Effect of Plant Density on Artemisia annua L. Biomass and Essential Oil Yield and Its Weed Seed Germination Suppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Climatic Parameters
2.3. Isolation of Essential Oils
2.4. GC and GC-MS Analysis
2.5. Phytotoxic Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Density on Morpho-Productive Traits of A. annua
3.2. Essential Oil Yield and Composition
3.3. Phytotoxic Activity
3.3.1. Effect on Germination
- The EO 20D 1st year showed significant inhibitory effects at all concentrations on P. oleracea (from 28.6 to 57.1%) and on A. fatua (97.0% at 1000 µg/mL). On P. rhoeas, it showed contrasting effects, with significant stimulations at low concentrations. On the other seeds, it showed weak inhibitory activities (up to 20.0%).
- The EO 20D 2nd year increased its inhibitory activity on P. rhoeas (up to 100%), P. oleracea (up to 61.3%), and S. alba (up to 73.6%) at all concentrations and on A. fatua (87.0% at 1000 µg/mL). L. multiflorum instead underwent stimulating effects. On the other seeds, it showed weak inhibitory activities (up to 20.0%).
- In the EO 40D 1st year, the greatest inhibitory effects were present at the highest concentrations on P. rhoeas (up to 46.0%) and P. oleracea (up to 100%). On the other seeds, it showed weak inhibitory activities (up to 20.0%), and in some cases, at low concentrations, stimulating activities.
- The EO 40D 2nd year’s inhibitory activity increased, especially at the highest concentrations, on P. rhoeas (up to 83.7%), P. oleracea (up to 100%), and S. alba (up to 70.0%). On the other seeds, it showed weak inhibitory activities (up to 22.2%), and only in rare cases, low concentrations, stimulating activities.
3.3.2. Effect on Radical Elongation
- The EO 20D 1st year significantly inhibited all seeds (up to 61.1%), except P. rhoeas, for which it showed very intense stimulating activities (−125%).
- The EO 20D 2nd year maintained the strong inhibitory activity of the first year, showing strong inhibitions also on P. rhoeas (up to 100%) and significant increases on T. durum (up to 80.5%).
- The EO 40D 1st year showed high inhibitory activities on all seeds, with inhibitions reaching 100.0%. Its activities were variable instead towards L. multiflorum and P. rhoeas, where for some concentrations there was a mild inhibition (up to 33.3%), and for others, there were strong stimulating effects (up to −133.3%).
- The EO 40D 2nd year expanded its inhibitory activity on all seeds, and it was particularly high on P. rhoeas and P. oleracea, with percentages of 100% at 500 and 1000 µg/mL. The very strong stimulating activities of the first year have disappeared and are now confined only to two cases with extremely low values (−2.0 and −14.3%).
3.3.3. Comparison with the Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EO | Essential oil |
References
