Suppression of Plastidial Glucan Phosphorylase (PHO1) Increases Drought Tolerance in Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Materials and Methods
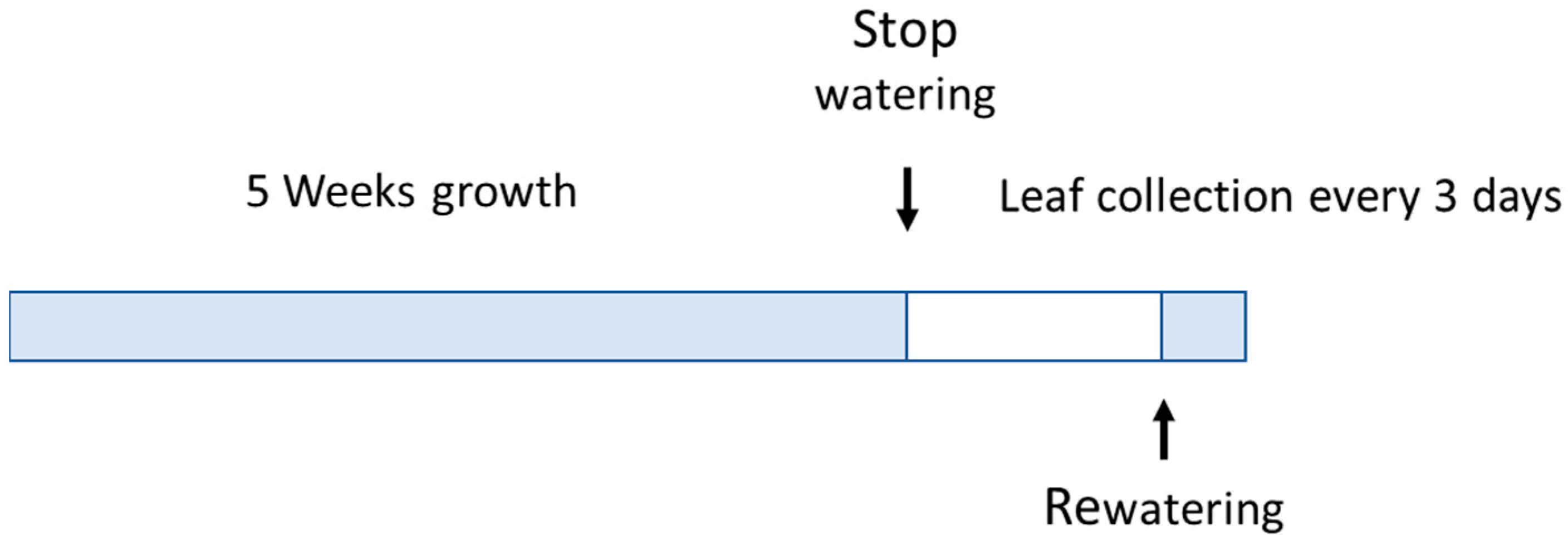
2.1. Plant Materials and Stress Conditions
2.2. Determination of Chlorophyll Content
2.3. Determination of Water Saturation Deficit (WSD)
2.4. Acquisition of Enzyme Extracts
2.5. Determination of Protein Content
2.6. Separation of Proteins by Electrophoresis under Nondenaturing Conditions
2.7. Glucan Phosphorylase Zymography and Determination of Intensity of PHO Activity
2.8. Determination of Hydrogen Peroxide (H2O2) Content
2.9. Sample Preparation and Carbohydrate Analysis
2.10. Starch Extraction and Morphology Analysis by Scanning Electron Microscope (SEM)
2.11. Laser Confocal Scanning Microscope (LCSM) Analysis
2.12. Statistical Analyses
3. Results
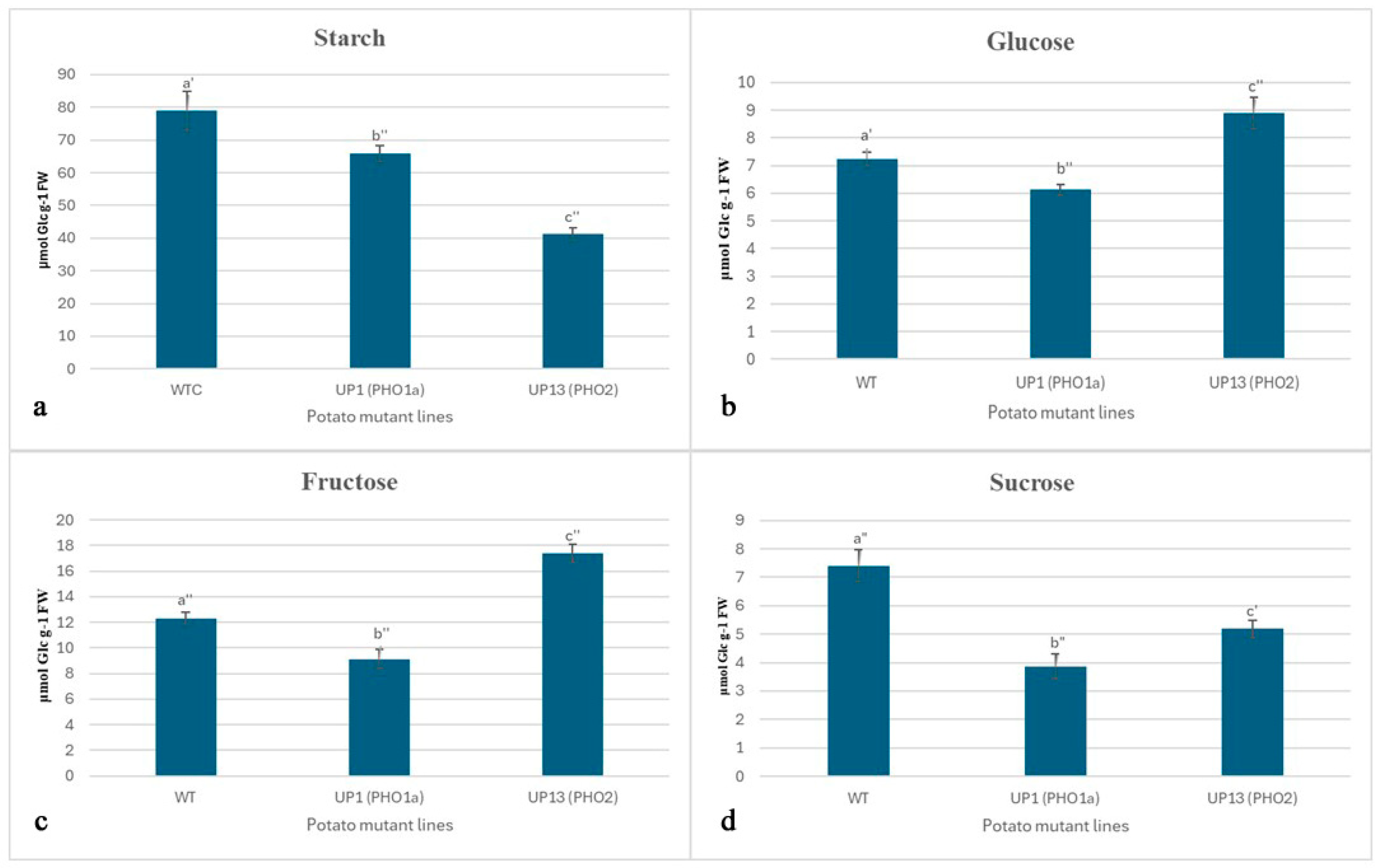
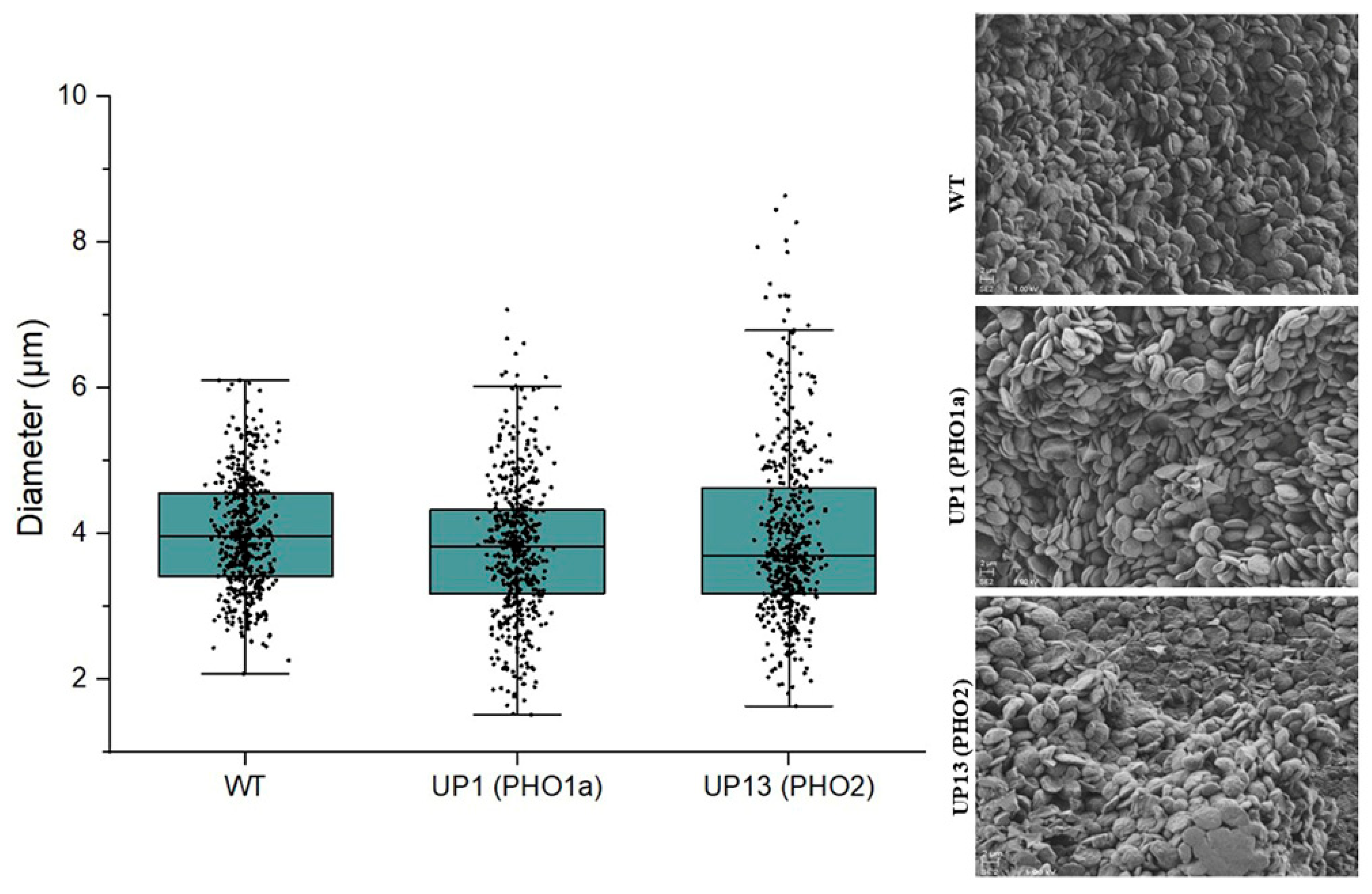
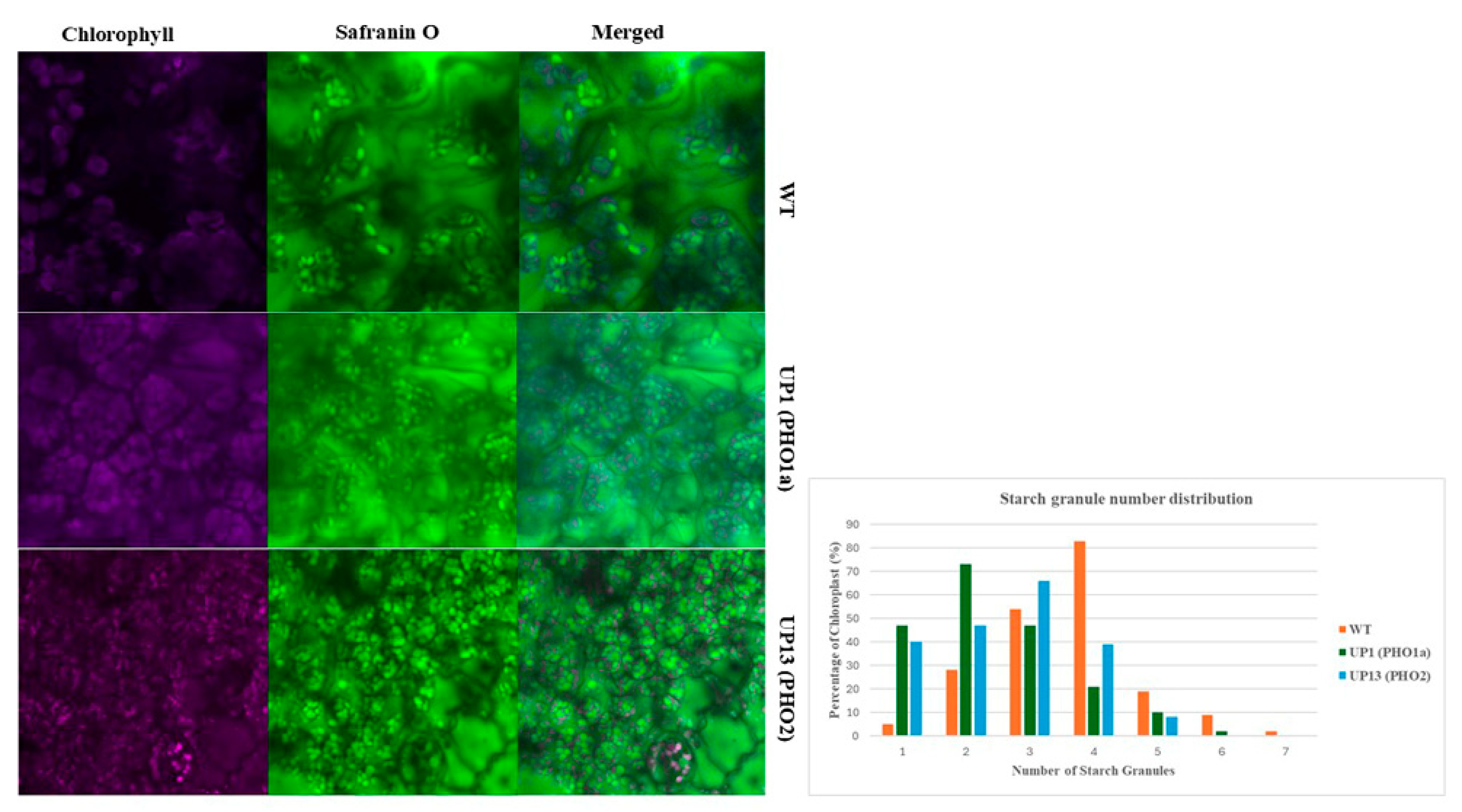
3.1. Potato Plants with Repressed PHO Had Reduced Leaf Starch Content and Fewer Starch Granules per Chloroplast
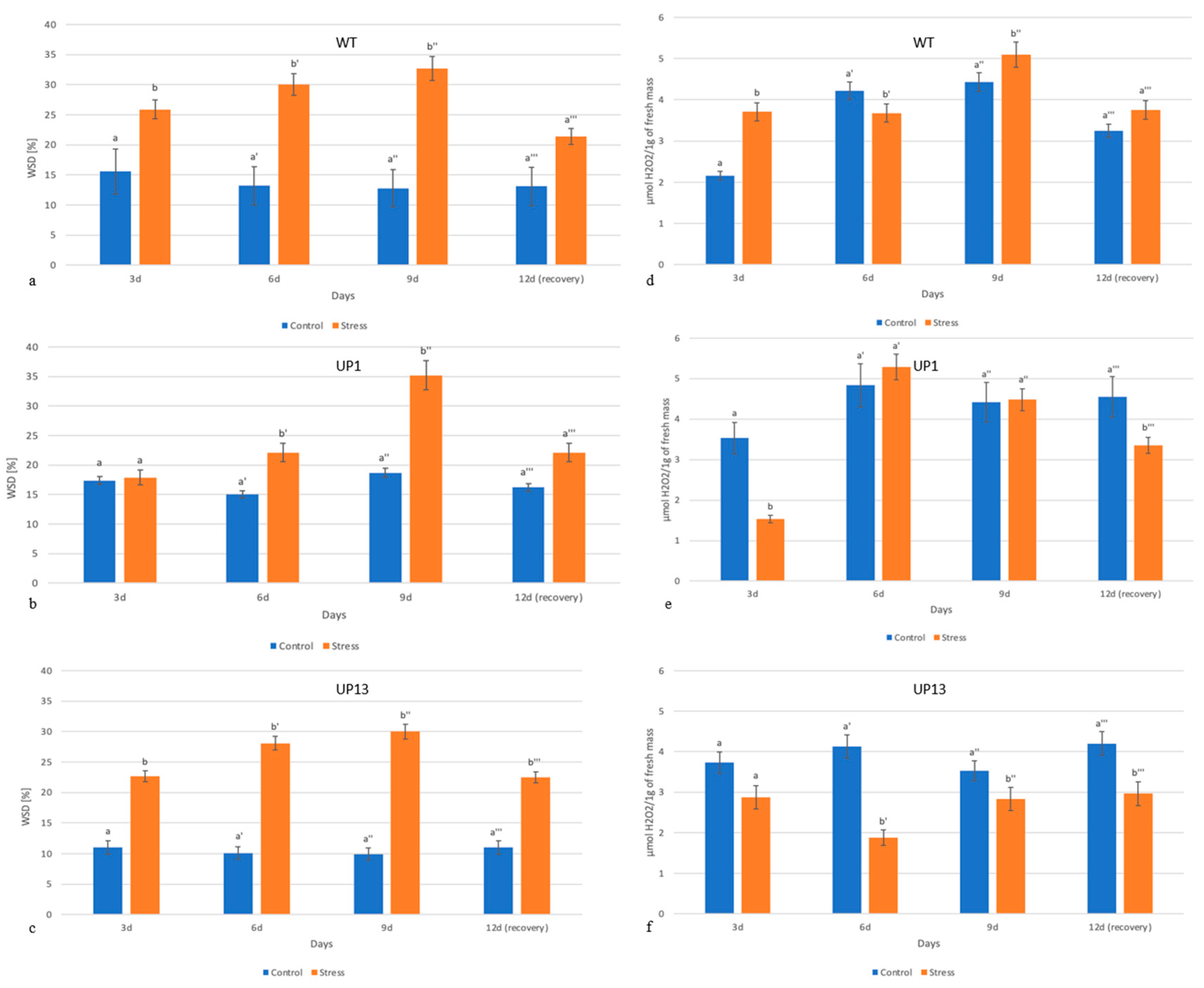
3.2. Plants with Reduced PHO1 Expression Had Delayed Water Deficiency and Less Hydrogen Peroxide Generation
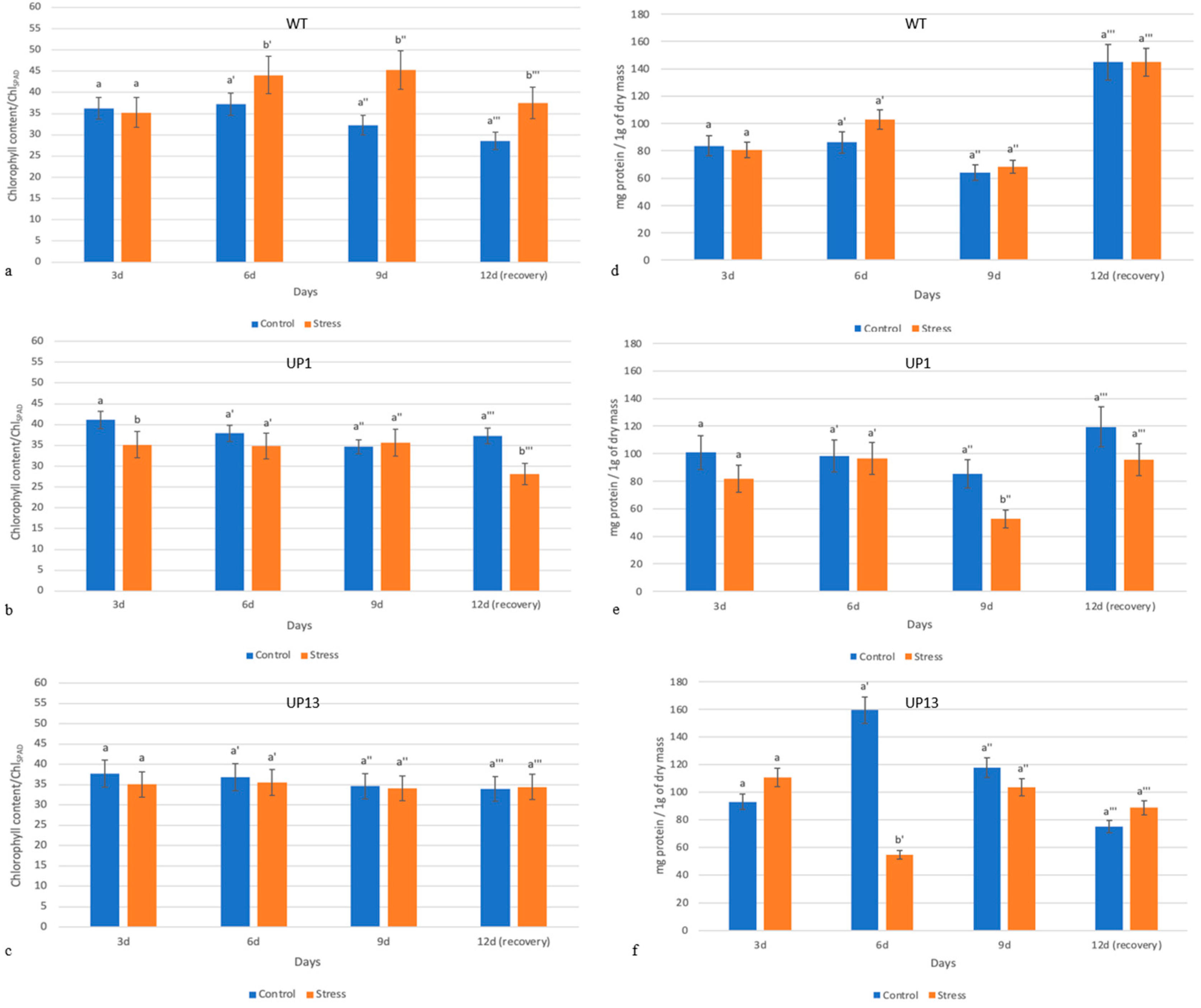
3.3. Chlorophyll Content Is Altered and Soluble Protein Amounts Are Decreased in Stressed Plants
3.4. PHO1 Activity Decreases under Water Deficiency Conditions in Wild-Type Plants Compared to Mutant Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef]
