The Effect of Nitrogen Dose and Plant Density Interactions on Potato Yield and Quality in Dry Cultivation: The Role of Photosynthesis and C–N Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Field Management
2.3. Measurement Index and Method
2.3.1. Photosynthetic Parameters and Photosynthetic Enzyme Activity of Potato Leaves
2.3.2. Nitrogen-Metabolizing Enzyme Activity of Potato Leaves
2.3.3. Starch Synthase Activity of Potato Leaves
2.3.4. Potato Yield and Yield Components
2.3.5. Potato Quality
2.4. Statistical Analysis
3. Results
3.1. Changes in Photosynthetic Parameters and Photosynthetic Enzyme Activities in Potato Leaves Under the Interaction of Nitrogen and Density
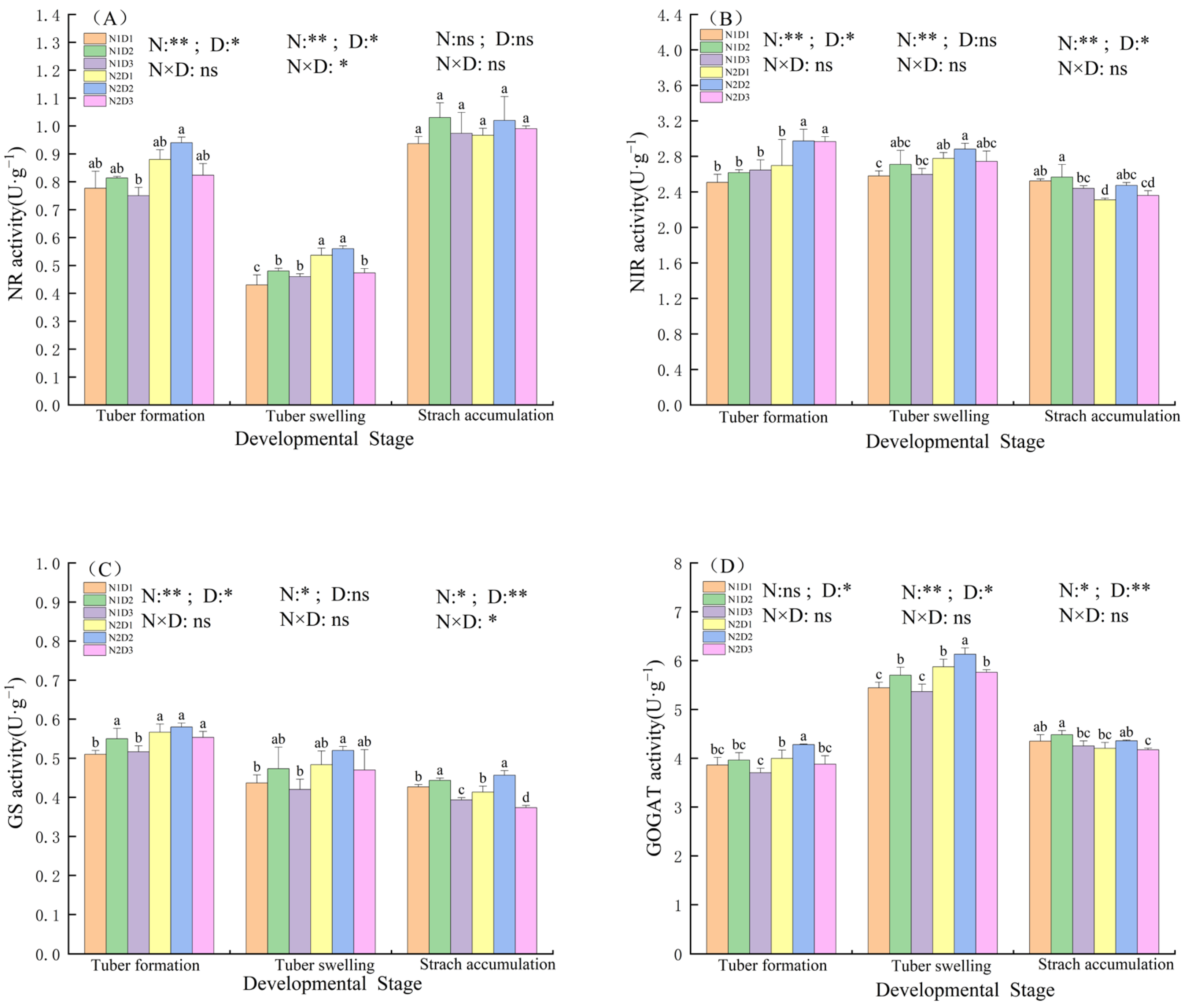
3.2. Changes in Nitrogen Metabolism Enzymes Activities in Potato Leaves Under the Interaction of Nitrogen and Density
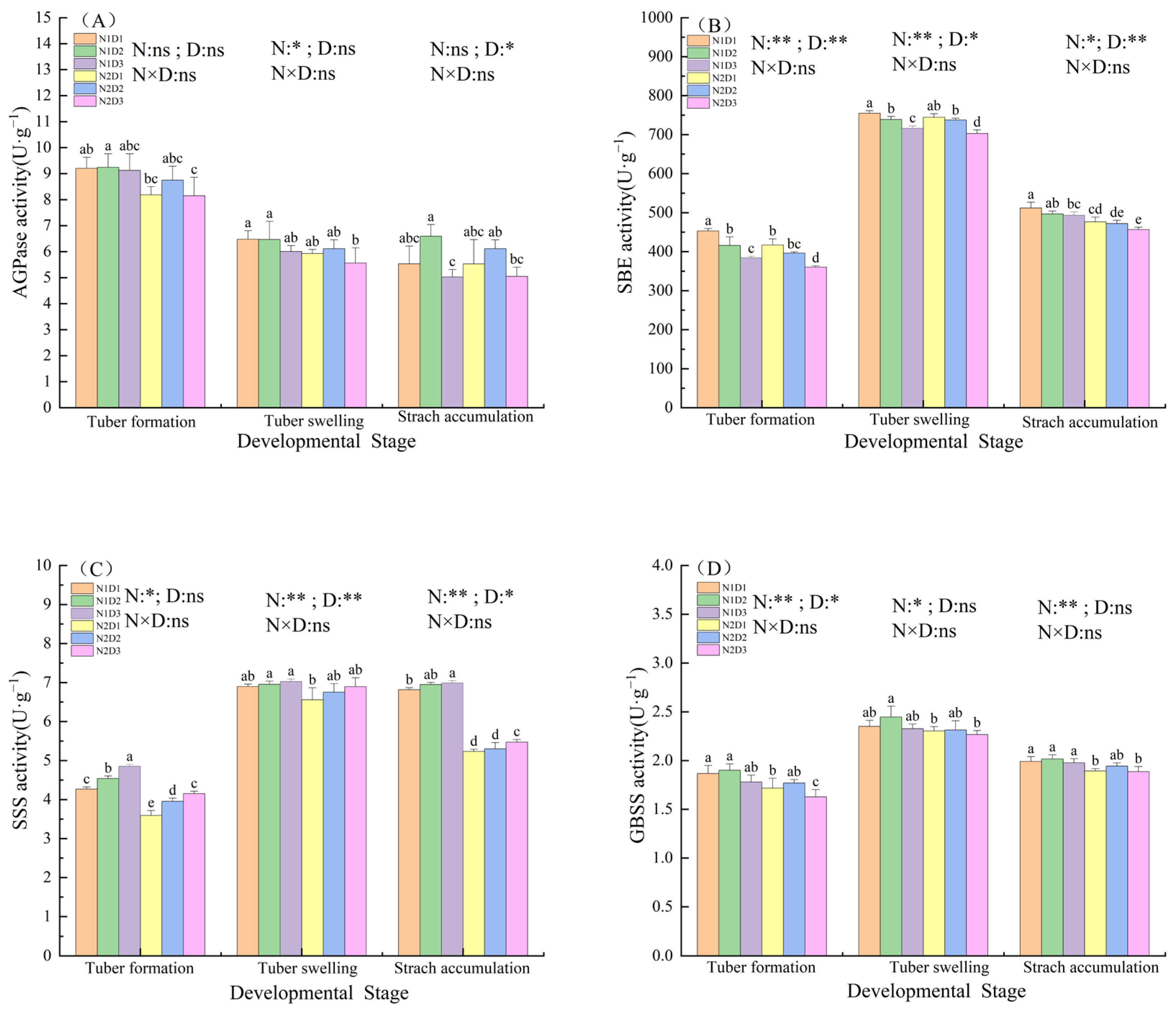
3.3. Changes in Starch Synthase Enzymes Activities in Potato Leaves Under the Interaction of Nitrogen and Density
3.4. Potato Yield and Yield Components Under the Interaction of Nitrogen and Density
3.5. Potato Quality Under the Interaction of Nitrogen and Density
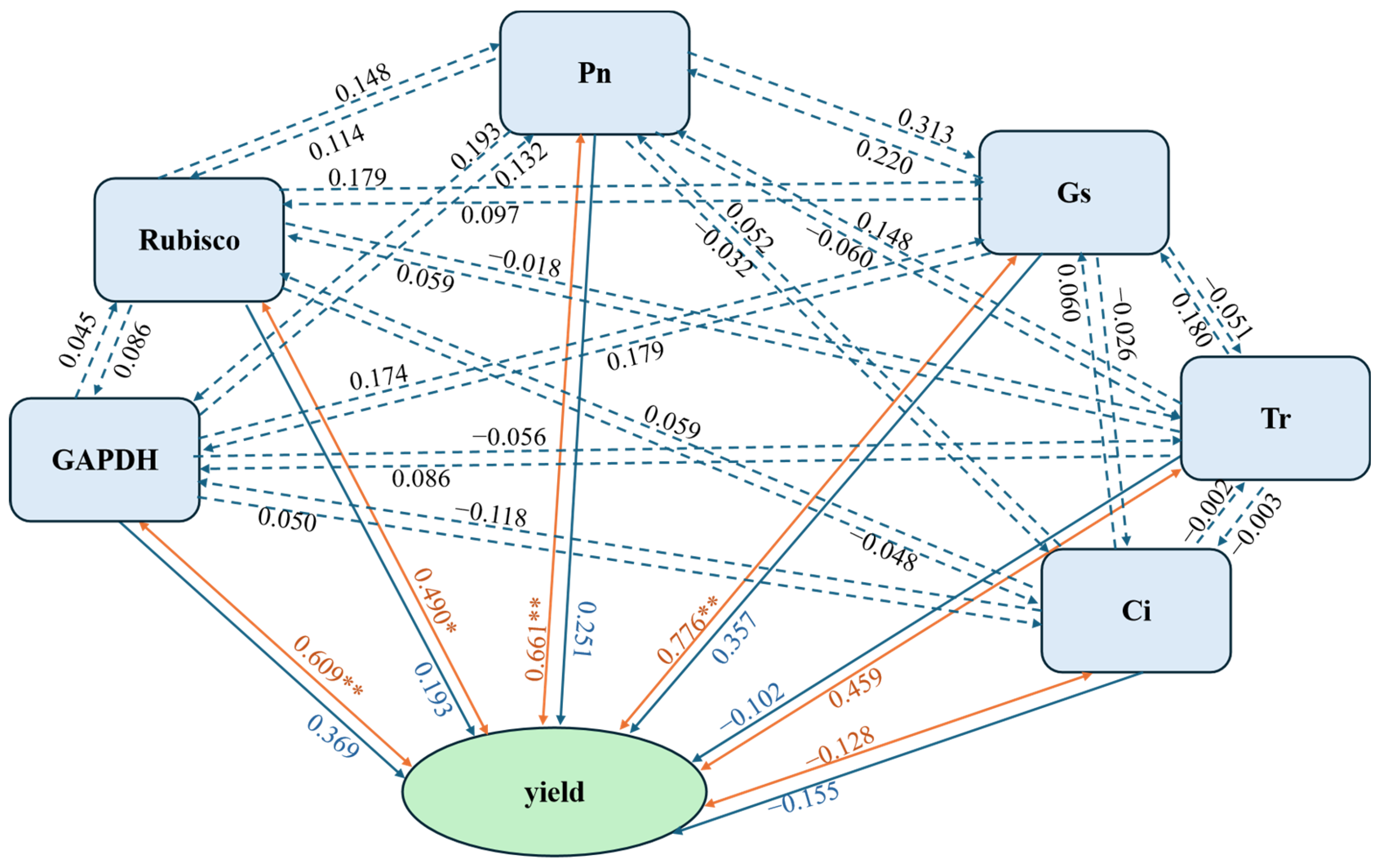
3.6. Correlation and Partial Least Squares Regression (PLS) Analysis of Photosynthetic Parameters, Photosynthetic Enzymes Activities, and Potato Tuber Yield Under the Interaction of Nitrogen and Density
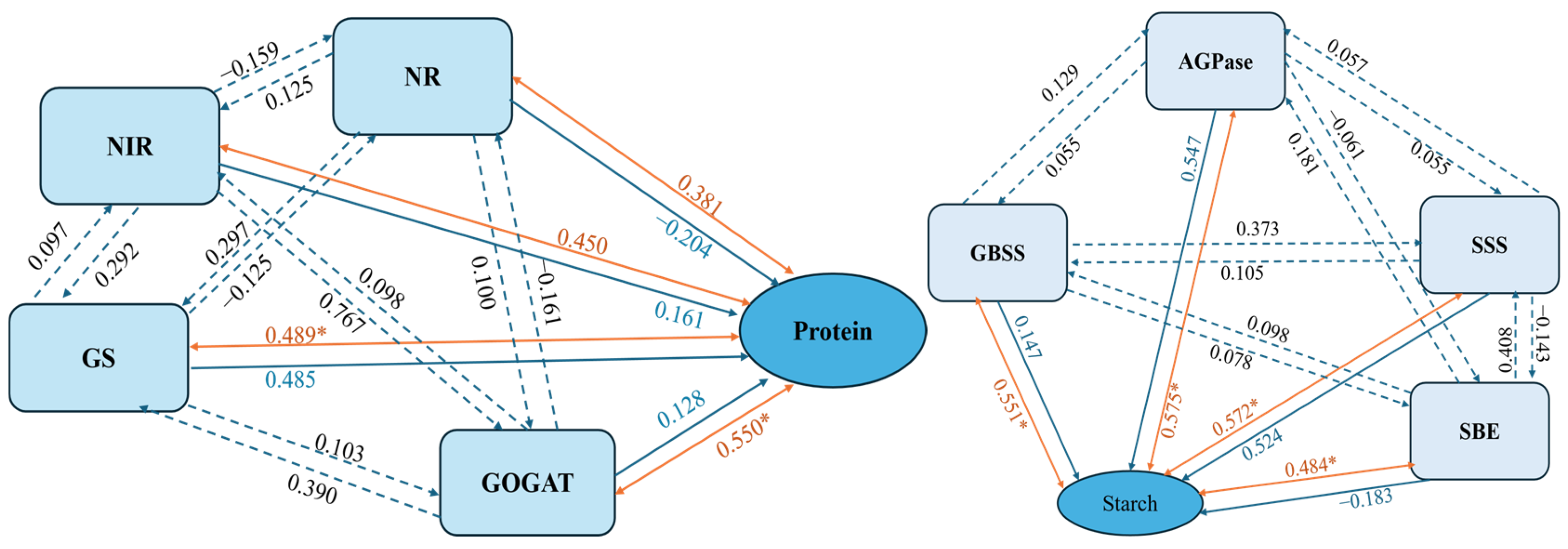
3.7. Correlation and Partial Least Squares Regression (PLS) Analysis of Nitrogen Metabolism, Starch Synthase Enzymes Activities, and Potato Tuber Yield Under the Interaction of Nitrogen and Density
3.8. Correlation and Partial Least Squares Regression (PLS) Analysis of Nitrogen Metabolism, Starch Synthase Enzymes Activities, and Potato Tuber Quality Under the Interaction of Nitrogen and Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xu, F.; Wu, Y.; Hu, H.H.; Dai, X.F. Progress of potato staple food research and