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crops Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Adhavan, P.; Kaur, G.; Princy, A.; Murugan, R. Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant Gram-positive, Gram-negative and Candida albicans. Ind. Crops Prod. 2017, 100, 106–116. [Google Scholar] [CrossRef]
- Romano, C.A.; Santana Paz, A.T.; de Paiva Brandão, M.L.; Sardeiro Vieira, T.E.; de Oliveira-Neto, J.R.; da Cunha, L.C.; dos Santos, A.H.; de Paula, J.R. Seasonal variation of essential oil from Murraya koenigii (Rutaceae) and insecticidal potential against Aedes aegypti (Diptera: Culicidae). Biochem. System. Ecol. 2014, 112, 104748. [Google Scholar] [CrossRef]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal Activity of Thirty Essential Oils to Control Pathogenic Fungi of Postharvest Decay. Antibiotics 2024, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Sotomayor, J.A.; Jordán, M.J. Salvia verbenaca L. essential oil: Variation of yield and composition according to collection site and phenophase. Biochem. System. Ecol. 2019, 82, 35–43. [Google Scholar] [CrossRef]
- Schmiderer, C.; Torres-Londoño, P.; Novak, J. Proof of geographical origin of Albanian sage by essential oil analysis. Biochem. System. Ecol. 2013, 51, 70–77. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crops Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-Central Italy). Ind. Crops Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Tibaldi, G.; Hazrati, S.; Hosseini, S.J.; Ertani, A.; Bulgari, R.; Nicola, S. Cultivation techniques and drying process can affect the inflorescence essential oil composition of three selections of Salvia officinalis. Ind. Crops Prod. 2022, 183, 114923. [Google Scholar] [CrossRef]
- Saki, A.; Mozafari, H.; Asl, K.K.; Sani, B.; Mirza, M. Plant Yield, Antioxidant Capacity and Essential Oil Quality of Satureja mutica Supplied with Cattle Manure and Wheat Straw in Different Plant Densities. Commun. Soil Sci. Plant Anal. 2019, 50, 2683–2693. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; La Bella, S.; Leto, C.; Inguanta, R.; Licata, M. Effects of plant density on the number of glandular trichomes and on yield and quality of essential oils from oregano. Nat. Prod. Commun. 2016, 11, 849–852. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Afrazeh, Z.; Azizi, R.; Sendi, J.J. Efficiency of Artemisia annua L. essential oil and its chitosan/tripolyphosphate or zeolite encapsulated form in controlling Sitophilus oryzae L. Pestic. Biochem. Physiol. 2023, 195, 105544. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Sendi, J.J.; Mahmoodi, N. The sweet wormwood essential oil and its two major constituents are promising for a safe control measure against fall webworm. Pestic. Biochem. Physiol. 2022, 184, 105124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; You, C.-X.; Yang, K.; Chen, R.; Wang, Y.; Wu, Y.; Geng, Z.-F.; Chen, H.-P.; Jiang, H.