- Cuellar-ortiz, S.M.; de la Paz Arrieta-montiel, M.A.R.I.A.; Acosta-Gallegos, J.O.R.G.E.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-R.; Jin, Y.-L.; Jung, W.-J.; Avice, J.-C.; Morvan-Bertrand, A.; Ourry, A.; Park, C.-W.; Kim, T.-H. Water-deficit accumulates sugars by starch degradation—not by de novo synthesis—in white clover leaves (Trifolium repens). Physiol. Plant 2008, 134, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005, 44, 730–743. [Google Scholar] [CrossRef]
- Hoermiller, I.I.; Naegele, T.; Augustin, H.; Stutz, S.; Weckwerth, W.; Heyer, A.G. Subcellular reprogramming of metabolism during cold acclimation in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 602–610. [Google Scholar] [CrossRef]
- Zanella, M.; Borghi, G.L.; Pirone, C.; Thalmann, M.; Pazmino, D.; Costa, A.; Santelia, D.; Trost, P.; Sparla, F. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 2016, 67, 1819–1826. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Yin, Y.-G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234–235, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Minami, A.; Arakawa, K.; Fujikawa, S.; Takezawa, D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J. Plant Physiol. 2005, 162, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.W.; Neuhaus, H.E. In concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Heiber, I.; Cai, W.; Baier, M. Linking chloroplast antioxidant defense to carbohydrate availability: The transcript abundance of stromal ascorbate peroxidase is sugar-controlled via ascorbate biosynthesis. Mol. Plant 2014, 7, 58–70. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Skirycz, A.; De Bodt, S.; Obata, T.; De Clercq, I.; Claeys, H.; De Rycke, R.; Andriankaja, M.; Van Aken, O.; Van Breusegem, F.; Fernie, A.R.; et al. Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol. 2010, 152, 226–244. [Google Scholar] [CrossRef]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. B-amylase1 and b-amylase3 are plastidic starch hydrolases in Arabidopsis that seem to be adapted for different thermal, pH, and stress conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef] [PubMed]
- He, J.-F.; Goyal, R.; Laroche, A.; Zhao, M.-L.; Lu, Z.-X. Water stress during grain development affects starch synthesis, composition and physicochemical properties in triticale. J. Cereal Sci. 2012, 56, 552–560. [Google Scholar] [CrossRef]
- Geigenberger, P.; Reimholz, R.; Geiger, M.; Merlo, L.; Canale, V.; Stitt, M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 1997, 201, 502–518. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M. Comparison of the effect of rapidly and gradually developing water-stress on carbohydrate metabolism in spinach leaves. Plant Cell Environ. 1991, 14, 939–946. [Google Scholar] [CrossRef]
- Villadsen, D.; Rung, J.H.; Nielsen, T.H. Osmotic stress changes carbohydrate partitioning and fructose-2,6-bisphosphate metabolism in barley leaves. Funct. Plant Biol. 2005, 32, 1033–1043. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Jimenez, R.; Arias, M.; Beckles, D. Effects of environmental factors on starch biosynthesis and composition. J. Cereal Sci. 2012, 56, 67–80. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef]
- Todaka, D.; Matsushima, H.; Morohashi, Y. Water stress enhances β-amylase activity in cucumber cotyledons. J. Exp. Bot. 2000, 51, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J.-H. PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Wang, Z.; Xu, G.; Zhu, Q. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 2004, 135, 1621–1629. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Yuan, L.; Wang, Z.; Yang, J.; Zhang, J. Post-anthesis alternate wetting and moderate soil drying enhances activities of key enzymes in sucrose-to-starch conversion in inferior spikelets of rice. J. Exp. Bot. 2012, 63, 215–227. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wang, B.; Wang, D.; Li, P.; Wang, L.; Yi, X.; Huang, Q.; Peng, M.; Guo, A. Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol. Cell Prot. 2013, 12, 2174–2195. [Google Scholar] [CrossRef]
- Pattanagul, W.; Thitisaksakul, M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Ind. J. Exp. Biol. 2008, 46, 736–742. [Google Scholar]
- Balibrea, M.; Dell’Amico Rodríguez, J.M.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Higuchi, K.; Kanai, M.; Tsuchiya, M.; Ishii, H.; Shibuya, N.; Fujita, N.; Nakamura, Y.; Suzui, N.; Fujimaki, S.; Miwa, E. Common reed accumulates starch in its stem by metabolic adaptation under Cd stress conditions. Front. Plant Sci. 2015, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Higuchi, K.; Hagihara, T.; Konishi, T.; Ishii, T.; Fujita, N.; Nakamura, Y.; Maeda, Y.; Yoshiba, M.; Tadano, T. Common reed produces starch granules at the shoot base in response to salt stress. New Phytol. 2007, 176, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Thorneycroft, D.; Schupp, N.; Chapple, A.; Weck, M.; Dunstan, H.; Haldimann, P.; Bechtold, N.; Smith, A.M.; Smith, S.M. Plastidial alpha-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol. 2004, 135, 849–858. [Google Scholar] [CrossRef]
- Sitnicka, D.; Orzechowski, S. Cold-induced starch degradation in potato leaves—Intercultivar differences in the gene expression and activity of key enzymes. Biol. Plantarum. 2014, 58, 659–666. [Google Scholar] [CrossRef]