industry development in China (Review). J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Villalba, A.; Martínez-Ispizua, E.; Morard, M.; Crespo-Sempere, A.; Albiach-Marti, M.R.; Calatayud, A.; Penella, C. Optimizing sweet potato production: Insights into the interplay of plant sanitation, virus influence, and cooking techniques for enhanced crop quality and food security. Front. Plant Sci. 2024, 15, 1357611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.Y.; Yuan, J.L.; Liang, L.J.; Zhang, F.; Wang, Y.P. Genotype × environment interactions for potato yield and quality traits: Identification of ideotypes adapted in different ecological regions of Northwest China. BMC Plant Biol. 2025, 25, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Zhang, J.F.; Li, T.; Xiong, S.T. Spatial Variability of Potato (Solanum tuberosum L.) Yield and quality on sand soil in the arid region of Northwest China. Appl. Ecol. Environ. Res. 2022, 20, 1969–1989. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; He, P.; Zhang, P.; Duan, Y. Yield and nutrient gap analysis for potato in northwest China. J. Agric. Sci. 2018, 156, 971–979. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.R.; Fu, J.T.; Zhang, X.Z.; Li, Y.; Shao, Y.T.; Xing, Y.Y.; Wang, X.K. Coupling effects of irrigation amount and fertilization rate on yield, quality, water and fertilizer use efficiency of different potato varieties in Northwest China. Agric. Water Manag. 2023, 287, 108446. [Google Scholar] [CrossRef]
- Conti, S.; Borrelli, C.; Maddaluno, P.; Carputo, D.; Caruso, G.; Frusciante, L. Effect of mulching and plant density on out-of-season organic potato growth, yield and quality. Adv. Hortic. Sci. 2013, 27, 115–121. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Luo, S.; Ma, X.; Li, Z.; Lin, Z.; Zhang, Z. Optimizing the optimal planting period for potato based on different water-temperature year types in the agro-pastoral ecotone of North China. Agriculture 2021, 11, 1061. [Google Scholar] [CrossRef]
