Estimation of Available Phosphorus Under Phosphorus Fertilization in Paddy Fields of a Cold Region Using Several Extraction Methods: A Case Study from Yamagata, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Analysis
2.2. Soil Analysis for Comparison of Available Phosphorus Analysis Methods
2.3. Pot Experiment to Assess the Effects of Accumulated Phosphorus
2.4. Statistical Analysis
3. Results
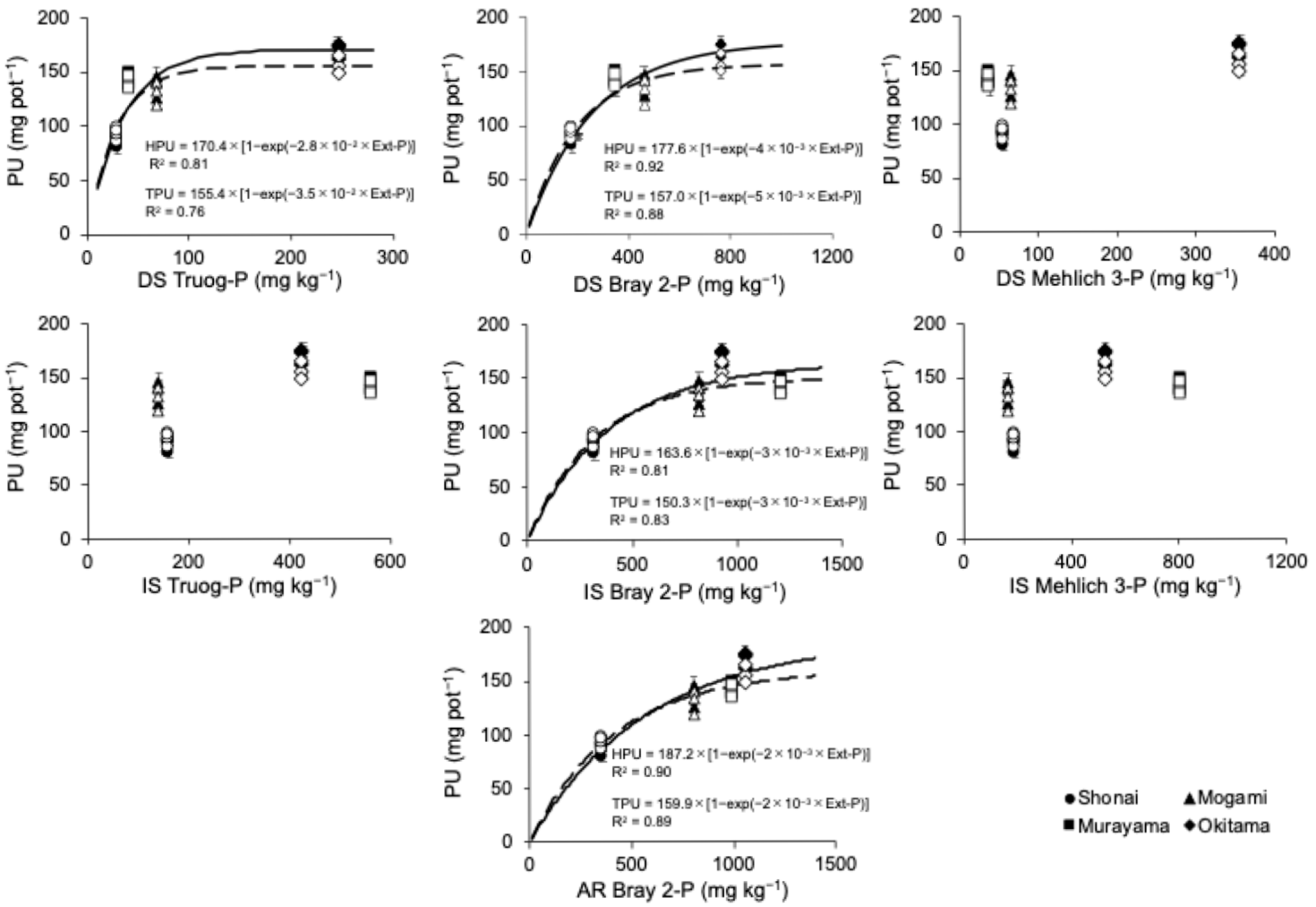
3.1. Comparison of Available Phosphorus Analysis Methods
3.2. Effect of Phosphorus Fertilization on Rice Growth and Development
4. Discussion
4.1. Correlation of Extraction Methods for Available P
4.2. Effects of P Treatment on Rice Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The Importance of Early Season Phosphorus Nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Shiga, H. Efficient Level of Phosphorus Fertility in Paddy Soils. Agric. Food Sci. 1981, 15, 227–241. [Google Scholar]
- Nishio, M. Recent Trend of Chemical Fertilizer Consumption in Japan. Jpn. J. Soil Sci. Plant Nutr. 2002, 73, 219–225. [Google Scholar]
- Mishima, S.-i.; Itahashi, S.; Kimura, R.; Inoue, T. Trends of Phosphate Fertilizer Demand and Phosphate Balance in Farmland Soils in Japan. Soil Sci. Plant Nutr. 2003, 49, 39–45. [Google Scholar] [CrossRef]
- Obara, H.; Nakai, M. Available Phosphate of Arable Lands in Japan. Changes of Soil Characteristics in Japanese Arable Lands (II). Jpn. J. Soil Sci. Plant Nutr. 2004, 75, 59–67. [Google Scholar]
- Goto, E.; Shu, M.; Michiko, N.; Osamu, I. The Present Condition of Chemical Properties of Paddy Soils in Hokkaido. Jpn. J. Soil Sci. Plant Nutr. 2003, 74, 475–483. [Google Scholar]
- Tohkairin, S. Fractionation and Availabilization of Phosphorus Accumulated in Paddy Soils with Application of Phosphatic Fertilizer in YAMAGATA Prefecture. Spec. Bull. Yamagata Prefect. Agric. Exp. Stn. 1992, 21, 1–48. [Google Scholar]
- Truog, E. The Determination of the Readily Available Phosphorus of Soils 1. Agron. J. 1930, 22, 874–882. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Shahandeh, H.; Hons, F.M.; Provin, T.L.; Pitt, J.L.; Waskom, J.S. Factors Affecting Mehlich III Soil Test Methodology for Extractable P. Commun. Soil Sci. Plant Anal. 2017, 48, 423–438. [Google Scholar] [CrossRef]
- Wuenscher, R.; Unterfrauner, H.; Peticzka, R.; Zehetner, F. A Comparison of 14 Soil Phosphorus Extraction Methods Applied to 50 Agricultural Soils from Central Europe. Plant Soil Environ. 2015, 61, 86–96. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H. A Method for Sectioning Saturated Soil Cores. Soil Sci. Soc. Am. J. 1976, 40, 611–612. [Google Scholar] [CrossRef]
- Upreti, K.; Joshi, S.R.; McGrath, J.; Jaisi, D.P. Factors Controlling Phosphorus Mobilization in a Coastal Plain Tributary to the Chesapeake Bay. Soil Sci. Soc. Am. J. 2015, 79, 826–837. [Google Scholar] [CrossRef]
- Uwasawa, M.; Uchida, Y. Soil Testing on Available Soil Phosphorus for Paddy Field in Cool Region of Japan. Bull. Tohoku Natl. Agric. Exp. Stn. 2011, 67, 77–95. [Google Scholar]