- Orawetz, T.; Malinova, I.; Orzechowski, S.; Fettke, J. Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol. Biochem. 2016, 100, 141–149. [Google Scholar] [CrossRef]
- Fettke, J.; Albrecht, T.; Hejazi, M.; Mahlow, S.; Nakamura, Y.; Steup, M. Glucose 1-phosphate is efficiently taken up by potato (Solanum tuberosum) tuber parenchyma cells and converted to reserve starch granules. New Phytol. 2010, 185, 663–675. [Google Scholar] [CrossRef]
- Fettke, J.; Leifels, L.; Brust, H.; Herbst, K.; Steup, M. Two carbon fluxes to reserve starch in potato (Solanum tuberosum L.) tuber cells are closely interconnected but differently modulated by temperature. J. Exp. Bot. 2012, 63, 3011–3029. [Google Scholar] [CrossRef]
- Flores-Castellanos, J.; Fettke, J. The plastidial glucan phosphorylase affects the maltooligosaccharide metabolism in parenchyma cells of potato (Solanum tuberosum L.) tuber discs. Plant Cell Physiol. 2023, 64, 422–432. [Google Scholar] [CrossRef]
- Castellanos, J.F.; Khan, A.; Fettke, J. Gradual analytics of starch-interacting proteins revealed the involvement of starch-phosphorylating enzymes during synthesis of storage starch in potato (Solanum tuberosum L.) tubers. Molecules 2023, 28, 6219. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Orzechowski, S.; Sitnicka, D.; Grabowska, A.; Compart, J.; Fettke, J.; Zdunek-Zastocka, E. Effect of short-term cold treatment on carbohydrate metabolism in potato leaves. IJMS 2021, 22, 7203. [Google Scholar] [CrossRef] [PubMed]
