Increasing Light Intensity Enhances Bacillus amyloliquefaciens PMB05-Mediated Plant Immunity and Improves Biocontrol of Bacterial Wilt
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions for Plants and Bacteria
2.2. Disease Severity Assay
2.3. Rapid Reactive Oxygen Species (ROS) Generation and Callose Deposition Assay
3. Results
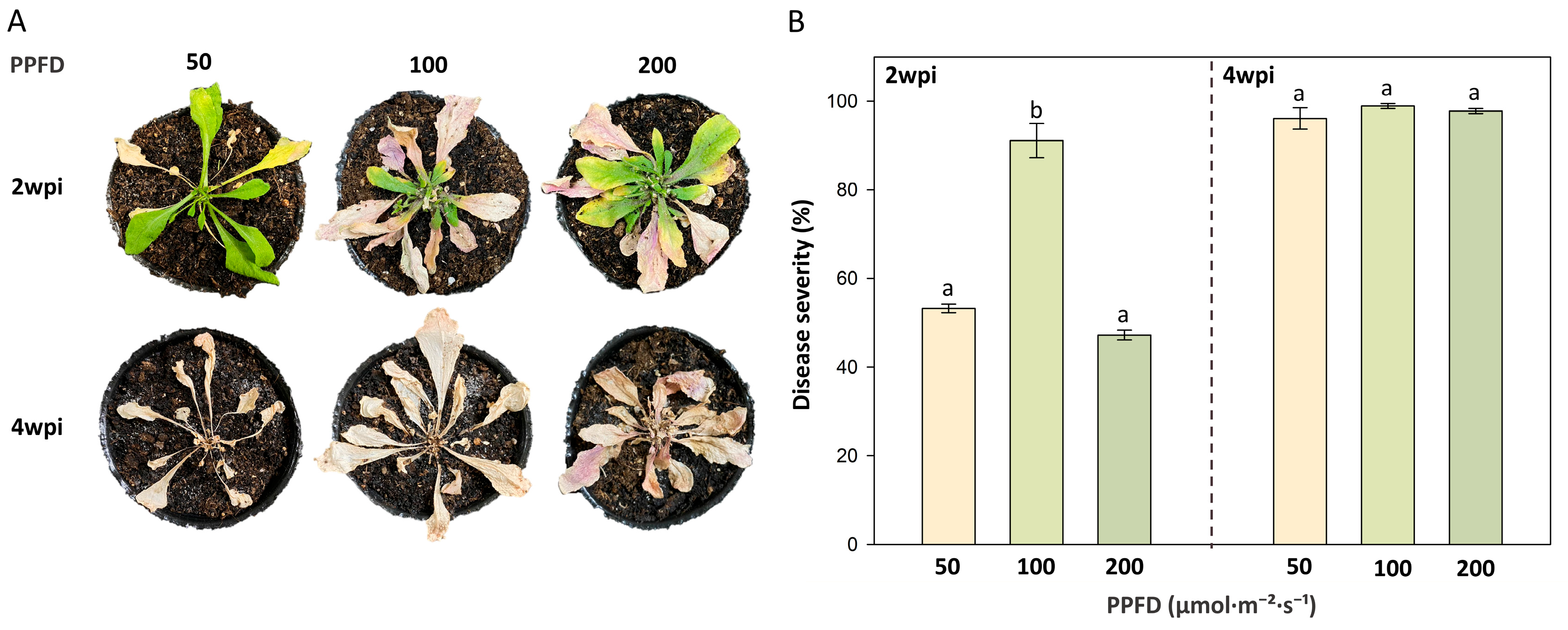
3.1. Occurrence of Bacterial Wilt in Arabidopsis thaliana Grown Under Different Light Intensities
3.2. PopW-Mediated ROS Generation in Arabidopsis thaliana Grown Under Different Light Intensities
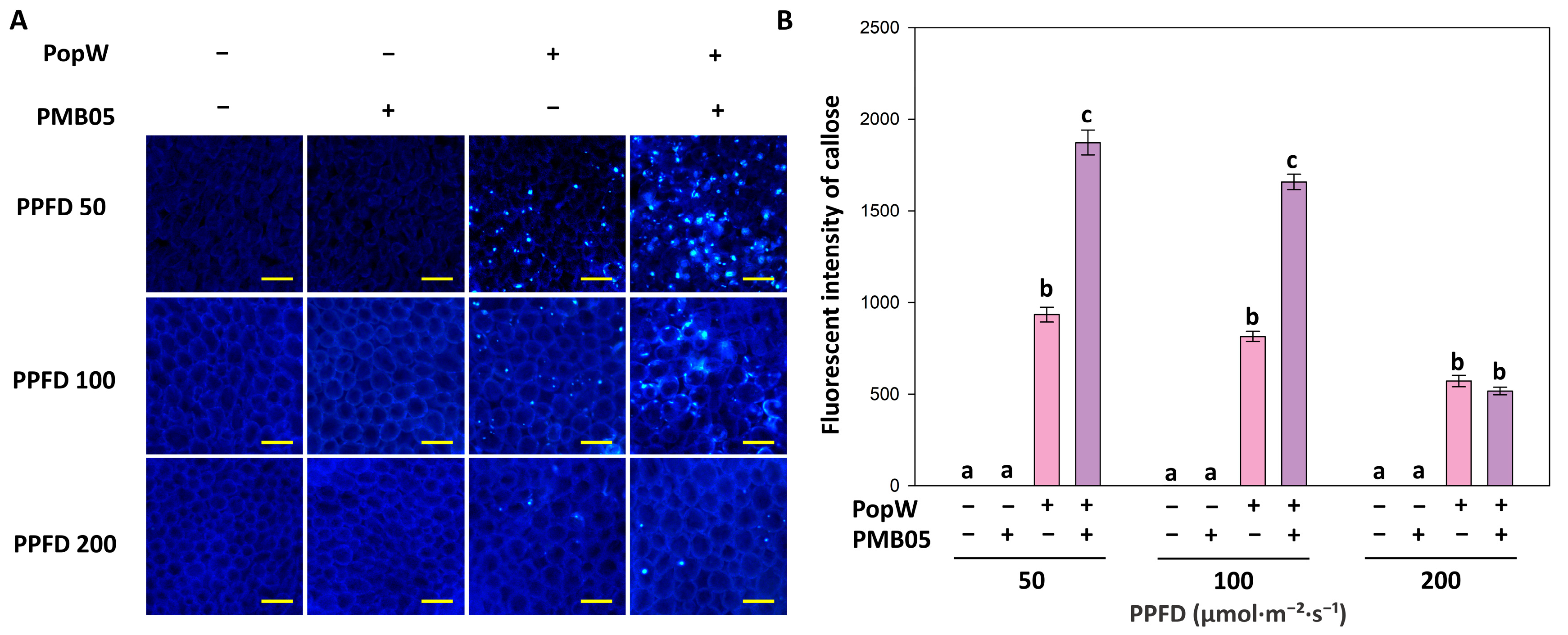
3.3. PopW-Mediated Callose Deposition in Arabidopsis thaliana Grown Under Different Light Intensities
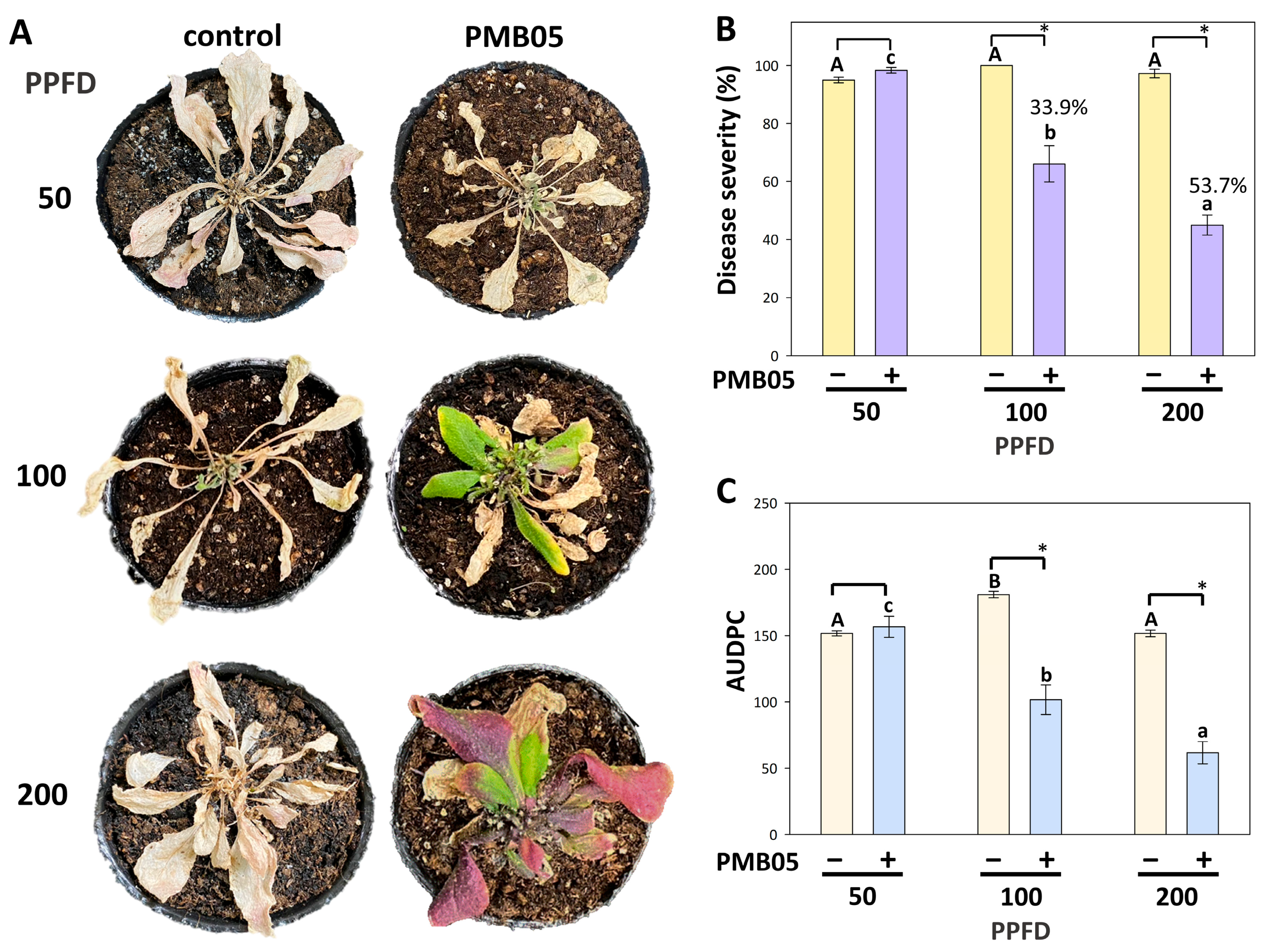
3.4. Effect of Bacillus amyloliquefaciens PMB05 on the Biocontrol of Bacterial Wilt in Arabidopsis thaliana Grown Under Different Light Intensities
3.5. Requirement of the Salicylic Acid Pathway for Bacillus amyloliquefaciens PMB05-Mediated Biocontrol of Bacterial Wilt Under High Light Intensity
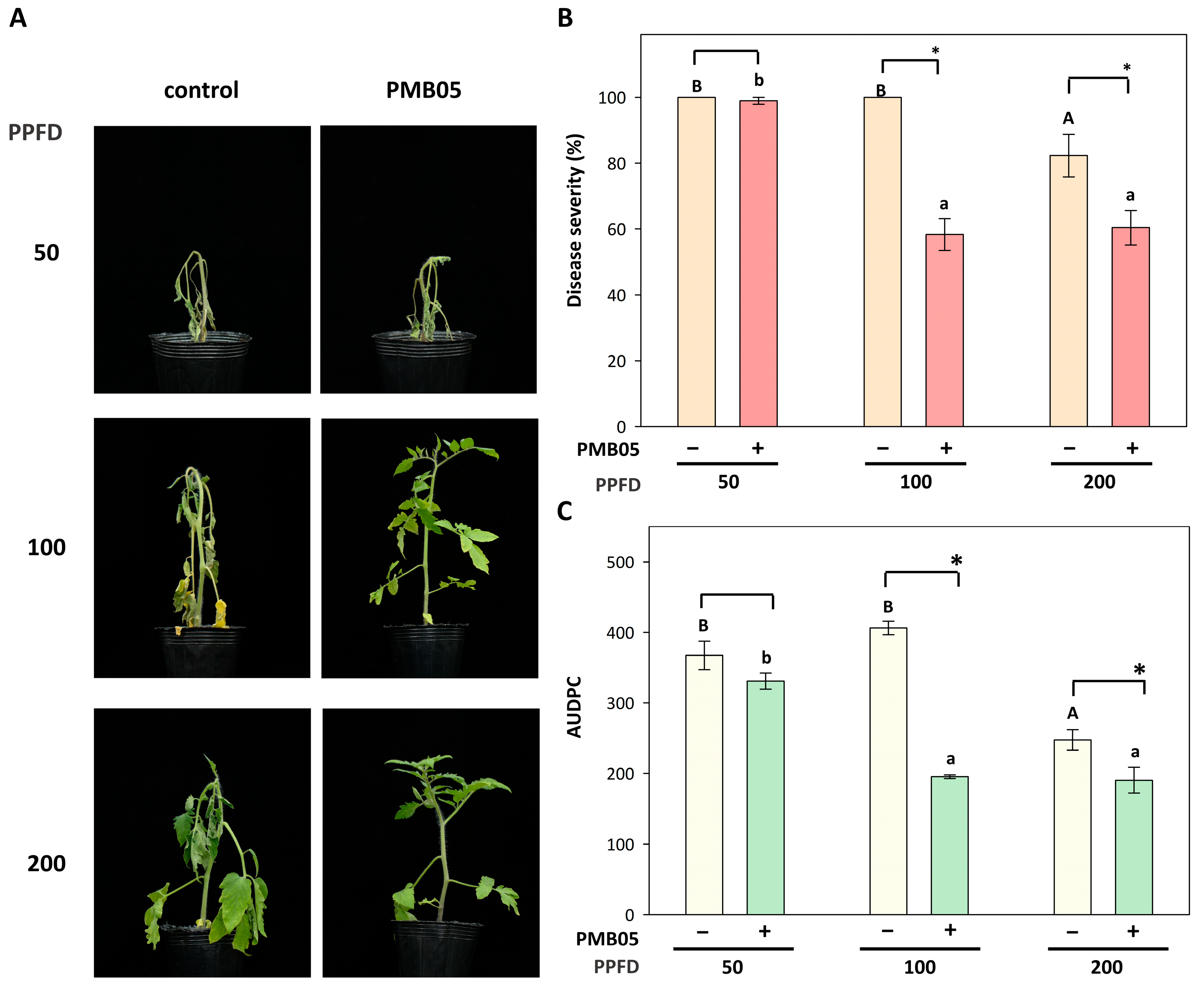
3.6. Effect of Bacillus amyloliquefaciens PMB05 on the Biocontrol of Bacterial Wilt in Tomato Plants Grown Under Different Light Intensities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, T.-H.; Chung, C.-Y.; Zheng, J.-L.; Chen, H.-H.; Liang, Y.-S.; Huang, T.-P.; Lin, Y.-H. Bacillus amyloliquefaciens Strain PMB05 Intensifies Plant Immune Responses to Confer Resistance Against Bacterial Wilt of Tomato. Phytopathology 2020, 110, 1877–1885. [Google Scholar] [CrossRef]