- Wang, C.L.; Shen, S.H.; Zhang, S.Y.; Li, Q.Z.; Yao, Y.B. Adaptation of potato production to climate change by optimizing sowing date in the Loess Plateau of central Gansu, China. J. Integr. Agric. 2015, 14, 398–409. [Google Scholar] [CrossRef]
- Qin, S.H.; Li, L.L.; Wang, D.; Zhang, J.L.; Pu, Y.L. Effects of limited supplemental irrigation with catchment rainfall on rain-fed potato in semi-arid areas on the Western Loess Plateau, China. Am. J. Potato Res. 2013, 90, 33–42. [Google Scholar] [CrossRef]
- Assunçao, N.S.; Fernandes, A.M.; Soratto, R.P.; Mota, L.H.S.O.; Ribeiro, N.P.; Leonel, M. Tuber Yield and quality of two potato cultivars in response to nitrogen fertilizer management. Potato Res. 2021, 64, 147–166. [Google Scholar] [CrossRef]
- Jiang, L.L.; Jin, G.H.; Zhang, G.Z.; Zhang, C.Y. Tuber yield and french fry processing quality response of potatoes to nitrogen rate. Potato Res. 2022, 65, 255–271. [Google Scholar] [CrossRef]
- Ghosh, U.; Hatterman-Valenti, H.; Chatterjee, A. Russet potato yield, quality, and nitrogen uptake with enhanced efficiency fertilizers. Agron. J. 2019, 111, 200–209. [Google Scholar] [CrossRef]
- Li, S.C.; Chen, H.Y.; Jiang, S.C.; Hu, F.Q.; Xing, D.Y.; Du, B. Selenium and nitrogen fertilizer management improves potato root function, photosynthesis, yield and selenium enrichment. Sustainability 2023, 15, 6060. [Google Scholar] [CrossRef]
- Liang, Q.G.; Chen, H.R.; Chang, H.L.; Liu, Y.; Wang, Q.N.; Wu, J.T.; Liu, Y.H.; Kumar, S.; Chen, Y.; Chen, Y.L.; et al. Influence of planting density on sweet potato storage root formation by regulating carbohydrate and lignin metabolism. Plants 2023, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lu, Y.; Zhao, Y.; Wang, Y.; Han, Z.; Han, Y.; Zhang, J. Analysis of relative expression of key enzyme genes and enzyme activity in nitrogen metabolic pathway of two genotypes of potato (Solanum tuberosum L.) under different nitrogen supply levels. Horticulturae 2022, 8, 769. [Google Scholar] [CrossRef]