- Fettke, J.; Eckermann, N.; Tiessen, A.; Geigenberger, P.; Steup, M. Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in leaves of Arabidopsis thaliana L.: Distinct SHG reside in the cytosol and in the apoplast. Plant J. 2005, 43, 568–585. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. AJAC 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Bergmeyer, H. Methoden der Enzymatischen Analyse, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1974. [Google Scholar]
- Jones, M.G.K.; Outlow, W.H.; Lowry, O.M. Enzymic assay of 10−7–10−14 moles of sucrose in plant tissues. Plant Physiol. 1977, 60, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Malinova, I.; Fettke, J. Reduced starch granule number per chloroplast in the dpe2/phs1 mutant is dependent on initiation of starch degradation. PLoS ONE 2017, 12, e0187985. [Google Scholar] [CrossRef] [PubMed]
- Malinova, I.; Mahlow, S.; Alseekh, S.; Orawetz, T.; Fernie, A.R.; Baumann, O.; Steup, M.; Fettke, J. Double knockout mutants of arabidopsis grown under normal conditions reveal that the plastidial phosphorylase isozyme participates in transitory starch metabolism. Plant Physiol. 2014, 164, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Fettke, J. Starch granules in Arabidopsis thaliana mesophyll and guard cells show similar morphology but differences in size and number. Int. J. Mol. Sci. 2021, 22, 5666. [Google Scholar] [CrossRef]
- Juzoń, K.; Skrzypek, E.; Czyczło-Mysza, I.; Marcińska, I. Effect of soil drought on the yield structure, protein and phenolics content in Pisum sativum and Lupinus luteus. Acta Agric. Hun. 2014, 61, 267–278. [Google Scholar] [CrossRef]