- Morales, P.; González, M.; Salvatierra-Martínez, R.; Araya, M.; Ostria-Gallardo, E.; Stoll, A. New Insights into Bacillus-Primed Plant Responses to a Necrotrophic Pathogen Derived from the Tomato-Botrytis Pathosystem. Microorganisms 2022, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Z.; Shao, J.; Xu, Z.; Liu, Y.; Xun, W.; Miao, Y.; Shen, Q.; Zhang, R. Biocontrol mechanisms of Bacillus: Improving the efficiency of green agriculture. Microb. Biotechnol. 2023, 16, 2250–2263. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, F.; Yin, J.; Peng, R.; Deng, J.; Shen, D.; Wu, J.; Liu, X.; Ma, H. Antifungal action and induction of resistance by Bacillus sp. strain YYC 155 against Colletotrichum fructicola for control of anthracnose disease in Camellia oleifera. Front. Microbiol. 2022, 13, 956642. [Google Scholar] [CrossRef]
- de Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hort. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Amsalem, L.; Freeman, S.; Rav-David, D.; Nitzani, Y.; Sztejnberg, A.; Pertot, I.; Elad, Y. Effect of climatic factors on powdery mildew caused by Sphaerotheca macularis f. sp. fragariae on strawberry. Eur. J. Plant Pathol. 2006, 114, 283–292. [Google Scholar] [CrossRef]
- Eskes, A.B. The effect of light intensity on incomplete resistance of coffee to Hemileia vastatrix. Netherlands J. Plant Pathol. 1982, 88, 191–202. [Google Scholar] [CrossRef]
- Shafia, A.; Sutton, J.C.; Yu, H.; Fletcher, R.A. Influence of preinoculation light intensity on development and interactions of Botrytis cinerea and Clonostachys rosea in tomato leaves. Can. J. Plant Pathol. 2010, 23, 346–357. [Google Scholar] [CrossRef]
