Regulatory Effects of Soil Microbes and Soil Properties on Ecosystem Multifunctionality Differ Among Grassland Types in the Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Sampling
2.2. Analysis of Soil Physicochemical Variables
2.3. Quantification of Ecosystem Multifunctionality
2.4. Soil DNA Extraction and High-Throughput Sequencing
2.5. Data Analysis
3. Results
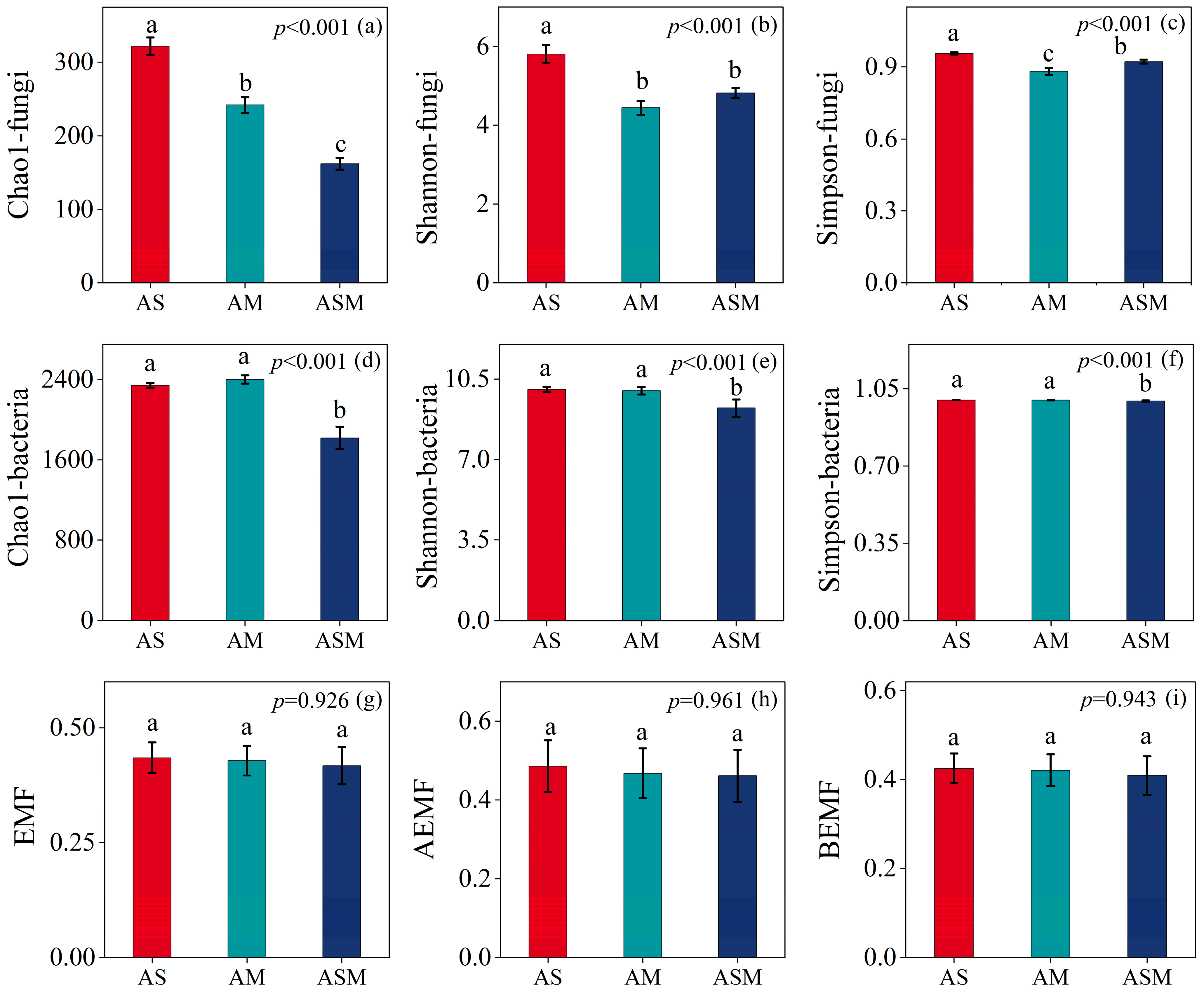
3.1. Soil Microbial Community Multidimensional Attributes and Ecosystem Multifunctionality
3.2. Relationship Between Soil Microbial Multidimensional Attributes and Ecosystem Multifunctionality
3.3. The Drivers of Ecosystem Multifunctionality
4. Discussion
4.1. Bacterial Community Composition and Soil pH Regulate EMF in Alpine Steppe
4.2. Fungal Network Complexity and Soil Moisture Regulate EMF in Alpine Meadow
4.3. Regulatory Effects of Soil Moisture and Bulk Density on EMF in Alpine Swamp Meadow
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Baquerizo, M.D.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Tang, R.; Zhu, B.; Su, J. Soil microbial diversity and network complexity sustain ecosystem multifunctionality following afforestation in a dry-hot valley savanna. Catena 2023, 231, 107329. [Google Scholar] [CrossRef]
- Luo, S.; Png, G.K.; Ostle, N.J.; Zhou, H.; Hou, X.; Luo, C.; Quinton, J.N.; Schaffner, U.; Sweeney, C.; Wang, D.; et al. Grassland degradation-induced declines in soil fungal complexity reduce fungal community stability and ecosystem multifunctionality. Soil Biol. Biochem. 2023, 176, 108865. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, J.; Lu, X.; Cai, Y.; Jiang, H.; Zhang, Q.; Zhang, K. Fungal-bacterial composition and network complexity determine soil multifunctionality during ecological restoration. Catena 2023, 230, 107251. [Google Scholar] [CrossRef]
- Gong, X.; Jarvie, S.; Wen, J.; Su, N.; Yan, Y.; Liu, Q.; Zhang, Q. Compared with soil fungal diversity and microbial network complexity, soil bacterial diversity drives soil multifunctionality during the restoration process. J. Environ. Manag. 2024, 354, 120379. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Xu, X.; Wang, C.; Liu, G.; Liang, C.; Zuo, X.; Wang, C.; Lv, Y.; Wang, R. Different facets of bacterial and fungal communities drive soil multifunctionality in grasslands spanning a 3500 km transect. Funct. Ecol. 2022, 36, 3120–3133. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Zhang, R.; Tian, D.; Wang, P.; Song, L.; Quan, Q.; Chen, C.; Niu, S.; Zhang, X.; et al. Soil microbial gene abundance rather than diversity and network complexity predominantly determines soil multifunctionality in Tibetan alpine grasslands along a precipitation gradient. Funct. Ecol. 2024, 38, 1210–1221. [Google Scholar] [CrossRef]
- Yang, W.-S.; Liu, Y.; Zhao, J.; Chang, X.; Wiesmeier, M.; Sun, J.; López-Vicente, M.; García-Ruiz, R.; Gómez, J.A.; Zhou, H.; et al. SOC changes were more sensitive in alpine grasslands than in temperate grasslands during grassland transformation in China: A meta-analysis. J. Clean Prod. 2021, 308, 127430. [Google Scholar] [CrossRef]
