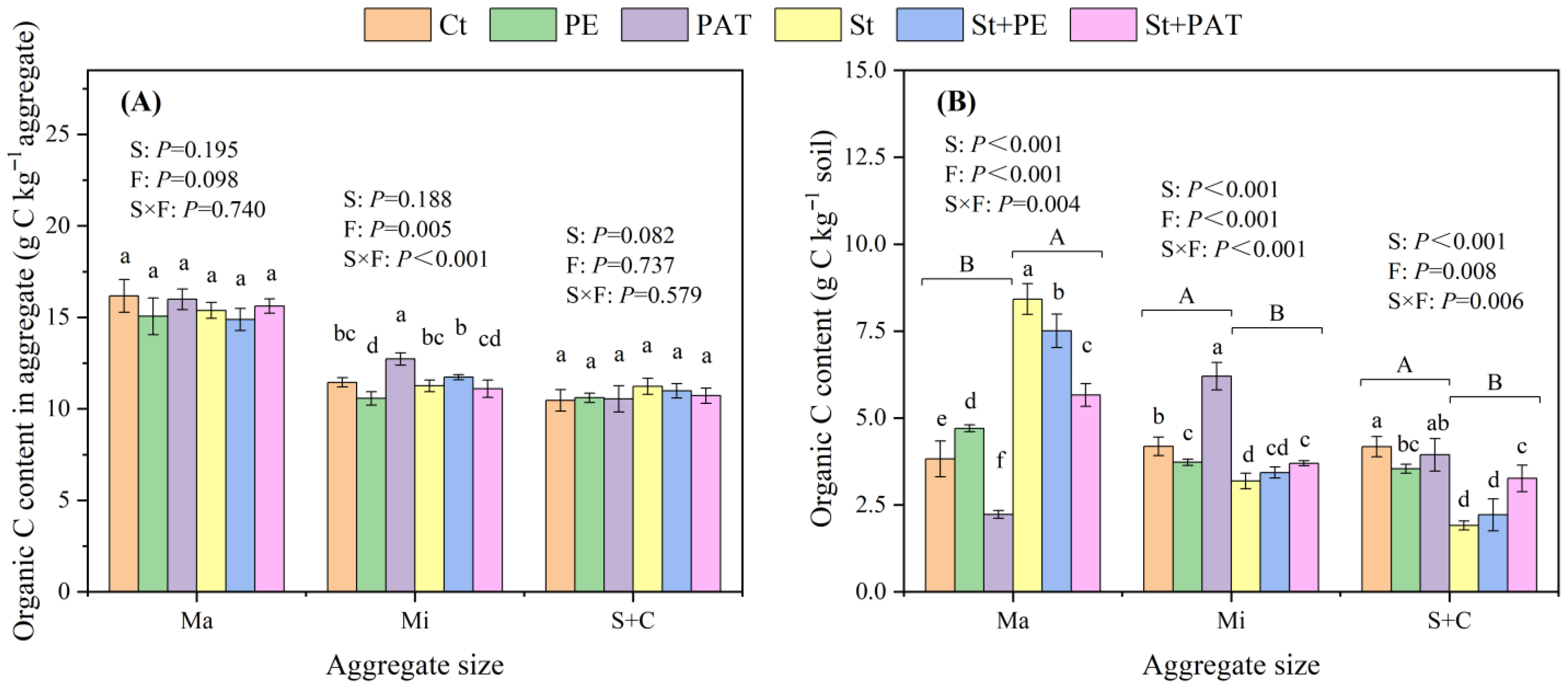
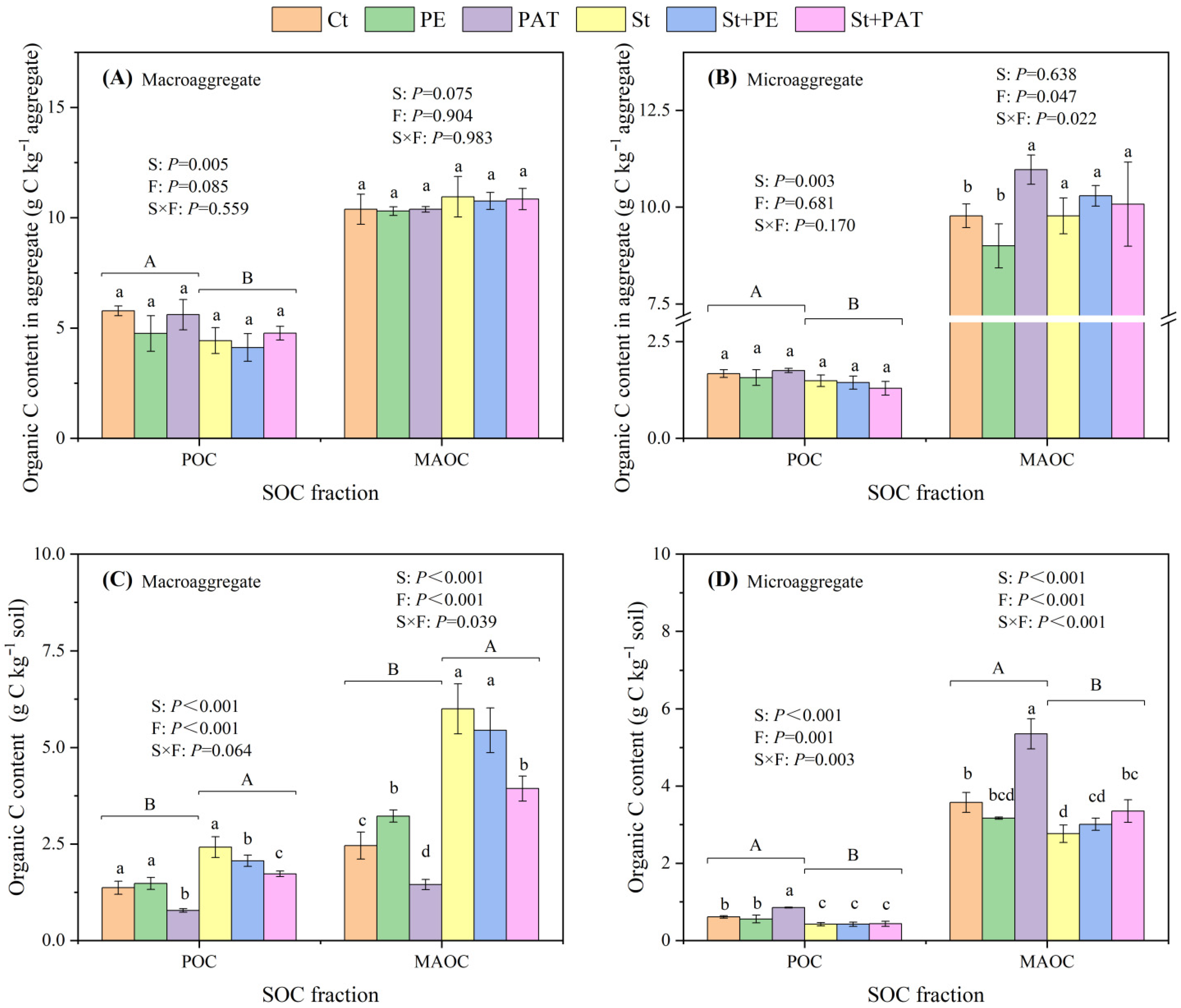
3.3. Soil Aggregation and SOC Distribution
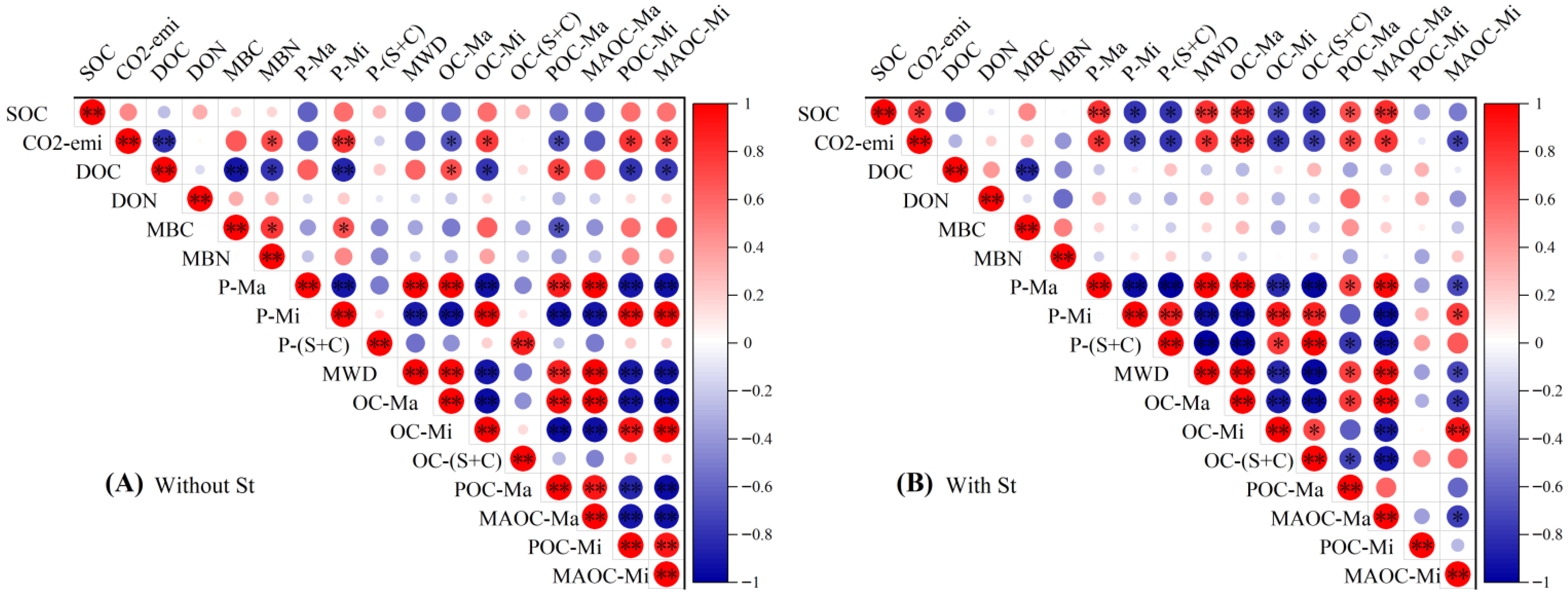
3.4. Correlation Analysis
4. Discussion
4.1. Organic C Mineralization
4.2. SOC Sequestration
4.3. Soil Aggregation and SOC Fractions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stockmann, U.; Padarian, J.; McBratney, A.; Minasny, B.; de Brogniez, D.; Montanarella, L.; Hong, S.Y.; Rawlins, B.G.; Field, D.J. Global soil organic carbon assessment. Glob. Food Secur. 2015, 6, 9–16. [Google Scholar] [CrossRef]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Li, X.S.; Qu, C.Y.; Li, Y.N.; Liang, Z.Y.; Tian, X.H.; Shi, J.L.; Ning, P.; Wei, G.H. Long-term effects of straw mulching coupled with N application on soil organic carbon sequestration and soil aggregation in a winter wheat monoculture system. Agron. J. 2021, 113, 2118–2131. [Google Scholar] [CrossRef]
- Yang, H.B.; Xiao, Q.; Huang, Y.P.; Cai, Z.J.; Li, D.C.; Wu, L.; Meersmans, J.; Colinet, G.; Zhang, W.J. Long-term manuring facilitates glomalin-related soil proteins accumulation by chemical composition shifts and macro-aggregation formation. Soil Tillage Res. 2014, 235, 105904. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, K.L.; Li, K.J.; Zheng, C.L.; Li, B.G. Simulating the effects of long-term iscontinuous and continuous fertilization with straw return on crop yields and soil organic carbon dynamics using the DNDC model. Soil Tillage Res. 2017, 165, 302–314. [Google Scholar] [CrossRef]
- Wu, L.; Xu, H.; Xiao, Q.; Huang, Y.P.; Suleman, M.M.; Zhu, P.; Kuzyakov, Y.K.; Xu, X.L.; Xu, M.G.; Zhang, W.J. Soil carbon balance by priming differs with single versus repeated addition of glucose and soil fertility level. Soil Biol. Biochem. 2020, 148, 107913. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.T.; Zhao, J.N.; Huang, J.J.; Wang, H.; Yu, Y.; Xiao, C.W. Root exudates mediate the processes of soil organic carbon input and efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef]
- Moretto, A.S.; Distel, R.A. Decomposition of and nutrient dynamics in leaf litter and roots of poaligularis and Stipagyneriodes. J. Arid. Environ. 2003, 55, 503–514. [Google Scholar] [CrossRef]