- Ikeda, T.; Okuda, M.; Ishihara, M.; Kon-No, Y. Effect of different light conditions on anatomical and histological features of galls in bacterial gall disease of Cerasus × yedoensis. Phytopathology 2024, 114, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, S.; Szechynska-Hebda, M.; Wituszynska, W.; Burdiak, P. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 2013, 36, 736–744. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Lai, I.-L.; Zheng, J.-L.; Lin, Y.-H. Using dynamic changes of chlorophyll fluorescence in Arabidopsis thaliana to evaluate plant immunity-intensifying Bacillus spp. strains. Phytopathology 2019, 109, 1566–1576. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Lin, S.-T.; Li, A.-T.; Li, S.-H.; Hsiao, C.-Y.; Lin, Y.-H. Bacillus amyloliquefaciens PMB05 increases resistance to bacterial wilt by activating MAPK and ROS pathway crosstalk in Arabidopsis thaliana. Phytopathology 2022, 6, 10.1094. [Google Scholar] [CrossRef]
- Postel, S.; Kemmerling, B. Plant systems for recognition of pathogen-associated molecular patterns. Semin. Cell Dev. Biol. 2009, 20, 1025–1031. [Google Scholar] [CrossRef]
- Oh, C.-S.; Beer, S.V. Molecular genetics of Erwinia amylovora involved in the development of fire blight. FEMS Microbiol. Lett. 2005, 253, 185–192. [Google Scholar] [CrossRef]
- Bauer, D.W.; Wei, Z.M.; Beer, S.V.; Collmer, A. Erwinia chrysanthemi harpinEch: An elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol. Plant-Microbe Interact. 1995, 8, 484–491. [Google Scholar] [CrossRef]
- Desikan, R.; Clarke, A.; Atherfold, P.; Hancock, J.T.; Neill, S.J. Harpin induces mitogen-activated protein kinase activity during defence response in Arabidopsis thaliana suspension cultures. Planta 1999, 210, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Zhou, X.; Wang, C.; Wang, C.; Fu, J.; Wei, T. Overexpression of a harpin-encoding gene popW from Ralstonia solanacearum primed antioxidant defenses with enhanced drought tolerance in tobacco plants. Plant Cell Rep. 2016, 35, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Li, J.