- Yao, Z.; Shi, L.; He, Y.; Peng, C.; Lin, Z.; Hu, M.; Yin, N.; Xu, H.; Zhang, D.; Shao, X. Grazing intensity, duration, and grassland type determine the relationship between soil microbial diversity and ecosystem multifunctionality in Chinese grasslands: A meta-analysis. Ecol. Indic. 2023, 154, 110801. [Google Scholar] [CrossRef]
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H.; et al. Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Delgado-Baquerizo, M.; Jayaramaiah, R.H.; Tao, D.; Wang, J.T.; Sáez-Sandino, T.; Liu, H.; Maestre, F.T.; Reich, P.B.; Singh, B.K. Aboveground and belowground biodiversity have complementary effects on ecosystem functions across global grasslands. PLoS Biol. 2024, 22, e3002736. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Liu, Y.; Xu, Q.; Zhu, C.; Ling, N.; Guo, J.; Li, R.; Huang, W.; Guo, S.; et al. Relative importance of altitude shifts with plant and microbial diversity to soil multifunctionality in grasslands of north-western China. Plant Soil 2024, 504, 545–560. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Cheng, J.; Wei, G.; Lin, Y. Above- and belowground biodiversity drives soil multifunctionality along a long-term grassland restoration chronosequence. Sci. Total Environ. 2021, 772, 145010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Trivedi, C.; Delgado-Baquerizo, M.; Hamonts, K.; Lai, K.; Reich, P.B.; Singh, B.K. Losses in microbial functional diversity reduce the rate of key soil processes. Soil Biol. Biochem. 2019, 135, 267–274. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, M.; Bai, S.H.; Xu, Z.; Liu, Y.; Chen, F.; Guo, X.; Luo, H.; Wang, S.; Xie, J.; et al. Successive mineral nitrogen or phosphorus fertilization alone significantly altered bacterial community rather than bacterial biomass in plantation soil. Appl. Microbiol. Biotechnol. 2020, 104, 7213–7224. [Google Scholar] [CrossRef]
- Fierer, N.; Ladau, J.; Clemente, J.C.; Leff, J.W.; Owens, S.M.; Pollard, K.S.; Knight, R.; Gilbert, J.A.; Mcculley, R.L. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 2013, 342, 621–624. [Google Scholar] [CrossRef]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Wang, J.; Dong, Z.; Meng, Z.; Xu, R.; Ji, Y.; Li, Y.; Chen, J.; Qi, X.; et al. Natural restoration enhances soil multitrophic network complexity and ecosystem functions in the Loess Plateau. Catena 2023, 226, 107059. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Change Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Gao, X.; Dong, S.; Xu, Y.; Li, Y.; Li, S.; Wu, S.; Shen, H.; Liu, S.; Fry, E.L. Revegetation significantly increased the bacterial-fungal interactions in different successional stages of alpine grasslands on the Qinghai-Tibetan Plateau. Catena 2021, 205, 105385. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J. The impacts of climate change and human activities on biogeochemical cycles on theQ inghai-T ibetanP lateau. Glob. Change Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; Zhang, H.; Li, J. Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau. Int. J. Environ. Res. Public Health 2022, 19, 13026. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, J.; Qu, G.; Li, R.; Wu, G. The drought-induced succession decreased ecosystem multifunctionality of alpine swamp meadow. Catena 2023, 231, 107358. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, L.; Jing, X.; Wang, J.; Shi, Y.; Chu, H.; He, J.S. Above- and belowground biodiversity jointly drive ecosystem stability in natural alpine grasslands on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2021, 30, 1418–1429. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, M.-A.; Shi, L.; Wu, Q.; Zhang, D.; Liu, G.; Shao, X.; Liu, D. Driving factors of plant and soil properties on ecosystem multifunctionality vary among grassland types in the Qinghai-Tibetan Plateau. Plant Soil 2025, 498, 337–352. [Google Scholar] [CrossRef]
- Liu, X.; Shi, X.; Zhang, S. Soil abiotic properties and plant functional diversity co-regulate the impacts of nitrogen addition on ecosystem multifunctionality in an alpine meadow. Sci. Total Environ. 2021, 780, 146476. [Google Scholar] [CrossRef]
- Manning, P.; van der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Liu, C.; Yu, Y.; Zong, M.; Duan, C. Soil microbial communities’ contributions to soil ecosystem multifunctionality in the natural restoration of abandoned metal mines. J. Environ. Manag. 2024, 353, 120244. [Google Scholar] [CrossRef]
- Wang, C.; Yu, W.; Ma, L.; Ye, X.; Erdenebileg, E.; Wang, R.; Huang, Z.; Indree, T.; Liu, G. Biotic and abiotic drivers of ecosystem multifunctionality: Evidence from the semi-arid grasslands of northern China. Sci. Total Environ. 2023, 887, 164158. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, W.; Tian, L.; Qu, G.; Wu, G. Warming differentially affects above- and belowground ecosystem functioning of the semi-arid alpine grasslands. Sci. Total Environ. 2024, 914, 170061. [Google Scholar] [CrossRef]
- Martin, M. CUTADAPT removes adapter sequences from highthroughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Guo, A.; Yue, P.; Guo, X.; Lv, P.; Zhao, S.; Zuo, X. Species diversity is a strong predictor of ecosystem multifunctionality under altered precipitation in desert steppes. Ecol. Indic. 2022, 137, 108762. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, L.; Wei, X.; Han, B.; Peng, C.; Yao, Z.; He, Y.; Xiao, Q.; Lu, X.; Deng, Y.; et al. Soil properties and fungal community jointly explain N2O emissions following N and P enrichment in an alpine meadow. Environ. Pollut. 2024, 344, 123344. [Google Scholar] [CrossRef]
- Yang, X.; Hu, H.-W.; Yang, G.-W.; Cui, Z.; Chen, Y. Crop rotational diversity enhances soil microbiome network complexity and multifunctionality. Geoderma 2023, 436, 116562. [Google Scholar] [CrossRef]
- Wang, C.; Pan, X.; Yu, W.; Ye, X.; Erdenebileg, E.; Wang, C.; Ma, L.; Wang, R.; Huang, Z.; Indree, T.; et al. Aridity and decreasing soil heterogeneity reduce microbial network complexity and stability in the semi-arid grasslands. Ecol. Indic. 2023, 151, 110342. [Google Scholar] [CrossRef]