- Li, Y.P.; Yan, Q.; Wang, J.; Shao, M.A.; Li, Z.Y.; Jia, H.Z. Biodegradable plastics fragments induce positive effects on the decomposition of soil organic matter. J. Hazard. Mater. 2024, 468, 133820. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Mason, K.E.; Goodall, T.; Taylor, A.; Zhao, P.; Otero-Farina, A.; Chen, H.; Peacock, C.L.; Ostle, N.J.; Griffiths, R. Microbial and mineral interactions decouple litter quality from soil organic matter formation. Nat. Commun. 2024, 15, 10036. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Yu, Y.X.; Velandia, M.; Hayes, D.G.; DeVetter, L.W.; Miles, C.A.; Flury, M. Chapter three biodegradable plastics as alternatives for polyethylene mulch films. Adv. Agron. 2024, 183, 121–192. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.B.; Hu, W.L.; Qin, X.h.; Ma, X.W.; Yan, C.R.; Wang, H.Y. The status and distribution characteristics of residual mulching film in Xinjiang, China. J. Integr. Agric. 2016, 15, 2639–2646. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Yu, Y.X.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowhury, I.; Flury, M. Minimal impacts of microplastics on soil physical properties under environmentally relevant concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef]
- Su, P.J.; Wang, J.; Zhang, D.; Chu, K.; Yao, Y.Z.; Sun, Q.Q.; Luo, Y.F.; Zhang, R.J.; Sun, X.P.; Wang, Z.C.; et al. Hierarchical and cascading changes in the functional traits of soil animals induced by microplastics: A meta-analysis. J. Hazard. Mater. 2022, 440, 129854. [Google Scholar] [CrossRef]
- Wang, K.; Min, W.; Markus, F.; Anna, G.; Lv, J.; Li, Q.; Jiang, R. Impact of long-term conventional and biodegradable film mulching on microplastic abundance, soil structure and organic carbon in a cotton field. Environ. Pollut. 2024, 356, 124367. [Google Scholar] [CrossRef]
- Liu, M.L.; Feng, J.G.; Shen, Y.W.; Zhu, B. Microplastics effects on soil biota are dependent on their properties: A meta-analysis. Soil Biol. Biochem. 2023, 178, 108940. [Google Scholar] [CrossRef]