- Nanzyo, M.; Takahashi, T.; Shoji, S. Rapid Method to Determine Available Phosphorus Content of Paddy Soils under Reducing Conditions Using Ascorbic Acid. Jpn. J. Soil Sci. Plant Nutr. 1996, 67, 73–77. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.H.; Chen, H.; Zhao, X.; Wang, D.; Wang, S.Q.; Ding, S.M. Small-Scale Interaction of Iron and Phosphorus in Flooded Soils with Rice Growth. Sci. Total Environ. 2019, 669, 911–919. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Kulhánek, M.; Balík, J.; Černý, J.; Vaněk, V. Evaluation of Phosphorus Mobility in Soil Using Different Extraction Methods. Plant Soil Environ. 2009, 55, 267–272. [Google Scholar] [CrossRef]
- van Rotterdam, A.M.D.; Bussink, D.W.; Temminghoff, E.J.M.; van Riemsdijk, W.H. Predicting the Potential of Soils to Supply Phosphorus by Integrating Soil Chemical Processes and Standard Soil Tests. Geoderma 2012, 189–190, 617–626. [Google Scholar] [CrossRef]
- Nawara, S.; Van Dael, T.; Merckx, R.; Amery, F.; Elsen, A.; Odeurs, W.; Vandendriessche, H.; Mcgrath, S.; Roisin, C.; Jouany, C.; et al. A Comparison of Soil Tests for Available Phosphorus in Long-Term Field Experiments in Europe. Eur. J. Soil Sci. 2017, 68, 873–885. [Google Scholar] [CrossRef]
- Nanzyo, M.; Kanno, H.; Obara, S. Effect of Reducing Conditions on P Sorption of Soils. Soil Sci. Plant Nutr. 2004, 50, 1023–1028. [Google Scholar] [CrossRef]
- Japan Meteorological Agency Database. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 12 September 2024).
- Japan Soil Inventory, Soil Map. Available online: https://soil-inventory.rad.naro.go.jp/ (accessed on 12 September 2024).
- Corey, R.B. A Textbook of Soil Chemical Analysis; Chemical Publishing Company: Revere, MA, USA, 1973; Volume 37. [Google Scholar]
- Nishikawa, T.; Li, K.; Inamura, T.; Nishikawa, T.; Li, K.; Nishikawa, T. Nitrogen Uptake by the Rice Plant and Changes in the Soil Chemical Properties in the Paddy Rice Field during Yearly Application of Anaerobically-Digested Manure for Seven Years. Plant Prod. Sci. 2014, 17, 237–244. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Childs, C.W. Estimation of Forms of Fe and Al: A Review, and Analysis of Contrasting Soils by Dissolution and Moessbauer Methods. Aust. J. Soil Res. 1988, 26, 121–144. [Google Scholar] [CrossRef]
- Gee, G.W.; Or, D. Particle-Size Analysis. In Methods of Soil Analysis, Part 4: Physical Methods; John Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 255–293. ISBN 9780891188933. [Google Scholar]
- Ishioka, G. Measurement of Nitrogen Release from Soil and Organic Materials under Flooded Conditions Using Aluminum-Deposited Bags. Jpn. J. Soil Sci. Plant Nutr. 2023, 94, 115–120. [Google Scholar]
- Frossard, E.; Morel, J.L.; Fardeau, J.C.; Brossard, M. Soil Isotopically Exchangeable Phosphorus: A Comparison between E and L Values. Soil Sci. Soc. Am. J. 1994, 58, 846–851. [Google Scholar] [CrossRef]
- Chuba, M. Breeding of a Rice Cultivar ‘Tsuyahime’ with Wide Adaptability and Superior Eating Quality, Using a New Appearance Evaluation Method of Cooked Rice. Breed. Res. 2022, 24, 52–58. [Google Scholar] [CrossRef]
- Fertilization Standards in Yamagata Prefecture. Available online: https://agrin.jp/documents/859/image1_file0119051020334431045.pdf (accessed on 12 September 2024).