- Hu, M.A.; Yao, Z.; Shi, L.; Wu, Q.; Tang, S.; Shao, X. Patchy degradation-induced changes in soil aggregates and organic carbon in an alpine swamp meadow on the Qinghai-Tibetan Plateau. Land Degrad. Dev. 2024, 35, 4713–4725. [Google Scholar] [CrossRef]
- Shipley, B.; Inouye, B.D. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 2013, 94, 560–564. [Google Scholar] [CrossRef]
- Pasari, J.R.; Levi, T.; Zavaleta, E.S.; Tilman, D. Several scales of biodiversity affect ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2013, 110, 10219–10222. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, N.; Zhang, S.; Zhu, X.; Wang, H.; Xiu, W.; Zhao, J.; Liu, H.; Zhang, H.; Yang, D. Soil bacterial community composition is altered more by soil nutrient availability than pH following long-term nutrient addition in a temperate steppe. Front. Microbiol. 2024, 15, 1455891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiao, Y.; Zhang, S.; Tang, J. Litter application increases soil multinutrient cycling in alpine meadow ecosystems on the Tibetan Plateau. Appl. Soil Ecol. 2024, 202, 105566. [Google Scholar] [CrossRef]
- Aguirre-von-Wobeser, E.; Rocha-Estrada, J.; Shapiro, L.R.; de la Torre, M. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales drives selection of bacterial community from soil by maize roots in a traditional milpa agroecosystem. PLoS ONE 2018, 13, e0208852. [Google Scholar] [CrossRef] [PubMed]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.; Singh, B.K. Microbial regulation of the soil carbon cycle: Evidence from gene-enzyme relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Pascault, N.; Ranjard, L.; Kaisermann, A.; Bachar, D.; Christen, R.; Terrat, S.; Mathieu, O.; Lévêque, J.; Mougel, C.; Henault, C.; et al. Stimulation of Different Functional Groups of Bacteria by Various Plant Residues as a Driver of Soil Priming Effect. Ecosystems 2013, 16, 810–822. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, G.; Zhang, C.; Wang, G. Bacterial richness is negatively related to potential soil multifunctionality in a degraded alpine meadow. Ecol. Indic. 2021, 121, 106996. [Google Scholar] [CrossRef]
- Maestre, F.T.; Eldridge, D.J.; Soliveres, S.; Kéfi, S.; Delgado-Baquerizo, M.; Bowker, M.A.; García-Palacios, P.; Gaitán, J.; Gallardo, A.; Lázaro, R.; et al. Structure and Functioning of Dryland Ecosystems in a Changing World. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 215–237. [Google Scholar] [CrossRef]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Cui, H.; Wagg, C.; Wang, X.; Liu, Z.; Liu, K.; Chen, S.; Chen, J.; Song, H.; Meng, L.; Wang, J.; et al. The loss of above- and belowground biodiversity in degraded grasslands drives the decline of ecosystem multifunctionality. Appl. Soil Ecol. 2022, 172, 104370. [Google Scholar] [CrossRef]
- Baquerizo, M.D.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Shen, R.F. Rare microbial communities drive ecosystem multifunctionality in acidic soils of southern China. Appl. Soil Ecol. 2023, 189, 104895. [Google Scholar] [CrossRef]