-Y.; Su, Y. Bioactivity of essential oil of Artemisia argyi Lévl. et Van. and its main compounds against Lasioderma serricorne. J. Oleo Sci. 2014, 63, 829–837. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Chifiriuc, C.M.; Badea, I.A.; Buleandra, M.; Lazar, V. Chemical composition and antipathogenic activity of Artemisia annua essential oil from Romania. Chem. Biodivers. 2015, 12, 1554–1564. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research advances on health effects of edible artemisia species and some sesquiterpene lactones constituents. Foods 2021, 10, 65. [Google Scholar] [CrossRef]
- Ur Rashid, M.; Alamzeb, M.; Ali, S.; Ullah, Z.; Shah, Z.A.; Naz, I.; Khan, M.R. The chemistry and pharmacology of alkaloids and allied nitrogen compounds from Artemisia species: A review. Phytother. Res. 2019, 33, 2661–2664. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Carlen, C.; Koch, S.; Christ, B.; Simonnet, X. Influence of planting density and development stage on photosynthetically absorbed radiation, biomass and artemisinin yield of Artemisia annua L. ‘Apollon’. Acta Hortic. 2023, 1358, 91–97. [Google Scholar] [CrossRef]
- Società Italiana della Scienza del Suolo—SISS. Metodi di Analisi Chimica del Suolo; Angeli, F., Ed.; Ministero delle Politiche Agricole e Forestali: Milan, Italy, 2000. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Irrigation and Drainage Paper 56. In Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998; Volume 56, p. e156. [Google Scholar]
- Ram, M.; Gupta, M.M.; Dwivedi, S.; Kumar, S. Effect of plant density on the yields of artemisinin and essential oil in Artemisia annua cropped under low input cost management in North-Central India. Planta Med. 1997, 63, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Jennings, W.; Shibamoto, T. Qualitative Analyisis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20 M phases. J. Chromatogr. 1980, 503, 1–24. [Google Scholar] [CrossRef]
- Goodner, K.I. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavour and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- McLafferty, F.W. The Wiley Registry of Mass Spectral Data, with Nist Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Scarcella, M.; Grassi, F.; Mastrorilli, M. Artemisia annua L.: Agro-techniques for semi-arid environments. Ital. J. Agron. 2011, 6, 166–170. [Google Scholar] [CrossRef]
- Zouari, S.; Ayad, I.; Fakhfakh, N.; Jdir, H.; Aloui, L.; Kossentini, M.; Rebai, A.; Zouari, N. Essential oil variation in wild populations of Artemisia saharae (Asteraceae) from Tunisia: Chemical composition, antibacterial and antioxidant properties. Bot. Stud. 2014, 55, 65. [Google Scholar] [CrossRef]
- Cabo, J.; Crespo, M.E.; Jimenez, J.; Navarro, C.; Risco, S. Seasonal variation of essential oil yield and composition of Thymus hyemalis. Planta Med. 1987, 53, 380–382. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition’. Ind. Crops Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Cherif, A.; Zoghlami, N. Effect of environmental conditions on chemical polymorphism and biological activities among Artemisia absinthium L. essential oil provenances grown in Tunisia. Ind. Crops Prod. 2015, 66, 96–102. [Google Scholar] [CrossRef]