- Yan, W.; Qin, J.; Jian, Y.; Liu, J.; Bian, C.; Jin, L.; Li, G. Analysis of potato physiological and molecular adaptation in response to different water and nitrogen combined regimes. Plants 2023, 12, 1671. [Google Scholar] [CrossRef]
- Gholamin, R.; Jamaati-e-Somarin, S.; Khayatnezhad, M.; Zabihi-e-Mahmoodabad, R. Quantitative and qualitative yield of potato tuber by used of nitrogen fertilizer and plant density. Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 310–318. [Google Scholar]
- Yari, A.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. Response of agronomical, physiological, apparent recovery nitrogen use efficiency and yield of potato tuber (Solanum tuberosum L.), to nitrogen and plant density. Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 16–21. [Google Scholar]
- Duan, W.X.; Zhang, H.Y.; Xie, B.T.; Wang, B.Q.; Hou, F.Y.; Li, A.X.; Dong, S.X.; Qin, Z.; Wang, Q.M.; Zhang, L.M. Nitrogen utilization characteristics and early storage root development in nitrogen-tolerant and nitrogen-susceptible sweet potato. Physiol. Plant. 2021, 173, 1090–1104. [Google Scholar] [CrossRef]
- Ding, K.X.; Shan, Y.; Wang, L.C.; Zhang, Y.; Tian, G.K. Transcriptomics combined with physiological analysis and metabolomics revealed the response of potato tuber formation to nitrogen. BMC Plant Biol. 2024, 24, 1109. [Google Scholar] [CrossRef]
- Kingori, G.G.; Nyamori, A.J.; Khasungu, I.D. Optimization of seed potato specific density, starch and dry matter contents and tuberization capacity of resultant plants through integrated irrigation, nitrogen and phosphorus management. J. Plant Sci. 2015, 3, 225–233. [Google Scholar]
- Wang, C.Y.; Meng, H.F.; Li, L.L.; Xie, J.H.; Wang, L.L.; Liu, B.X.; Tian, X.; Bao, X.Y. Effects of nitrogen-dense interaction on photosynthetic characteristics, dry matter accumulation and yield of dryland potatoes. Agric. Res. Arid Area. 2024, 42, 118–127. (In Chinese) [Google Scholar]