- Wei, Y.; Jing, X.; Su, F.; Li, Z.; Wang, F.; Guo, H. DoespH matter for ecosystem multifunctionality? An empirical test in a semi-arid grassland on the Loess Plateau. Funct. Ecol. 2022, 36, 1739–1753. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J. The links between ecosystem multifunctionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol. Lett. 2011, 14, 1134–1142. [Google Scholar] [CrossRef]
- Maynard, D.S.; Crowther, T.W.; Bradford, M.A. Fungal interactions reduce carbon use efficiency. Ecol. Lett. 2017, 20, 1034–1042. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, D.; Wang, J.; Wang, Y.; Zhu, H.; Wu, Y.; Fang, L.; Bing, H. Fungal community determines soil multifunctionality during vegetation restoration in metallic tailing reservoir. J. Hazard. Mater. 2024, 478, 135438. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Change Biol. 2020, 26, 4506–4520. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al-Sadi, A.M.; Al-Shehi, M.; Al-Hinai, S.; Robinson, M.D. Diversity of free-living and lichenized fungal communities in biological soil crusts of the Sultanate of Oman and their role in improving soil properties. Soil Biol. Biochem. 2013, 57, 695–705. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Jia, X.; Wang, M.; Ding, J.; Cheng, L.; Bao, F.; Wu, B. Network analysis reveals the strengthening of microbial interaction in biological soil crust development in the Mu Us Sandy Land, northwestern China. Soil Biol. Biochem. 2020, 144, 107782. [Google Scholar] [CrossRef]
- Miehe, G.; Schleuss, P.-M.; Seeber, E.; Babel, W.; Biermann, T.; Braendle, M.; Chen, F.; Coners, H.; Foken, T.; Gerken, T.; et al. The Kobresia pygmaea ecosystem of the Tibetan highlands—Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem. Sci. Total Environ. 2019, 648, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Zhu, Y.N.; Singh, B.K. Fungal richness contributes to multifunctionality in boreal forest soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Z.; Yao, Z.; Peng, C.; Hu, M.; Yin, N.; Lu, X.; Zhou, H.; Liu, K.; Shao, X. Increased precipitation rather than warming increases ecosystem multifunctionality in an alpine meadow. Plant Soil 2023, 498, 357–370. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Yin, X.; Wubuli, S.; Feng, J.; Wang, C.; Wu, P.; Degen, A. Decadal warming-induced changes in abiotic factors and multitrophic diversity drive soil multifunctionality in an alpine meadow. Geoderma 2024, 450, 117035. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, L.; William, K.; Chang, Q.; Wang, H.; D’Odorico, P. Observed increasing water constraint on vegetation growth over the last three decades. Nat. Commun. 2021, 12, 3777. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, S.; Gao, X.; Yang, M.; Li, S.; Shen, H.; Xiao, J.; Han, Y.; Zhang, J.; Li, Y.; et al. Aboveground community composition and soil moisture play determining roles in restoring ecosystem multifunctionality of alpine steppe on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2021, 305, 107163. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, T.; Li, R.; Li, X.; Tian, L. Grazing effect on growing season ecosystem respiration and its temperature sensitivity in alpine grasslands along a large altitudinal gradient on the central Tibetan Plateau. Agric. For. Meteorol. 2016, 218, 114–121. [Google Scholar] [CrossRef]