- Khan, S.; Sahar, A.; Tariq, T.; Sameen, A.; Tariq, F. Essential oils in plants: Plant physiology, the chemical composition of the oil, and natural variation of the oils (chemotaxonomy and environmental effects, etc.). In Essential Oils; Academic Press: Cambridge, UK, 2023; pp. 1–23. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Effect of environmental factors on essential oil biosynthesis, chemical stability, and yields. In Plant Essential Oils: From Traditional to Modern-Day Application; Springer Nature: Singapore, 2023; pp. 225–247. [Google Scholar]
- Xie, D.Y.; Ma, D.M.; Judd, R.; Jones, A.L. Artemisinin biosynthesis in Artemisia annua and metabolic engineering: Questions, challenges, and perspectives. Phytochem. Rev. 2016, 15, 1093–1110. [Google Scholar] [CrossRef]
- Arsenault, P.R.; Vail, D.R.; Wobbe, K.K.; Weathers, P.J. Effect of sugars on artemisinin production in Artemisia annua L.: Transcription and metabolite measurements. Molecules 2010, 15, 2302–2318. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol. Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Choudhary, S.M.; Naeem, M.; Masroor, M.; Khan, A.; Tariq Aftab, T. A review of medicinal and aromatic plants and their secondary metabolites status under abiotic stress. J. Med. Plants Stud. 2019, 7, 99–106. [Google Scholar]
- Soni, R.; Shankar, G.; Mukhopadhyay, P.; Gupta, V. A concise review on Artemisia annua L.: A major source of diverse medicinal compounds. Ind. Crops Prod. 2022, 184, 115072. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a traditional plant brought to light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Das, S. Artemisia annua (Qinghao): A pharmacological review. Int. J. Pharm. Sci. Res. 2012, 3, 4573. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential oil of Artemisia annua L.: An extraordinary component with numerous antimicrobial properties. Evid.-Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Polito, F.; Di Mercurio, M.; Rizzo, S.; Di Vito, M.; Sanguinetti, M.; Urbani, A.; Bugli, F.; De Feo, V. Artemisia spp. essential oils: From their ethnobotanical use to unraveling the microbiota modulation potential. Plants 2024, 13, 967. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E.; Della Porta, G. An optimized process for SC-CO2 extraction of antimalarial compounds from Artemisia annua L. J. Supercrit. Fluids 2017, 128, 89–93. [Google Scholar] [CrossRef]
- Donato, R.; Santomauro, F.; Bilia, A.R.; Flamini, G.; Sacco, C. Antibacterial activity of Tuscan Artemisia annua essential oil and its major components against some foodborne pathogens. LWT-Food Sci. Technol. 2015, 64, 1251–1254. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Cioni, P.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Rahimi, M.; Bidarnamani, F.; Shabanipoor, M. Effects of allelopathic three medicinal plants on germination and seeding growth of Portulaca oleracea. Biol. Forum 2015, 7, 1520–1523. [Google Scholar]