- Zhang, F.; Chen, M.R.; Zheng, Y.; Xie, Y.X.; Xing, Y.Y. Optimizing irrigation and fertilization to simultaneously improve potato tuber yield, water and fertilizer use efficiency and net income in northwest China. Agronomy 2024, 14, 1124. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Zhang, Z.A. Experimental Instructionf Plant Physiology; China Agricultural Press: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Campbell, W.; Remmler, J. Regulation of corn leaf nitrate reductase: I. immunochemical methods for analysis of the rnzyme’s protein component. Plant Physiol. 1986, 80, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Oaks, A.; Wallace, W.; Stevens, D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972, 50, 649–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamachi, K.; Yamaya, T.; Mae, T.; Ojima, K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991, 96, 411–417. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Cheng, F.M.; Zhong, L.J.; Sun, Z.X. Effect of temperature at grain-filling stage on starch biosynthetic metabolism in developing rice grains of early-indica. Sci. Agric. Sin. 2003, 36, 492–501. (In Chinese) [Google Scholar]
- Li, T.G.; Shen, B.; Chen, N.; Luo, Y.K. Effect of Q-enzyme on the chalkiness formation of rice grain. Acta Agron. Sin. 1997, 23, 338–344. (In Chinese) [Google Scholar]
- Zhou, C.T.; Zhang, R.Y.; Shi, M.F.; Zhang, W.N.; Yuan, W.Y. Effect of different iron fertilizer forms iron fertilizer on endogenous hormones, yield and quality of potato. J. Northwest AF Univ. 2022, 50, 42–49. (In Chinese) [Google Scholar]
- Yao, Y.B.; Lei, J.; Niu, H.Y.; Zhang, X.Y. Combined impact of elevated CO2 concentration and climatic warming on potato yield and quality in semi-arid regions. Potato Res. 2021, 64, 97–113. [Google Scholar]