- Peng, F.; Xue, X.; You, Q.; Huang, C.; Dong, S.; Liao, J.; Duan, H.; Tsunekawa, A.; Wang, T. Changes of soil properties regulate the soil organic carbon loss with grassland degradation on the Qinghai-Tibet Plateau. Ecol. Indic. 2018, 93, 572–580. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Tian, L.; Qu, G.; Wu, G. Microtopographic heterogeneity mediates the soil respiration response to grazing in an alpine swamp meadow on the Tibetan Plateau. Catena 2022, 213, 106158. [Google Scholar] [CrossRef]
- Döbert, T.F.; Bork, E.W.; Apfelbaum, S.; Cameron, N.; Carlyle, C.N.; Chang, S.X.; Khatri-Chhetri, U.; Silva Sobrinho, L.; Thompson, R.; Boyce, M.S. Adaptive multi-paddock grazing improves water infiltration in Canadian grassland soils. Geoderma 2021, 401, 115314. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Ding, J.; Fu, B.; Zhao, W.; Han, Y.; Zhou, A.; Liu, Y.; Eldridge, D. Ecosystem multifunctionality is more related to the indirect effects than to the direct effects of human management in China’s drylands. J. Environ. Manag. 2024, 368, 122259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, M.; Zhang, H.; Wang, N.; Yu, Z.; Xu, H.; Huang, P. Degradation-driven bacterial homogenization closely associated with the loss of soil multifunctionality in alpine meadows. Agric. Ecosyst. Environ. 2023, 344, 108284. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zheng, K.; Han, C.; Li, L.; Sheng, H.; Ma, Z. Changes in soil carbon and nitrogen stocks following degradation of alpine grasslands on theQinghai-Tibetan Plateau: A meta-analysis. Land Degrad. Dev. 2021, 32, 1262–1273. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Wei, X.; Liu, C.; Shi, L.; Hu, M.; Liu, G.; Shao, X. Regulatory Effects of Soil Microbes and Soil Properties on Ecosystem Multifunctionality Differ Among Grassland Types in the Qinghai-Tibetan Plateau. Agriculture 2025, 15, 1410. https://doi.org/10.3390/agriculture15131410
Yao Z, Wei X, Liu C, Shi L, Hu M, Liu G, Shao X. Regulatory Effects of Soil Microbes and Soil Properties on Ecosystem Multifunctionality Differ Among Grassland Types in the Qinghai-Tibetan Plateau. Agriculture. 2025; 15(13):1410. https://doi.org/10.3390/agriculture15131410
Chicago/Turabian StyleYao, Zeying, Xiaoting Wei, Chunyang Liu, Lina Shi, Meng’ai Hu, Guihe Liu, and Xinqing Shao. 2025. "Regulatory Effects of Soil Microbes and Soil Properties on Ecosystem Multifunctionality Differ Among Grassland Types in the Qinghai-Tibetan Plateau" Agriculture 15, no. 13: 1410. https://doi.org/10.3390/agriculture15131410
APA StyleYao, Z., Wei, X., Liu, C., Shi, L., Hu, M., Liu, G., & Shao, X. (2025). Regulatory Effects of Soil Microbes and Soil Properties on Ecosystem Multifunctionality Differ Among Grassland Types in the Qinghai-Tibetan Plateau. Agriculture, 15(13), 1410. https://doi.org/10.3390/agriculture15131410