- Kashiwagura, M.; Sato, Y.; Uno, T.; Tajima, R.; Ito, T.; Saito, M. Microscale Digestion of Plant Samples with Sulicacid and Oxygen Peroxide. Bull. Integr. Field Sci. Cent. 2015, 30, 1–2. [Google Scholar] [CrossRef]
- R Core Team. R Core Team 2023 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 5 December 2023).
- Ando, K.; Yamaguchi, N.; Nakamura, Y.; Kasuya, M.; Taki, K. Speciation of Phosphorus Accumulated in Fertilized Cropland of Aichi Prefecture in Japan with Different Soil Properties by Sequential Chemical Extraction and P K-Edge XANES. Soil Sci. Plant Nutr. 2021, 67, 150–161. [Google Scholar] [CrossRef]
- Nanzyo, M. Progress and Prospect of the Research on Paddy Soil Management under Various Rice Growing System. 1. Progress in Nutrient Behavior and Management Research on Paddy Soil (2) Phosphorus. Jpn. J. Soil Sci. Plant Nutr. 1996, 67, 317–321. [Google Scholar]
- Yan, X.; Wei, Z.; Wang, D.; Zhang, G.; Wang, J. Phosphorus Status and Its Sorption-Associated Soil Properties in a Paddy Soil as Affected by Organic Amendments. J. Soils Sediments 2015, 15, 1882–1888. [Google Scholar] [CrossRef]
- Nishida, M.; Yoshida, K.; Takahashi, T. Estimation of Changes in Available Soil Phosphate under Submerged Conditions Associated with Temperature during the Tillering Stage of Rice Plant in the Cool Climate Region of Japan. Commun. Soil Sci. Plant Anal. 2018, 49, 1695–1706. [Google Scholar] [CrossRef]
- Shiga, H. Effect of Phosphorus Fertility of Soils and Phosphate Application on Rice Culture in Cool Region. Part 1 Measurement of Phosphorus Supplying Ability of Paddy Soils. Res. Bull. Hokkaido Natl. Agric. Exp. Stn. 1973, 105, 31–49. [Google Scholar]