- Knudsmark Jessing, K.; Duke, S.O.; Cedergreeen, N. Potential ecological roles of artemisinin produced by Artemisia annua L. J. Chem. Ecol. 2014, 40, 100–117. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Asplund, R.O. Some quantitative aspects of the phytotoxicity of monoterpenes. Weed Sci. 1969, 17, 454–455. [Google Scholar] [CrossRef]
- Wang, H.; Lin, W.; Zhang, D.; Yang, R.; Zhou, W.; Qi, Z. Phytotoxicity of chemical compounds from Cinnamomum camphora pruning waste in germination and plant cultivation. Int. J. Environ. Res. Public Health 2022, 19, 11617. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yamaji, K.; Kobayashi, K. Allelopathic activity of camphor released from camphor tree (Cinnamomum camphora). Allelopath. J. 2011, 27, 123–132. [Google Scholar]
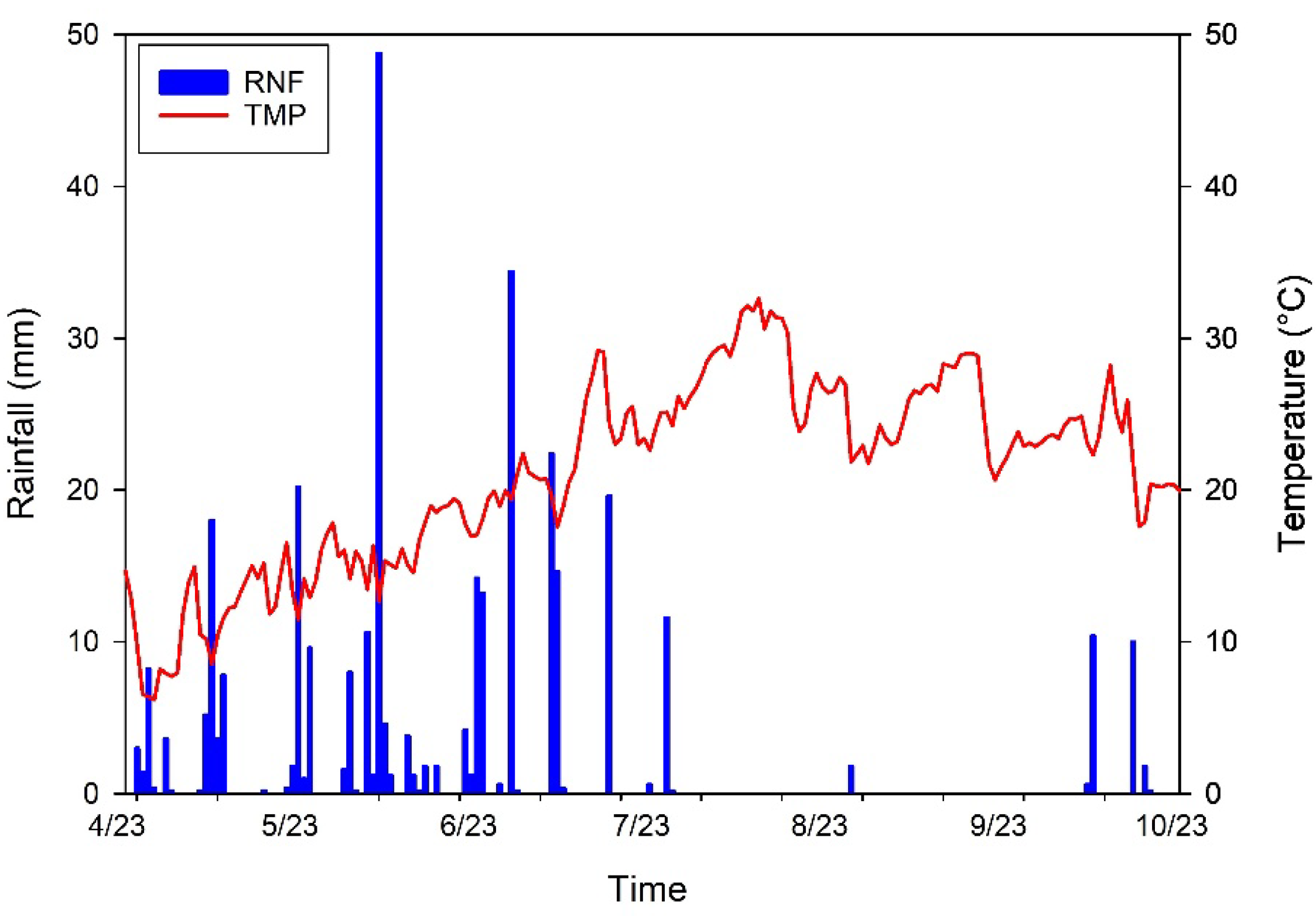
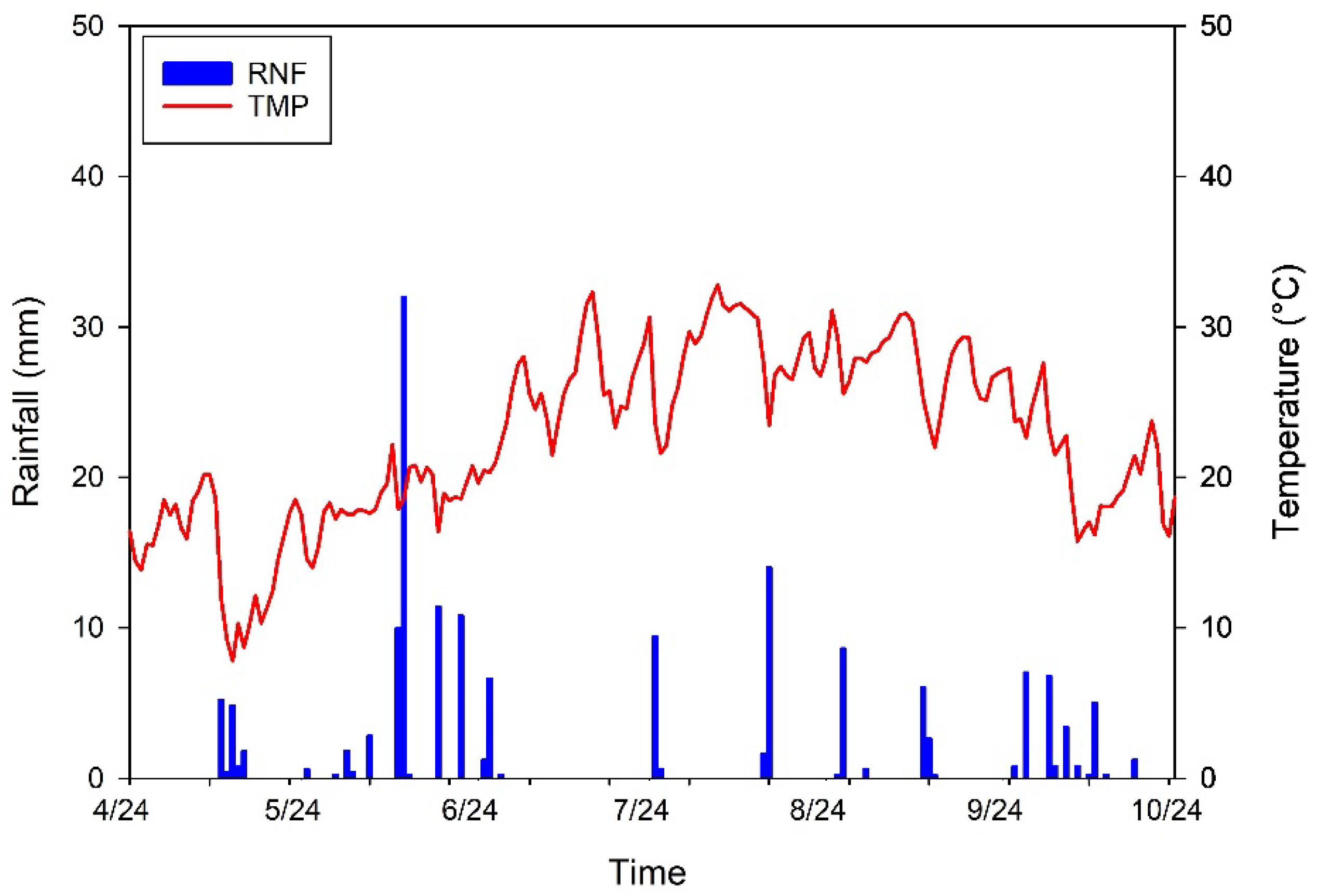
| Parameters | Values |
|---|---|
| Sand (%) | 37.1 |
| Silt (%) | 26.0 |
| Clay (%) | 36.9 |
| pH (in water) | 8.2 |
| Total CaCO3 (%) | 5.0 |
| Organic matter (%) | 2.28 |
| Cation exchange capacity (meq/100 g) | 25.16 |
| Active CaCO3 (%) | 2.5 |
| Total N (‰) | 0.138 |
| Electrical conductivity (mS/cm) | 0.188 |
| Exchangeable K (ppm) | 862 |
| Assimilable P (ppm) | 7 |
| Year | Plant Densities | Plant Height (cm) | Stems Per Plant (n.) | Fresh Biomass (g/m2) | Dry Matter Content (%) | Dry Biomass (g/m2) |
|---|---|---|---|---|---|---|
| 2023 | 20D | 176.0 ± 1.2 | 2.5 ± 0.2 | 3683.5 ± 185.2 | 40.4 ± 0.7 | 1489.1 ± 99.1 |
| 2023 | 40D | 156.8 ± 1.7 | 3.5 ± 0.1 | 3071.3 ± 199.0 | 40.6 ± 0.2 | 1248.5 ± 36.4 |
| 2024 | 20D | 95.6 ± 4.0 | 3.1 ± 0.4 | 1840.1 ± 99.1 | 44.0 ± 0.1 | 810.3 ± 28.4 |
| 2024 | 40D | 95.5 ± 1.0 | 4.9 ± 0.4 | 1211.1 ± 98.3 | 47.1 ± 0.6 | 571.6 ± 19.1 |
| Significance | ** | * | ** | ** | ** | |
| % | Ki a | Ki b | Identification c | |||||
|---|---|---|---|---|---|---|---|---|
| 20D 1st Year | 20D 2nd Year | 40D 1st Year | 40D 2nd Year | |||||
| Yield | 0.117 | 0.430 | 0.157 | 0.550 | ||||
| 1 | α-Pinene | 0.20 | - | 0.67 | - | 862 | 1025 | 1,2,3 |
| 2 | Camphene | 0.23 | 1.10 | 0.83 | 0.77 | 874 | 1068 | 1,2,3 |
| 3 | β-Pinene | 0.38 | 2.07 | 0.89 | 1.34 | 899 | 1110 | 1,2,3 |
| 4 | β-Myrcene | - | 3.67 | - | 2.27 | 920 | 1145 | 1,2 |
| 5 | Yomogi alcohol | 0.65 | 1.18 | 0.84 | 1.38 | 928 | 1395 | 1,2 |
| 6 | p-Cymene | 0.35 | - | 0.51 | - | 946 | 1270 | 1,2,3 |
| 7 | Eucalyptol | 4.70 | 13.14 | 11.19 | 8.97 | 951 | 1211 | 1,2,3 |
| 8 | Artemisia ketone | 8.05 | 50.32 | 14.01 | 65.77 | 985 | 1344 | 1,2 |
| 9 | Artemisia alcohol | 0.27 | 2.77 | 1.08 | 2.73 | 1002 | 1510 | 1,2 |
| 10 | p-Mentha-trans-2,8-dien-1-ol | - | 2.37 | - | 1.42 | 1025 | 1639 | 1,2 |