- Bailey-Serres, J.; Pierik, R.; Ruban, A.; Wingler, A. The dynamic plant: Capture, transformation, and management of energy. Plant Physiol. 2018, 176, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.N.; Ma, H.Y.; Wang, K.X.; Tang, M.X.; Zhang, K.Q.; Jahandad, A.; Saba, T.; Fang, X.T.; Shahzad, M.A.; Ansar, M.; et al. Deciphering nitrogen dynamics in aeroponics: Physio-biochemical and enzymatic responses influencing nitrogen use efficiency in contrasting potato genotypes. Sci. Hortic. 2024, 338, 113768. [Google Scholar] [CrossRef]
- Cheng, S.H.; Su, Z.H.; Xie, C.H.; Liu, J. Effects of variationin activities of starch-sugar metabolic enzymes on reducing sugar accumulation and processing quality of potato tubers. Sci. Agric. Sin. 2004, 37, 1904–1910. (In Chinese) [Google Scholar]
- Akkamis, M.; Caliskan, S. Responses of yield, quality and water use efficiency of potato grown under different drip irrigation and nitrogen levels. Sci. Rep. 2023, 13, 9911. [Google Scholar] [CrossRef]
- Getie, A.T.; Dechassa, N.; Tana, T. Response of potato (Solanum tuberosum L.) Yield and yield components to nitrogen fertilizer and planting density at haramaya, eastern ethiopia. J. Plant Sci. 2015, 3, 320–328. [Google Scholar]
- Tsibart, A.S.; Dillen, J.; Van Craenenbroeck, L.; Elsen, A.; Postelmans, A.; van de Ven, G.; Saeys, W. Scenarios for precision nitrogen management in potato: Impact on yield, tuber quality and post-harvest nitrate residues in the soil. Field Crops Res. 2024, 319, 109648. [Google Scholar] [CrossRef]
- Vasilyev, A.A. Dependence of the yield and quality of potato tubers in the forest-steppe zone of Southern Urals on the level of mineral nutrition and planting density. Russ. Agric. Sci. 2014, 40, 422–425. [Google Scholar] [CrossRef]





| Indirect Coefficient | ||||
|---|---|---|---|---|
| Yield Components | The Coefficient of Direct Regression with y | Direct Coefficient | X1→y | X2→y |
| X1 | 0.627 | 0.374 | — | −0.2031 |
| X2 | 0.615 | −0.0261 | 0.2908 | — |