- Golterman, H.L. Fractionation of Sediment Phosphate with Chelating Compounds: A Simplification, and Comparison with Other Methods. Hydrobiologia 1996, 335, 87–95. [Google Scholar] [CrossRef]
- Marschner, H. Functions of Mineral Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2002; pp. 313–404. [Google Scholar]
- Yan, X.; Wei, Z.; Hong, Q.; Lu, Z.; Wu, J. Phosphorus Fractions and Sorption Characteristics in a Subtropical Paddy Soil as Influenced by Fertilizer Sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, G.H.; Feng, Y.K.; Sun, Q.Z.; Witt, C.; Dobermann, A. Changes in Soil Phosphorus Fractions in a Calcareous Paddy Soil under Intensive Rice Cropping. Plant Soil 2006, 288, 141–154. [Google Scholar] [CrossRef]
- Nishigaki, T.; Sugihara, S.; Kobayashi, K.; Hashimoto, Y.; Kilasara, M.; Tanaka, H.; Watanabe, T.; Funakawa, S. Fractionation of Phosphorus in Soils with Different Geological and Soil Physicochemical Properties in Southern Tanzania. Soil Sci. Plant Nutr. 2018, 64, 291–299. [Google Scholar] [CrossRef]
- Darke, A.K.; Walbridge, M.R. Al and Fe Biogeochemistry in a Floodplain Forest: Implications for P Retention. Biogeochemistry 2000, 51, 1–32. [Google Scholar] [CrossRef]
- Nanzyo, M.; Yamasaki, S. Phosphorus-Bearing Mineral in Fresh, Andesite and Rhyolite Tephras in Northern Part of Japan. Phosphorus Res. Bull. 1998, 8, 95–100. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Wang, D.; Zhang, H.; Zhang, G.; Wei, Z. Organic Amendments Affect Phosphorus Sorption Characteristics in a Paddy Soil. Agric. Ecosyst. Environ. 2013, 175, 47–53. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Jdrgensen, S.S.; Moberg, J.P.; Raben-lange, B. Influence of Organic Matter on Phosphate Adsorption by Aluminium and Iron Oxides in Sandy Soils. J. Soil Sci. 1990, 41, 443–449. [Google Scholar] [CrossRef]