- Li, C.T.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.Q.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Flury, M.; Schaeffer, S.M.; Xu, Y.D.; Wang, J.K. Does long-term use of biodegradable plastic mulch affect soil carbon stock? Resour. Conserv. Recycl. 2021, 175, 105895. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 118211. [Google Scholar] [CrossRef]
- Huang, F.Y.; Wang, B.F.; Li, Z.Y.; Liu, Z.H.; Wu, P.; Wang, J.Y.; Ye, X.; Zhang, P.; Jia, Z.K. Continuous years of biodegradable film mulching enhances the soil environment and maize yield sustainability in the dryland of northwest China. Field Crops Res. 2022, 288, 108698. [Google Scholar] [CrossRef]
- Zhao, X.L.; Liu, Z.H.; Cong, C.Y.; Han, J.Q. Application of various methods to extract microplastic from typical soils in China. Environ. Sci. 2021, 42, 4872–4879. (In Chinese) [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring microbial biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Six, J.; Callewaert, P.; Lenders, S.; De Gryze, S.; Morris, S.J.; Gregorich, E.G.; Paul, E.A.; Paustian, K. Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Sci. Soc. Am. J. 2002, 66, 1981–1987. [Google Scholar] [CrossRef]
- Zhang, W.J.; Munkholm, L.J.; Liu, X.; An, T.T.; Xu, Y.D.; Ge, Z.; Xie, N.H.; Li, A.M.; Dong, Y.Q.; Peng, C.; et al. Soil aggregate microstructure and microbial community structure mediate soil organic carbon accumulation: Evidence from one-year field experiment. Geoderma 2023, 430, 116324. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; SSSA Book Series; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.J.; Wei, W.J.; He, Z.L.; Kuzyakov, Y.K.; Bold, R.; Hu, R.G. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Mou, Z.J.; Kuang, L.H.; Zhang, J.; Li, Y.; Wu, W.J.; Liang, C.; Hui, D.F.; Lambers, H.; Sardans, J.; Peñuelas, J.; et al. Nutrient availability and stoichiometry mediate microbial effects on soil carbon sequestration in tropical forests. Soil Biol. Biochem. 2023, 186, 109186. [Google Scholar] [CrossRef]