-R.; Li, A.-T.; Li, S.-H.; Blanco, S.D.; Chen, S.-Y.; Lai, Y.-R.; Shi, M.-Q.; Lin, T.-C.; Su, J.-F.; et al. A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes. Plants 2024, 13, 2185. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Huang, H.-E.; Chen, Y.-R.; Liao, P.-L.; Chen, C.-L.; Feng, T.-Y. C-terminal region of plant ferredoxin-like protein is required to enhance resistance to bacterial disease in Arabidopsis thaliana. Phytopathology 2011, 101, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Simko, I.; Piepho, H.-P. The Area Under the Disease Progress Stairs: Calculation, Advantage, and Application. Phytopathology 2011, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Kim, B.-S.; French, E.; Caldwell, D.; Harrington, E.J.; Iyer-Pascuzzi, A.S. Bacterial wilt disease: Host resistance and pathogen virulence mechanisms. Physiol. Mol. Plant Pathol. 2016, 95, 37–43. [Google Scholar] [CrossRef]
- Li, J.; Huang, B.; Wang, Q.; Li, Y.; Fang, W.; Yan, D.; Guo, M.; Cao, A. Effect of fumigation with chloropicrin on soil bacterial communities and genes encoding key enzymes involved in nitrogen cycling. Environ. Pollut. 2017, 227, 534–542. [Google Scholar] [CrossRef]
- Mao, L.; Wang, Q.; Yan, D.; Ma, T.; Liu, P.; Shen, J.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Evaluation of Chloropicrin as a Soil Fumigant against Ralstonia solanacarum in Ginger (Zingiber officinale Rosc.) Production in China. PLoS ONE 2014, 9, e91767. [Google Scholar] [CrossRef]