- Kang, J.; Hesterberg, D.; Osmond, D.L. Soil Organic Matter Effects on Phosphorus Sorption: A Path Analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, Phosphorus, and Potassium Fertilization Affects the Flowering Time of Rice (Oryza Sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Guo, H.S.; Xie, Q.; Fei, J.F.; Chua, N.H. MicroRNA Directs MRNA Cleavage of the Transcription Factor NAC1 to Downregulate Auxin Signals for Arabidopsis Lateral Root Development. Plant Cell 2005, 17, 1376–1386. [Google Scholar] [CrossRef]
- Panten, K.; Godlinski, F.; Schroetter, S.; Hofmeier, M. Variability of P Uptake by Plants. In Phosphorus in Agriculture: 100% Zero; Springer: Dordrecht, The Netherlands, 2016; pp. 155–178. ISBN 9789401776127. [Google Scholar]
- Shiga, H.; Yamaguchi, Y.; Awazaki, H. Effect of Phosphorus Fertility of Soil and Phosphate Application on Rice Culture in Cool Region. Part 2 Response of Rice Plant Growth to the Amounts of Extractable Phosphorus in Flooded Soils under Field Conditions. Res. Bull. Hokkaido Natl. Agric. Exp. Stn. 1976, 113, 95–107. [Google Scholar]
- Ito, T.; Kikawa, N.; Saigusa, M. Phosphorus Sorption and Bioavailability in Allophanic and Non AllophanicAndosol. Pedologist 2011, 55, 84–88. [Google Scholar]
- Tanikawa, N. Effect of Available Phosphorus or Phosphorus Fractions on Paddy Rice Growth in Tillering Stage. Tohoku Agric. Res. 2016, 69, 11–12. [Google Scholar]
- Nasukawa, H.; Tajima, R.; Muacha, B.I.J.; Filomena Pereira, M.C.; Naruo, K.; Nakamura, S.; Fukuda, M.; Ito, T.; Homma, K. Analyzing Soil-Available Phosphorus by the Mehlich-3 Extraction Method to Recommend a Phosphorus Fertilizer Application Rate for Maize Production in Northern Mozambique. Plant Prod. Sci. 2019, 22, 211–214. [Google Scholar] [CrossRef]