| 11 | Butanoic acid, 2-methyl-, 3-methyl-3-butenyl ester | 0.10 | - | - | - | 1032 | 1,2 | |
| 12 | trans-Pinocarveol | 0.12 | - | - | - | 1049 | 1661 | 1,2 |
| 13 | Camphor | 1.95 | 6.26 | 6.25 | 4.32 | 1054 | 1515 | 1,2,3 |
| 14 | 4,8-dimethyl-,1,3,7-Nonatriene | - | 1.67 | - | 1.04 | 1060 | 1309 | 1,2 |
| 15 | 2,6-dimethyl-1,5,7-Octatrien-3-ol | - | - | - | 0.97 | 1064 | 1,2 | |
| 16 | Borneol | 0.11 | 1.61 | - | - | 1076 | 1700 | 1,2,3 |
| 17 | Terpinen-4-ol | 0.76 | 0.54 | 1.30 | - | 1088 | 1601 | 1,2 |
| 18 | α-Terpineol | 0.32 | - | - | - | 1094 | 1694 | 1,2,3 |
| 19 | Myrtenol | 0.21 | - | 0.38 | - | 1100 | 1790 | 1,2 |
| 20 | cis-3-Hexenyl isovalerate | 0.12 | - | - | - | 1141 | 1,2 | |
| 21 | Carvacrol,methyl ether | 0.14 | - | - | - | 1197 | 1599 | 1,2 |
| 22 | p-Menth-en-3,8-diol | 0.16 | - | 0.39 | - | 1198 | 1,2 | |
| 23 | α-Copaene | 0.56 | - | 0.53 | - | 1269 | 1491 | 1,2,3 |
| 24 | Butanoic acid, 3-methyl-, phenylmethyl ester | 0.95 | - | 1.08 | - | 1283 | 1902 | 1,2 |
| 25 | Benzyl isovalerate | - | 0.55 | - | - | 1286 | 1851 | 1,2 |
| 26 | cis-Jasmone | 0.18 | - | - | - | 1293 | 1933 | 1,2 |
| 27 | trans-Caryophyllene | 11.65 | 1.52 | 5.97 | 1.48 | 1303 | 1588 | 1,2 |
| 28 | α-Humulene | 0.77 | - | 0.46 | - | 1337 | 1667 | 1,2 |
| 29 | Amorpha-4,11-diene | 0.76 | - | 0.45 | - | 1345 | 1,2 | |
| 30 | trans-β-Farnesene | 0.17 | 0.54 | - | - | 1347 | 1665 | 1,2 |
| 31 | α-Acoradiene | 0.41 | - | 0.55 | - | 1360 | 1690 | 1,2 |
| 32 | Germacrene D | - | 1.06 | 3.43 | 0.87 | 1364 | 1708 | 1,2 |
| 33 | γ-Muurolene | 3.84 | - | - | - | 1365 | 1690 | 1,2 |
| 34 | β-Selinene | 34.35 | 8.2 | 37.53 | 5.38 | 1369 | 1717 | 1,2 |
| 35 | δ-Selinene | 0.15 | - | - | - | 1376 | 1756 | 1,2 |
| 36 | Bicyclogermacrene | 0.26 | - | - | - | 1379 | 1734 | 1,2 |
| 37 | Indipone | 0.25 | - | - | - | 1381 | 1,2 | |
| 38 | γ-Patchoulene | 0.42 | - | - | - | 1397 | 1664 | 1,2 |
| 39 | δ-Cadinene | 0.18 | - | - | - | 1402 | 1749 | 1,2 |
| 40 | cis-Nerolidol | 0.12 | - | - | - | 1407 | 2007 | 1,2 |
| 41 | trans-Nerolidol | 0.22 | - | - | - | 1445 | 2036 | 1,2 |
| 42 | Palustrol | 0.25 | - | - | - | 1449 | 1953 | 1,2 |
| 43 | Spathulenol | 0.76 | - | 0.74 | - | 1452 | 2127 | 1,2 |
| 44 | Caryophyllene oxide | 2.16 | - | 1.04 | - | 1457 | 1986 | 1,2 |
| 45 | Isoaromadendrene epoxide | 2.31 | - | 1.8 | - | 1460 | 1807 | 1,2 |
| 46 | Globulol | 0.18 | - | - | - | 1484 | 2082 | 1,2 |
| 47 | Longiborneol | 1.11 | - | 0.49 | - | 1493 | 1,2 | |
| 48 | Junenol | 0.12 | - | - | - | 1496 | 1,2 | |
| 49 | 10-epi-γ-Eudesmol | 0.35 | - | - | - | 1498 | 1624 | 1,2 |
| 50 | allo-Aromadendrene epoxide | 0.25 | - | - | - | 1497 | 2095 | 1,2 |
| 51 | Cubenol | 2.12 | - | 1.29 | - | 1500 | 2068 | 1,2 |
| 52 | Cedr-8(15)-en-9-α-ol | 0.35 | - | - | - | 1519 | - | 1,2 |
| 53 | 7-epi-α-Eudesmol | 0.24 | - | - | - | 1526 | 2224 | 1,2 |
| 54 | Ylangenal | 0.25 | - | - | - | 1529 | 1,2 | |
| 55 | Aromadendrene oxide-(2) | 1.43 | - | 0.56 | - | 1532 | 1,2 | |