| Treatments | Protein (mg·g−1) | Starch (%) | Sugar (%) | Vitamin C (mg·100 g−1) | Calcium (mg·kg−1) |
|---|---|---|---|---|---|
| N1D1 | 2.33 ± 0.13 b | 18.55 ± 0.12 b | 0.3 ± 0.01 b | 15.64 ± 0.1 b | 14.96 ± 0.05 ab |
| N1D2 | 2.51 ± 0.1 a | 19.1 ± 0.14 a | 0.3 ± 0.01 b | 15.91 ± 0.09 a | 15.29 ± 0.5 a |
| N1D3 | 2.12 ± 0.04 c | 18.3 ± 0.11 c | 0.31 ± 0.01 b | 15.82 ± 0.03 ab | 15.23 ± 0.42 a |
| N2D1 | 2.4 ± 0.08 ab | 18.33 ± 0.17 bc | 0.33 ± 0.01 a | 15.13 ± 0.1 c | 14.45 ± 0.28 b |
| N2D2 | 2.4 ± 0.08 ab | 18.36 ± 0.09 bc | 0.34 ± 0.02 a | 14.86 ± 0.24 d | 14.79 ± 0.07 ab |
| N2D3 | 2.25 ± 0.04 bc | 18.2 ± 0.07 c | 0.35 ± 0.01 a | 14.79 ± 0.07 d | 14.73 ± 0.18 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Wang, C.; Li, L.; Bao, X.; Tian, X.; Xie, J.; Wang, L.; Luo, Z. The Effect of Nitrogen Dose and Plant Density Interactions on Potato Yield and Quality in Dry Cultivation: The Role of Photosynthesis and C–N Metabolism. Agriculture 2025, 15, 2065. https://doi.org/10.3390/agriculture15192065
Meng H, Wang C, Li L, Bao X, Tian X, Xie J, Wang L, Luo Z. The Effect of Nitrogen Dose and Plant Density Interactions on Potato Yield and Quality in Dry Cultivation: The Role of Photosynthesis and C–N Metabolism. Agriculture. 2025; 15(19):2065. https://doi.org/10.3390/agriculture15192065
Chicago/Turabian StyleMeng, Haofeng, Chunyan Wang, Lingling Li, Xiaoyan Bao, Xin Tian, Junhong Xie, Linlin Wang, and Zhuzhu Luo. 2025. "The Effect of Nitrogen Dose and Plant Density Interactions on Potato Yield and Quality in Dry Cultivation: The Role of Photosynthesis and C–N Metabolism" Agriculture 15, no. 19: 2065. https://doi.org/10.3390/agriculture15192065
APA StyleMeng, H., Wang, C., Li, L., Bao, X., Tian, X., Xie, J., Wang, L., & Luo, Z. (2025). The Effect of Nitrogen Dose and Plant Density Interactions on Potato Yield and Quality in Dry Cultivation: The Role of Photosynthesis and C–N Metabolism. Agriculture, 15(19), 2065. https://doi.org/10.3390/agriculture15192065