- Zhang, H.; Kariuki, S.; Schroder, J.L.; Payton, M.E.; Focht, C. Interlaboratory Validation of the Mehlich 3 Method for Extraction of Plant-Available Phosphorus. J. AOAC Int. 2009, 92, 91–102. [Google Scholar] [CrossRef]
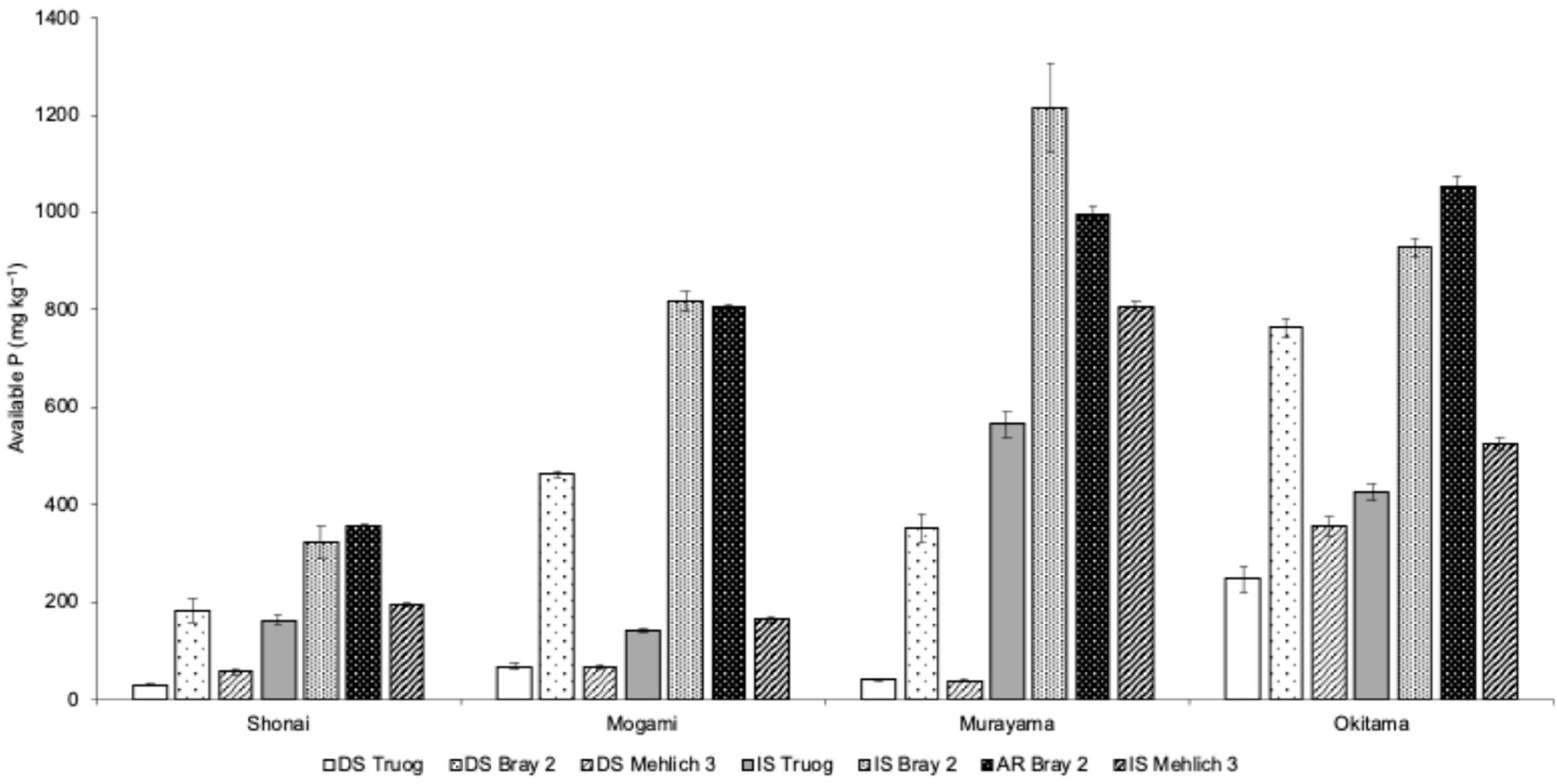
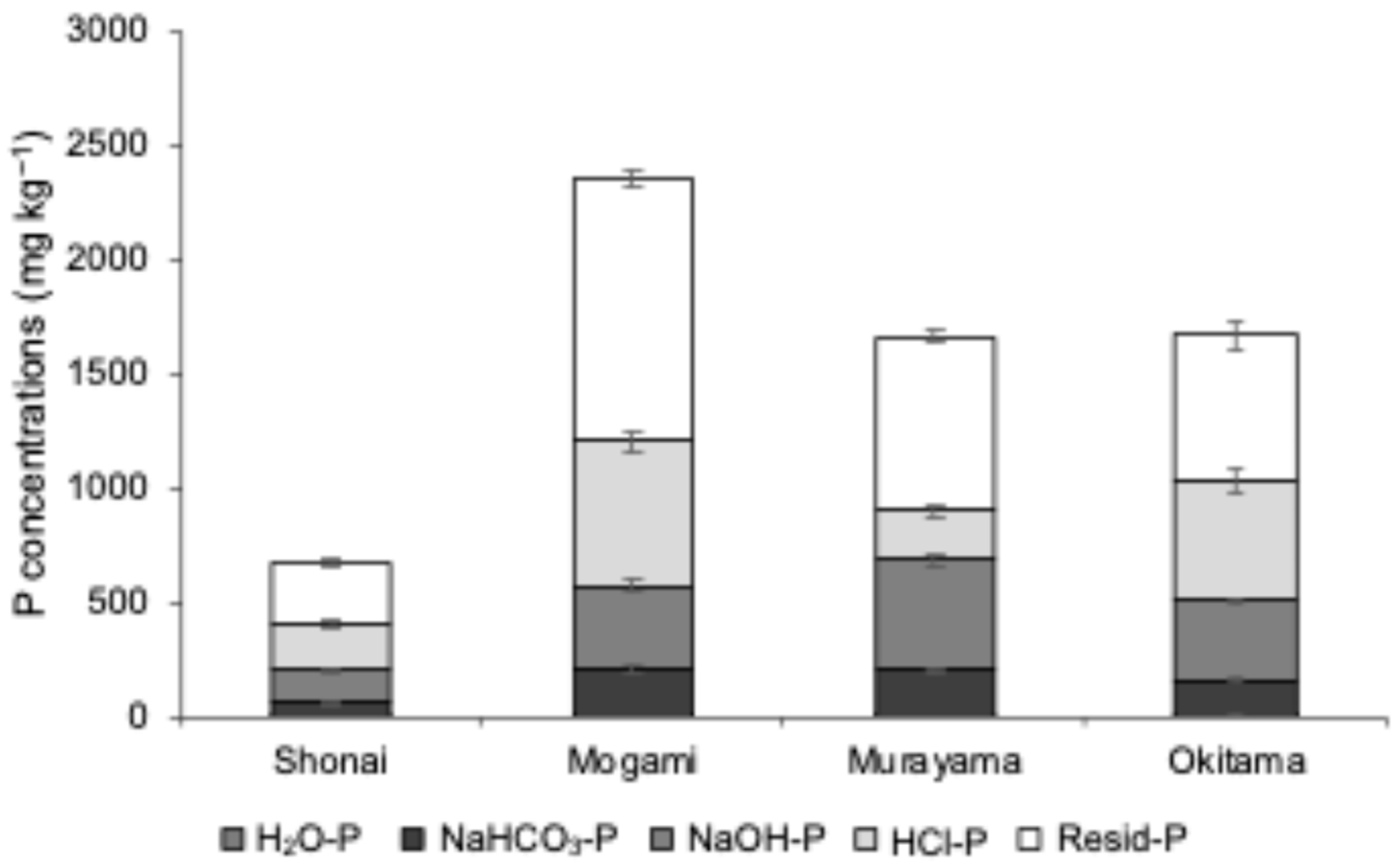
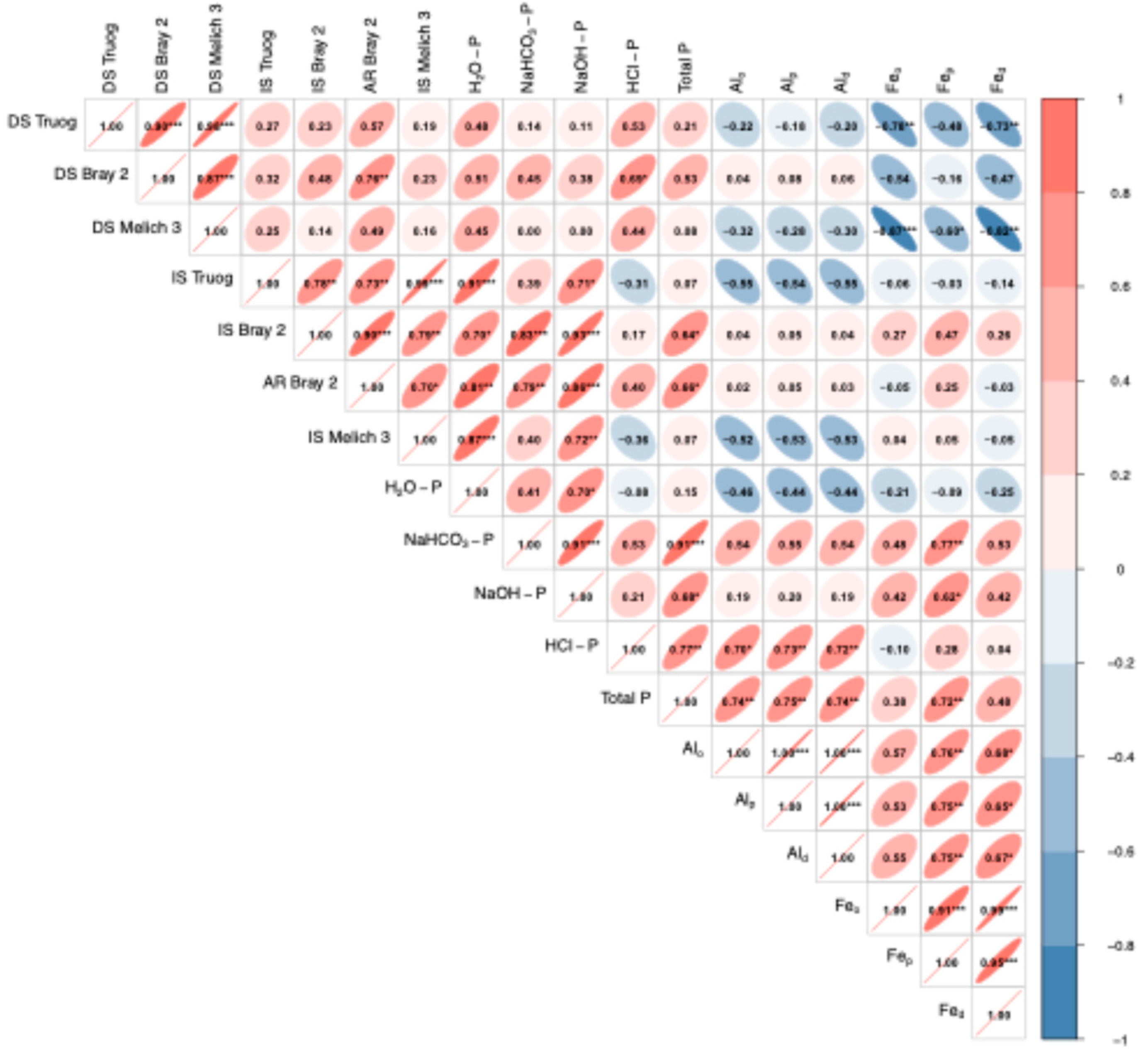
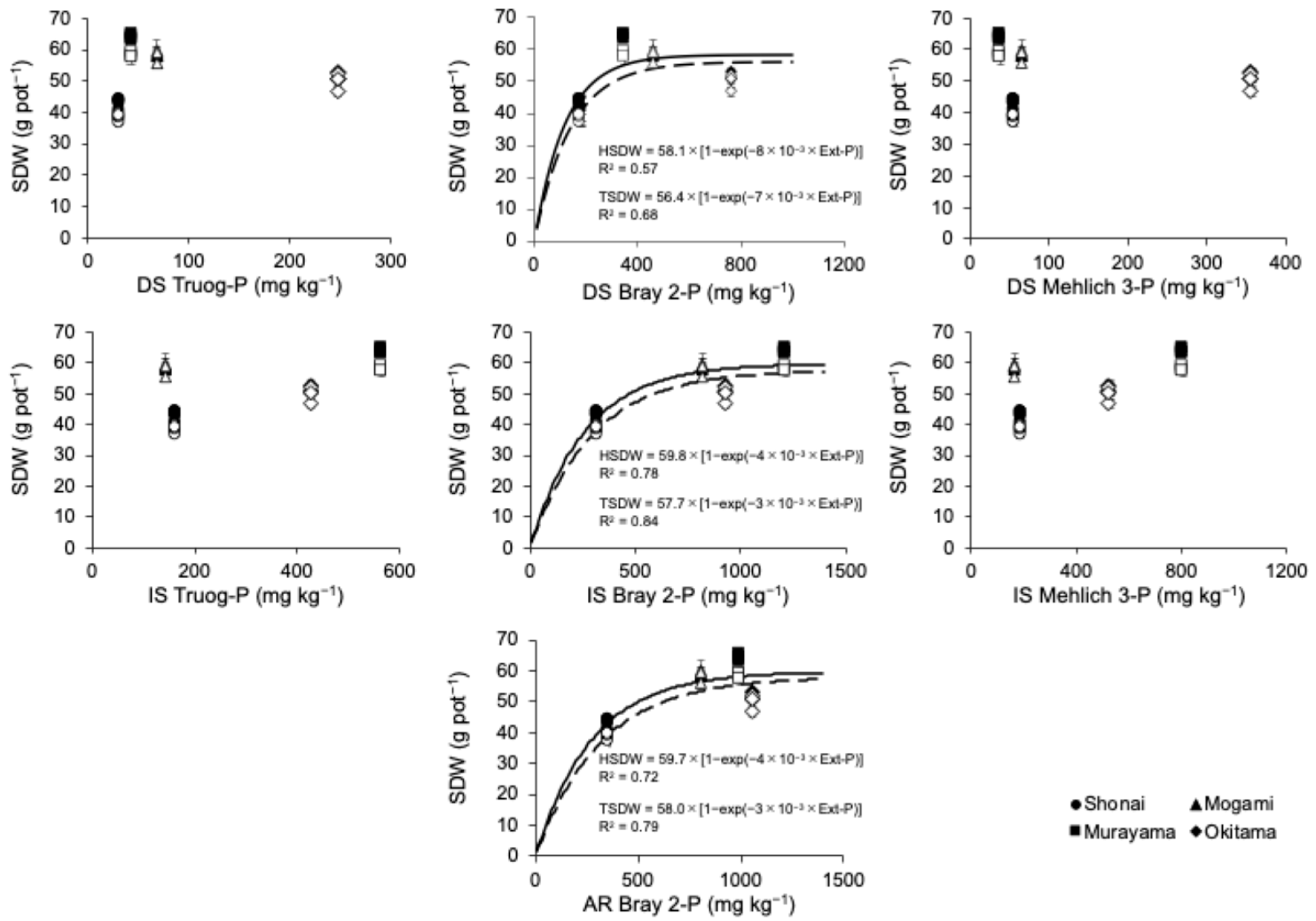
| Location | pH | EC | Total | CEC | Exchangeable Cation | Available N | Particle Size Distribution | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | Ca | Mg | K | Clay | Silt | Sand | |||||
| (μS cm−1) | (%) | (cmolc kg−1) | (cmolc kg−1) | (mg kg−1) | (%) | |||||||
| Shonai | 5.9 | 126.4 | 2.0 | 0.1 | 12.3 | 7.4 | 1.6 | 0.6 | 50.3 | 7.6 | 18.8 | 73.6 |
| Mogami | 5.8 | 35.2 | 7.2 | 0.5 | 27.7 | 5.9 | 1.2 | 0.6 | 79.6 | 26.0 | 48.5 | 25.6 |
| Murayama | 5.6 | 28.0 | 4.2 | 0.3 | 24.5 | 8.7 | 2.2 | 0.9 | 95.3 | 26.1 | 46.1 | 27.7 |
| Okitama | 5.7 | 25.5 | 3.0 | 0.3 | 15.0 | 9.1 | 1.9 | 0.4 | 77.5 | 13.0 | 29.9 | 57.1 |
| Location | Active Al | Active Fe | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alo | Alp | Ald | Feo | Fep | Fed | |||||||||||||
| (g kg−1) | (g kg−1) | |||||||||||||||||
| Shonai | 2.62 | ± | 0.06 | 0.93 | ± | 0.02 | 2.56 | ± | 0.02 | 7.07 | ± | 0.09 | 2.88 | ± | 0.01 | 13.38 | ± | 0.07 |
| Mogami | 17.99 | ± | 0.19 | 16.15 | ± | 0.21 | 14.18 | ± | 0.07 | 11.04 | ± | 0.03 | 9.74 | ± | 0.03 | 19.45 | ± | 0.05 |
| Murayama | 3.58 | ± | 0.05 | 1.81 | ± | 0.26 | 3.24 | ± | 0.09 | 11.13 | ± | 0.03 | 8.04 | ± | 0.17 | 17.99 | ± | 0.19 |
| Okitama | 2.45 | ± | 0.02 | 1.46 | ± | 0.03 | 2.68 | ± | 0.04 | 3.19 | ± | 0.02 | 2.43 | ± | 0.01 | 9.35 | ± | 0.18 |