| 56 | Alloaromadendrene oxide-(1) | 0.24 | - | - | - | 1545 | 1,2 | |
| 57 | (1R,7S)-Germacra-4(15),5,10(14)-trien-1β-ol | 1.11 | - | 0.46 | - | 1550 | 1,2 | |
| 58 | Eudesm-7(11)-en-4-ol | 0.14 | - | - | - | 1558 | 2271 | 1,2 |
| 59 | 8-α-11-Elemodiol | 0.2 | - | - | - | 1579 | 1,2 | |
| 60 | Aristolone | 1.13 | - | 0.61 | - | 1586 | 1,2 | |
| 61 | α-Costol | 0.12 | - | - | - | 1599 | 2604 | 1,2 |
| 62 | γ-Eudesmol acetate | 0.17 | - | - | - | 1600 | 2174 | 1,2 |
| 63 | 8-Cedren-13-ol acetate | 0.8 | - | - | - | 1603 | 1,2 | |
| 64 | Isovalencenol | 1.89 | - | - | - | 1626 | 1,2 | |
| 65 | Acid cis-thujopsenic | 0.16 | - | - | - | 1693 | 1,2 | |
| 66 | 8S,13-Cedranediol | 0.16 | - | - | - | 1694 | 1,2 | |
| 67 | 11,12-dihydroxy-Valencene | 0.44 | - | - | - | 1731 | 1,2 | |
| 68 | Phytol | 0.85 | - | - | - | 1951 | 2622 | 1,2 |
| Total | 93.35 | 98.57 | 95.33 | 98.71 | ||||
| Monoterpene hydrocarbons | 1.16 | 6.84 | 2.90 | 4.38 | ||||
| Oxygenated monoterpenes | 17.60 | 78.19 | 35.83 | 84.59 | ||||
| Sesquiterpene hydrocarbons | 53.52 | 11.32 | 48.92 | 7.73 | ||||
| Oxygenated sesquiterpenes | 18.78 | - | 6.99 | - | ||||
| Others | 2.29 | 2.22 | 0.69 | 2.01 | ||||
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 3.8 | 7.0 | 0.0 | −42.6 | 28.6 | 0.0 | 0.0 |
| 250 | 0.0 | 10.0 | 0.0 | −12.8 | 38.6 | 0.0 | 10.0 |
| 500 | −16.3 | 20.0 | 7.0 | 21.3 | 42.8 | 20.0 | 20.0 |
| 1000 | 0.0 | 20.0 | 97.0 | 29.8 | 57.1 | 20.0 | 20.0 |
| Radical Length (cm) | |||||||
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 16.7 | 12.0 | 3.7 | −125.0 | 15.6 | 0.0 | 17.0 |
| 250 | 5.6 | 32.0 | 18.5 | −125.0 | 28.1 | 0.0 | 18.9 |
| 500 | 11.1 | 36.0 | 25.9 | −25.0 | 40.6 | 8.7 | 26.4 |
| 1000 | 61.1 | 52.0 | 25.9 | 0.0 | 59.4 | 17.4 | 34.0 |
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | −13.0 | 8.0 | 0.0 | −9.3 | 50.0 | 10.0 | 0.0 |
| 250 | −7.8 | 31.1 | 10.0 | 60.5 | 47.5 | 10.0 | 0.0 |
| 500 | −13.0 | 69.0 | 20.0 | 69.8 | 58.8 | 10.0 | 10.0 |
| 1000 | −7.8 | 73.6 | 87.0 | 100.0 | 61.3 | 20.0 | 20.0 |
| Radical Length (cm) | |||||||
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 4.4 | 42.9 | 3.2 | −71.4 | 7.14 | 8.5 | 39.0 |
| 250 | 55.6 | 57.1 | 12.9 | 71.4 | 16.7 | 16.9 | 65.9 |
| 500 | 46.7 | 57.1 | 19.4 | 42.9 | 35.7 | 30.5 | 73.2 |
| 1000 | 66.7 | 64.3 | 25.8 | 100.0 | 57.1 | 37.3 | 80.5 |
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | −3.8 | −4.3 | −12.5 | −35.1 | 6.98 | 0.0 | 10.0 |
| 250 | −12.5 | 0.0 | −12.5 | 0.0 | 16.28 | 10.0 | 20.0 |
| 500 | 0.0 | 3.23 | 0.0 | 46.0 | 69.8 | 20.0 | 20.0 |
| 1000 | 8.8 | 10.8 | 0.0 | 46.0 | 100 | 20.0 | 30.0 |
| Radical Length (cm) | |||||||
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 7.7 | 40.0 | 13.0 | −100.0 | 19.1 | 18.2 | 47.4 |
| 250 | 38.5 | 48.0 | 26.1 | 33.3 | 42.9 | 24.2 | 56.1 |
| 500 | −107.7 | 60.0 | 30.4 | −33.3 | 61.9 | 23.8 | 77.2 |
| 1000 | 30.8 | 68.0 | 39.1 | −133.0 | 100.0 | 28.6 | 86.0 |