- Watkinson, J.I.; Hendricks, L.; Sioson, A.A.; Heath, L.S.; Bohnert, H.J.; Grene, R. Tuber development phenotypes in adapted and acclimated, drought-stressed Solanum tuberosum ssp. Andigena have distinct expression profiles of genes associated with carbon metabolism. Plant Physiol. Biochem. 2008, 46, 34–45. [Google Scholar]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J. Exp. Bot. 2001, 52, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Greve, B.; Pusch, K.; Kossmann, J.; Buchner, P.; Wobus, U.; Steup, M. Homodimers and heterodimers of Pho1-type phosphorylase isoforms in Solanum tuberosum L. as revealed by sequence-specific antibodies. Eur. J. Bioch 1998, 251, 343–352. [Google Scholar] [CrossRef]
- Shoaib, N.; Liu, L.; Ali, A.; Mughal, N.; Yu, G.; Huang, Y. Molecular functions and pathways of plastidial starch phosphorylase (PHO1) in starch metabolism: Current and future perspectives. IJMS 2021, 22, 10450. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Pieczyński, M.; Wyrzykowska, A.; Kalaji, H.M.; Sieczko, L.; Szweykowska-Kulińska, Z.; Zagdańska, B. Divergent strategies displayed by potato (Solanum tuberosum L.) cultivars to cope with soil drought. J. Agric. Crop Sci. 2018, 204, 13–30. [Google Scholar] [CrossRef]
- Mori, I.C.; Schroeder, J.I. Reactive Oxygen Species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 2004, 135, 702–708. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
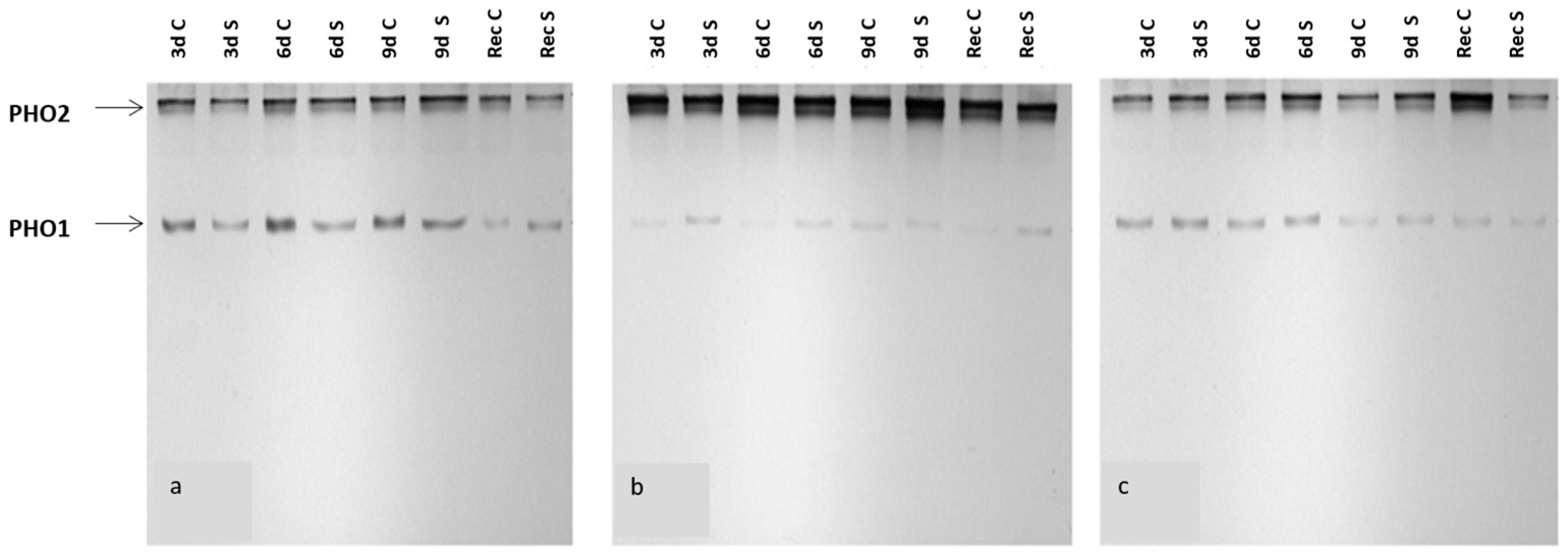
| A | ||||||||
| Sample | 3 d C | 3 d S | 6 d C | 6 d S | 9 d C | 9 d S | Rec C | Rec S |
| PHO2 activity | 100% | 65% | 100% | 105% | 100% | 148% | 100% | 76% |
| PHO1 activity | 100% | 46% | 100% | 39% | 100% | 84% | 100% | 197% |
| Total PHO activity | 100% | 57% | 100% | 75% | 100% | 120% | 100% | 93% |
| B | ||||||||
| Sample | 3 d C | 3 d S | 6 d C | 6 d S | 9 d C | 9 d S | Rec C | Rec S |
| PHO2 activity | 100% | 83% | 100% | 92% | 100% | 107% | 100% | 67% |