| Variety | Location | Treatment | SDW | P Concentration | PU | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g pot−1) | (%) | (mg pot−1) | |||||||||
| Haenuki | Shonai | P0 | 40.4 | ± | 2.7 | 0.20 | ± | 0.004 | 80.5 | ± | 2.7 |
| P1/2 | 43.2 | ± | 1.9 | 0.21 | ± | 0.010 | 92.1 | ± | 1.9 | ||
| P1 | 44.1 | ± | 1.4 | 0.20 | ± | 0.005 | 88.5 | ± | 1.4 | ||
| P4/3 | 44.1 | ± | 1.1 | 0.21 | ± | 0.004 | 91.7 | ± | 1.1 | ||
| P2 | 42.7 | ± | 0.1 | 0.21 | ± | 0.011 | 90.2 | ± | 0.1 | ||
| Mogami | P0 | 58.2 | ± | 2.9 | 0.24 | ± | 0.011 | 141.3 | ± | 2.9 | |
| P1/2 | 58.3 | ± | 1.5 | 0.25 | ± | 0.008 | 146.7 | ± | 1.5 | ||
| P1 | 58.8 | ± | 2.2 | 0.22 | ± | 0.007 | 126.2 | ± | 2.2 | ||
| Murayama | P0 | 62.9 | ± | 0.7 | 0.22 | ± | 0.004 | 139.4 | ± | 0.7 | |
| P1/2 | 64.9 | ± | 1.5 | 0.23 | ± | 0.007 | 149.7 | ± | 1.5 | ||
| P1 | 63.9 | ± | 1.2 | 0.21 | ± | 0.003 | 137.7 | ± | 1.2 | ||
| Okitama | P0 | 50.6 | ± | 2.5 | 0.34 | ± | 0.020 | 175.2 | ± | 2.5 | |
| P1/2 | 50.7 | ± | 1.2 | 0.34 | ± | 0.003 | 174.1 | ± | 1.2 | ||
| P1 | 53.0 | ± | 0.6 | 0.31 | ± | 0.020 | 162.3 | ± | 0.6 | ||
| Tsuyahime | Shonai | P0 | 38.0 | ± | 1.2 | 0.26 | ± | 0.011 | 97.0 | ± | 2.0 |
| P1/2 | 39.2 | ± | 0.6 | 0.25 | ± | 0.012 | 98.2 | ± | 4.3 | ||
| P1 | 36.7 | ± | 1.5 | 0.23 | ± | 0.003 | 85.9 | ± | 4.4 | ||
| P4/3 | 38.5 | ± | 1.0 | 0.24 | ± | 0.008 | 93.0 | ± | 5.4 | ||
| P2 | 39.0 | ± | 0.4 | 0.25 | ± | 0.003 | 96.0 | ± | 2.1 | ||
| Mogami | P0 | 55.8 | ± | 1.6 | 0.21 | ± | 0.002 | 119.5 | ± | 4.5 | |
| P1/2 | 59.8 | ± | 3.5 | 0.24 | ± | 0.018 | 141.0 | ± | 1.9 | ||
| P1 | 59.2 | ± | 1.1 | 0.23 | ± | 0.003 | 133.7 | ± | 4.2 | ||
| Murayama | P0 | 58.2 | ± | 1.4 | 0.24 | ± | 0.003 | 137.8 | ± | 4.7 | |
| P1/2 | 59.0 | ± | 2.0 | 0.25 | ± | 0.010 | 145.9 | ± | 1.9 | ||
| P1 | 57.3 | ± | 1.8 | 0.23 | ± | 0.008 | 134.4 | ± | 7.8 | ||