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 0.0 | 18.9 | 0.0 | −16.3 | 11.3 | 0.0 | 0.0 |
| 250 | 27.6 | 41.1 | 0.0 | 30.2 | 28.3 | 10.0 | 10.0 |
| 500 | 4.6.0 | 55.6 | 7.8 | 83.7 | 43.4 | 10.0 | 10.0 |
| 1000 | −3.4.0 | 70.0 | 22.2 | 83.7 | 100.0 | 10.0 | 20.0 |
| Radical Length (cm) | |||||||
| L. multiflorum | S. alba | A. fatua | P. rhoeas | P. oleracea | V. lens | T. durum | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment (µg/mL) | |||||||
| 125 | 4.0 | 50.0 | 25.0 | −14.3 | 23.8 | 13.7 | 50.0 |
| 250 | 24.0 | 58.3 | 33.3 | 14.3 | 38.1 | 23.5 | 67.3 |
| 500 | −2.0 | 50.0 | 58.3 | 100.0 | 66.7 | 39.2 | 71.2 |
| 1000 | 12.0 | 33.3 | 66.7 | 100.0 | 100.0 | 47.1 | 76.9 |
| EO | Process | Most Sensitive Species | Maximum Inhibition Observed (%) | Most Effective Concentration (µg/Ml) |
|---|---|---|---|---|
| EO 20D 1st year | Germination | A. fatua, P. oleracea | 97.0 (A. fatua) | 1000 |
| Radical elongation | P. rhoeas, T. durum | 61.1 (L. multiflorum) | 1000 | |
| EO 20D 2nd year | Germination | P. rhoeas, S. alba, A. fatua | 100.0 (P. rhoeas) | 1000 |
| Radical elongation | P. rhoeas, T. durum | 100.0 (P. rhoeas) | 1000 | |
| EO 40D 1st year | Germination | P. rhoeas, P. oleracea | 100.0 (P. oleracea) | 1000 |
| Radical elongation | T. durum, P. oleracea | 100.0 (P. oleracea) | 1000 | |
| EO 40D 2nd year | Germination | P. rhoeas, P. oleracea | 100.0 (P. oleracea, P. rhoeas) | 1000 |
| Radical elongation | P. rhoeas, P. oleracea, T. durum | 100.0 (P. rhoeas, P. oleracea) | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polito, F.; Denora, M.; Casiello, D.; Casiero, P.; Cardone, L.; Candido, V.; Perniola, M.; De Feo, V.; Palombo, V.; Delfine, S. Effect of Plant Density on Artemisia annua L. Biomass and Essential Oil Yield and Its Weed Seed Germination Suppression. Agriculture 2025, 15, 1330. https://doi.org/10.3390/agriculture15131330
Polito F, Denora M, Casiello D, Casiero P, Cardone L, Candido V, Perniola M, De Feo V, Palombo V, Delfine S. Effect of Plant Density on Artemisia annua L. Biomass and Essential Oil Yield and Its Weed Seed Germination Suppression. Agriculture. 2025; 15(13):1330. https://doi.org/10.3390/agriculture15131330
Chicago/Turabian StylePolito, Flavio, Michele Denora, Donato Casiello, Pierluigi Casiero, Loriana Cardone, Vincenzo Candido, Michele Perniola, Vincenzo De Feo, Valentino Palombo, and Sebastiano Delfine. 2025. "Effect of Plant Density on Artemisia annua L. Biomass and Essential Oil Yield and Its Weed Seed Germination Suppression" Agriculture 15, no. 13: 1330. https://doi.org/10.3390/agriculture15131330
APA StylePolito, F., Denora, M., Casiello, D., Casiero, P., Cardone, L., Candido, V., Perniola, M., De Feo, V., Palombo, V., & Delfine, S. (2025). Effect of Plant Density on Artemisia annua L. Biomass and Essential Oil Yield and Its Weed Seed Germination Suppression. Agriculture, 15(13), 1330. https://doi.org/10.3390/agriculture15131330