- Zhang, G.H.; Liu, D.; Lin, J.J.; Kumar, A.; Jia, K.T.; Tian, X.X.; Yu, Z.G.; Zhu, B. Priming effects induced by degradable microplastics in agricultural soils. Soil Biol. Biochem. 2023, 180, 109006. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, Y.Y.; Yu, Y.X.; Yao, H.Y. Effect of nonbiodegradable microplastics on soil respiration and enzyme activity: A meta–analysis. Appl. Soil Ecol. 2023, 184, 104770. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.; Wang, E.; Zhou, T.; Xin, P. Residual plastic mulch fragments effects on soil physical properties and water flow behavior in the Minqin Oasis, northwestern China. Soil Tillage Res. 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Tian, X.M.; Fan, H.; Wang, J.Q.; Ippolito, J.; Li, Y.B.; Feng, S.; An, M.; Zhang, F.; Wang, K. Effect of polymer materials on soil structure and organic carbon under drip irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Kong, X.; Jin, D.; Jin, S.; Wang, Z.; Yin, H.; Xu, M.; Deng, Y. Responses of bacterial community to dibutyl phthalate pollution in a soil- vegetable ecosystem. J. Hazard. Mater. 2018, 353, 142–150. [Google Scholar] [CrossRef]
- Wang, P.Y.; Song, T.J.; Bu, J.S.; Zhang, Y.Q.; Liu, J.X.; Zhao, J.B.; Zhang, T.K.; Xi, J.; Xu, J.; Li, L.; et al. Does bacterial community succession within the polyethylene film plastisphere drive biodegradation? Sci. Total Environ. 2022, 824, 153884. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.G.; Wadsworth, L.C.; Sintim, H.Y.; Flury, M.; English, M.; Schaeffer, S.; Saxton, A.M. Effect of diverse weathering conditions on the physicochemical properties of biodegradable plastic mulches. Polym. Test. 2017, 62, 454–467. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhao, C.X.; Zhang, N.H.; Wang, J.; Li, Z.Y.; Uwiragiye, Y.; Fallah, N.; Crowther, T.W.; Huang, Y.; Xu, Y.; et al. Degradable film mulching increases soil carbon sequestration in major Chinese dryland agroecosystems. Nat. Commun. 2025, 16, 5029. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.S.; Lynch, L.; Xie, H.T.; Bao, X.L.; Liang, C. Tradeoffs among microbial life history strategies influence the fate of microbial residues in subtropical forest soils. Soil Biol. Biochem. 2021, 153, 108112. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mader, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Chen, L.Y.; Han, L.F.; Sun, K.; Chen, G.C.; Ma, C.X.; Zhang, B.A.; Cao, Y.N.; Xing, B.S.; Yang, Z.F. Molecular transformation of dissolved organic carbon of rhizosphere soil induced by flooding and copper pollution. Geoderma 2022, 407, 115563. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Atere, C.T.; Gunina, A.; Zhu, Z.K.; Xiao, M.L.; Liu, S.L.; Kuzyakov, Y.; Chen, L.; Deng, Y.W.; Wu, J.S.; Ge, T.D. Organic matter stabilization in aggregates and density fractions in paddy soil depending on long-term fertilization: Tracing of pathways by 13C natural abundance. Soil Biol. Biochem. 2020, 149, 107931. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Conte, P.; Haberhauer, G.; Gerzabek, M.H. Increased soil organic carbon sequestration through hydrophobic protection by humic substances. Soil Biol. Biochem. 2002, 34, 1839–1851. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Wang, P.Y.; Wang, Y.B.; Zhou, R.; Kiprotich, K.; Alex, N.M.; Liu, S.T.; Wang, W.; Su, Y.Z.; Xiong, Y.C. Fate of plastic film residues in agro-ecosystem and its effects on aggregate-associated soil carbon and nitrogen stocks. J. Hazard. Mater. 2021, 416, 125954. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Du, J.; Chen, J.; Shi, J.; Tian, X. Biodegradable Plastic Film Residues Impede Soil Organic Carbon Sequestration and Macroaggregate-Associated Carbon Storage in Agricultural Soil. Agriculture 2025, 15, 2121. https://doi.org/10.3390/agriculture15202121
Li X, Du J, Chen J, Shi J, Tian X. Biodegradable Plastic Film Residues Impede Soil Organic Carbon Sequestration and Macroaggregate-Associated Carbon Storage in Agricultural Soil. Agriculture. 2025; 15(20):2121. https://doi.org/10.3390/agriculture15202121
Chicago/Turabian StyleLi, Xiushuang, Junli Du, Juan Chen, Jianglan Shi, and Xiaohong Tian. 2025. "Biodegradable Plastic Film Residues Impede Soil Organic Carbon Sequestration and Macroaggregate-Associated Carbon Storage in Agricultural Soil" Agriculture 15, no. 20: 2121. https://doi.org/10.3390/agriculture15202121
APA StyleLi, X., Du, J., Chen, J., Shi, J., & Tian, X. (2025). Biodegradable Plastic Film Residues Impede Soil Organic Carbon Sequestration and Macroaggregate-Associated Carbon Storage in Agricultural Soil. Agriculture, 15(20), 2121. https://doi.org/10.3390/agriculture15202121