| Okitama | P0 | 52.2 | ± | 2.0 | 0.30 | ± | 0.006 | 155.3 | ± | 3.0 | |
| P1/2 | 46.7 | ± | 1.6 | 0.32 | ± | 0.005 | 149.7 | ± | 5.7 | ||
| P1 | 50.6 | ± | 1.2 | 0.33 | ± | 0.016 | 165.6 | ± | 4.7 | ||
| Variety | *** | *** | ns | ||||||||
| Soil | *** | *** | *** | ||||||||
| Treatment | ns | * | ns | ||||||||
| Variety × Soil | ** | *** | ** | ||||||||
| Variety × Treatment | ns | ns | ns | ||||||||
| Soil × Treatment | ns | ns | ns | ||||||||
| Variety × Soil × Treatment | ns | ns | ns | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsumuraya, S.; Nasukawa, H.; Tajima, R. Estimation of Available Phosphorus Under Phosphorus Fertilization in Paddy Fields of a Cold Region Using Several Extraction Methods: A Case Study from Yamagata, Japan. Agriculture 2025, 15, 1453. https://doi.org/10.3390/agriculture15131453
Tsumuraya S, Nasukawa H, Tajima R. Estimation of Available Phosphorus Under Phosphorus Fertilization in Paddy Fields of a Cold Region Using Several Extraction Methods: A Case Study from Yamagata, Japan. Agriculture. 2025; 15(13):1453. https://doi.org/10.3390/agriculture15131453
Chicago/Turabian StyleTsumuraya, Shuhei, Hisashi Nasukawa, and Ryosuke Tajima. 2025. "Estimation of Available Phosphorus Under Phosphorus Fertilization in Paddy Fields of a Cold Region Using Several Extraction Methods: A Case Study from Yamagata, Japan" Agriculture 15, no. 13: 1453. https://doi.org/10.3390/agriculture15131453
APA StyleTsumuraya, S., Nasukawa, H., & Tajima, R. (2025). Estimation of Available Phosphorus Under Phosphorus Fertilization in Paddy Fields of a Cold Region Using Several Extraction Methods: A Case Study from Yamagata, Japan. Agriculture, 15(13), 1453. https://doi.org/10.3390/agriculture15131453