- Pesonen, M.; Vähäkangas, K. Chloropicrin-induced toxicity in the respiratory system. Toxicol. Lett. 2020, 323, 10–18. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Keharia, H. Phytostimulation and biocontrol potential of Gram-positive endospore-forming Bacilli. Planta 2021, 254, 49. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Bauer, Z.; Regenass, M.; Boller, T.; Felix, G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 2006, 18, 465–476. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.; Boller, T.; Felix, G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in Arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef]
- Cheaib, A.; Killiny, N. Photosynthesis Responses to the Infection with Plant Pathogens. Mol. Plant-Microbe Interact. 2025, 38, 9–29. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Karpinski, S.; Mullineaux, P.M. The influence of the light environment and photosynthesis on oxidative signalling responses in plant-biotrophic pathogen interactions. Plant Cell Environ. 2005, 28, 1046–1055. [Google Scholar] [CrossRef]
- Suárez, J.C.; Vanegas, J.I.; Contreras, A.T.; Anzola, J.A.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Chlorophyll Fluorescence Imaging as a Tool for Evaluating Disease Resistance of Common Bean Lines in the Western Amazon Region of Colombia. Plants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Yang, H.; Luo, P. Changes in Photosynthesis Could Provide Important Insight into the Interaction between Wheat and Fungal Pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Jones, A.M.; Sklenář, J.; Robatzek, S.; Weber, A.P. Molecular crosstalk between PAMP-triggered immunity and photosynthesis. Mol. Plant Microbe Interact. 2012, 25, 1083–1092. [Google Scholar] [CrossRef]