| PHO1 activity | 100% | 227% | 100% | 123% | 100% | 79% | 100% | 133% |
| Total PHO activity | 100% | 91% | 100% | 93% | 100% | 105% | 100% | 71% |
| C | ||||||||
| Sample | 3 d C | 3 d S | 6 d C | 6 d S | 9 d C | 9 d S | Rec C | Rec S |
| PHO2 activity | 100% | 130% | 100% | 118% | 100% | 159% | 100% | 29% |
| PHO1 activity | 100% | 102% | 100% | 116% | 100% | 114% | 100% | 90% |
| Total PHO activity | 100% | 118% | 100% | 117% | 100% | 149% | 100% | 35% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, J.; Khan, A.; Rękowska, A.; Nowak, P.; Zdunek-Zastocka, E.; Fettke, J.; Orzechowski, S. Suppression of Plastidial Glucan Phosphorylase (PHO1) Increases Drought Tolerance in Potato (Solanum tuberosum L.). Agriculture 2024, 14, 1491. https://doi.org/10.3390/agriculture14091491
Paprocka J, Khan A, Rękowska A, Nowak P, Zdunek-Zastocka E, Fettke J, Orzechowski S. Suppression of Plastidial Glucan Phosphorylase (PHO1) Increases Drought Tolerance in Potato (Solanum tuberosum L.). Agriculture. 2024; 14(9):1491. https://doi.org/10.3390/agriculture14091491
Chicago/Turabian StylePaprocka, Julia, Arsalan Khan, Agnieszka Rękowska, Paulina Nowak, Edyta Zdunek-Zastocka, Joerg Fettke, and Sławomir Orzechowski. 2024. "Suppression of Plastidial Glucan Phosphorylase (PHO1) Increases Drought Tolerance in Potato (Solanum tuberosum L.)" Agriculture 14, no. 9: 1491. https://doi.org/10.3390/agriculture14091491
APA StylePaprocka, J., Khan, A., Rękowska, A., Nowak, P., Zdunek-Zastocka, E., Fettke, J., & Orzechowski, S. (2024). Suppression of Plastidial Glucan Phosphorylase (PHO1) Increases Drought Tolerance in Potato (Solanum tuberosum L.). Agriculture, 14(9), 1491. https://doi.org/10.3390/agriculture14091491