- Galvez-Valdivieso, G.; Fryer, M.J.; Lawson, T.; Slattery, K.; Truman, W.; Smirnoff, N.; Asami, T.; Davies, W.J.; Jones, A.M.; Baker, N.R.; et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 2009, 21, 2143–2162. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and Function of Defense-Related Callose Deposition in Plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Chen, X.; Wang, F.; Hsiao, C.-Y.; Yang, C.-Y.; Lin, S.-T.; Wu, L.-H.; Chen, Y.-K.; Liang, Y.-S.; Lin, Y.-H. Bacillus amyloliquefaciens strains control strawberry anthracnose through antagonistic activity and plant immune response intensification. Biol. Control 2021, 157, 104592. [Google Scholar] [CrossRef]
- Bayles, C.J.; Ghemawat, M.S.; Aist, J.R. Inhibition by 2-deoxy-D-glucose of callose formation, papilla deposition, and resistance to powdery mildew in an ml-o barley mutant. Physiol. Mol. Plant Pathol. 1990, 36, 63–72. [Google Scholar] [CrossRef]
- Sanmartín, N.; Pastor, V.; Pastor-Fernández, J.; Flors, V.; Pozo, M.J.; Sánchez-Bel, P. Role and mechanisms of callose priming in mycorrhiza-induced resistance. J. Exp. Bot. 2020, 71, 2769–2781. [Google Scholar] [CrossRef]
- de Vallavieille-Pope, C.; Huber, L.; Leconte, M.; Bethenod, O. Preinoculation Effects of Light Quantity on Infection Efficiency of Puccinia striiformis and P. triticina on Wheat Seedlings. Phytopathology 2002, 92, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Feri dit Frey, N.; Garcia, A.V.; Bigeard, J.; Zaag, R.; Bueso, E.; Garmier, M.; Pateyron, S.; de Tauzia-Moreau, M.-L.; Brunaud, V.; Balzergue, S.; et al. Functional analysis of Arabidopsis immune-related MAPKs uncover a role for MPK3 as negative regulator of inducible defences. Genome Biol. 2014, 15, R87. [Google Scholar]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, J.; Wang, P.; Liang, X.; Li, J.; Wu, C.; Fang, W.; Ding, S.; Shao, S.; Shi, K. Transcriptomic and genetic approaches reveal that low-light-induced disease susceptibility is related to cellular oxidative stress in tomato. Hortic. Res. 2023, 10, uhad173. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Zhao, F.-F.; Zhang, M.; Lin, H.-H.; Xi, D.-H. Effects of Light Quality on the Interaction between Cucumber Mosaic Virus and Nicotiana tabacum. J. Phytopathol. 2015, 163, 1002–1013. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-H.; Li, A.-T.; Shi, M.-Q.; Lu, Y.-X.; Hong, L.-Y.; Chung, H.-Y.; Lin, Y.-H. Increasing Light Intensity Enhances Bacillus amyloliquefaciens PMB05-Mediated Plant Immunity and Improves Biocontrol of Bacterial Wilt. Agriculture 2025, 15, 2110. https://doi.org/10.3390/agriculture15202110
Li S-H, Li A-T, Shi M-Q, Lu Y-X, Hong L-Y, Chung H-Y, Lin Y-H. Increasing Light Intensity Enhances Bacillus amyloliquefaciens PMB05-Mediated Plant Immunity and Improves Biocontrol of Bacterial Wilt. Agriculture. 2025; 15(20):2110. https://doi.org/10.3390/agriculture15202110
Chicago/Turabian StyleLi, Sin-Hua, Ai-Ting Li, Ming-Qiao Shi, Yi-Xuan Lu, Li-Ya Hong, Hsing-Ying Chung, and Yi-Hsien Lin. 2025. "Increasing Light Intensity Enhances Bacillus amyloliquefaciens PMB05-Mediated Plant Immunity and Improves Biocontrol of Bacterial Wilt" Agriculture 15, no. 20: 2110. https://doi.org/10.3390/agriculture15202110
APA StyleLi, S.-H., Li, A.-T., Shi, M.-Q., Lu, Y.-X., Hong, L.-Y., Chung, H.-Y., & Lin, Y.-H. (2025). Increasing Light Intensity Enhances Bacillus amyloliquefaciens PMB05-Mediated Plant Immunity and Improves Biocontrol of Bacterial Wilt. Agriculture, 15(20), 2110. https://doi.org/10.3390/agriculture15202110






