Bacteria Affect the Distribution of Soil-Dissolved Organic Matter on the Slope: A Long-Term Experiment in Black Soil Erosion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Plot Setup
2.2. Sample Collection
2.3. DOM Extraction
2.4. UV–Visible Spectrum Determination
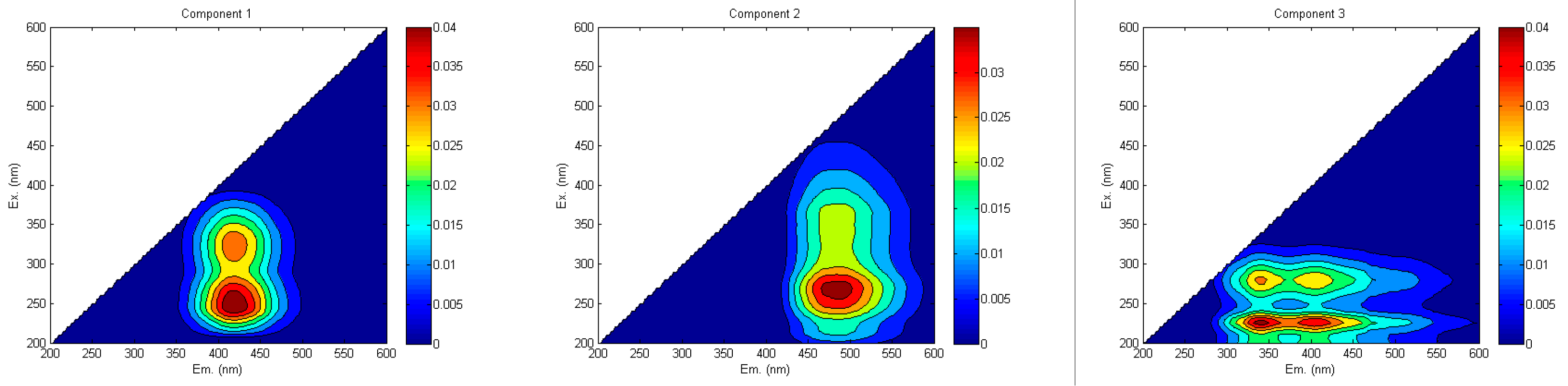
2.5. Fluorescence Spectrum Determination and Analysis
2.6. Bacteria Characteristics Analysis
2.7. Statistical Analysis
3. Results
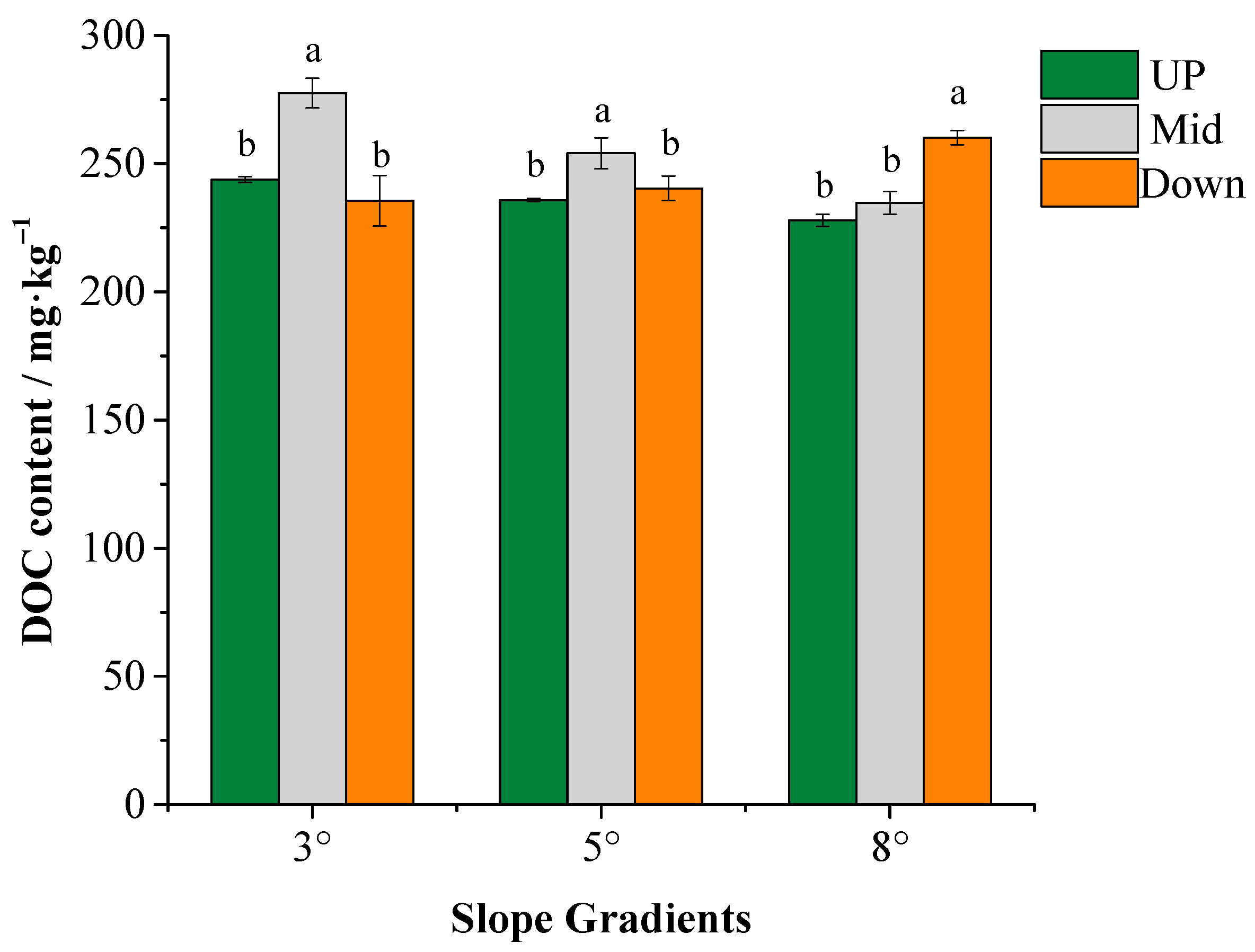
3.1. Characteristics of DOM Content and Composition
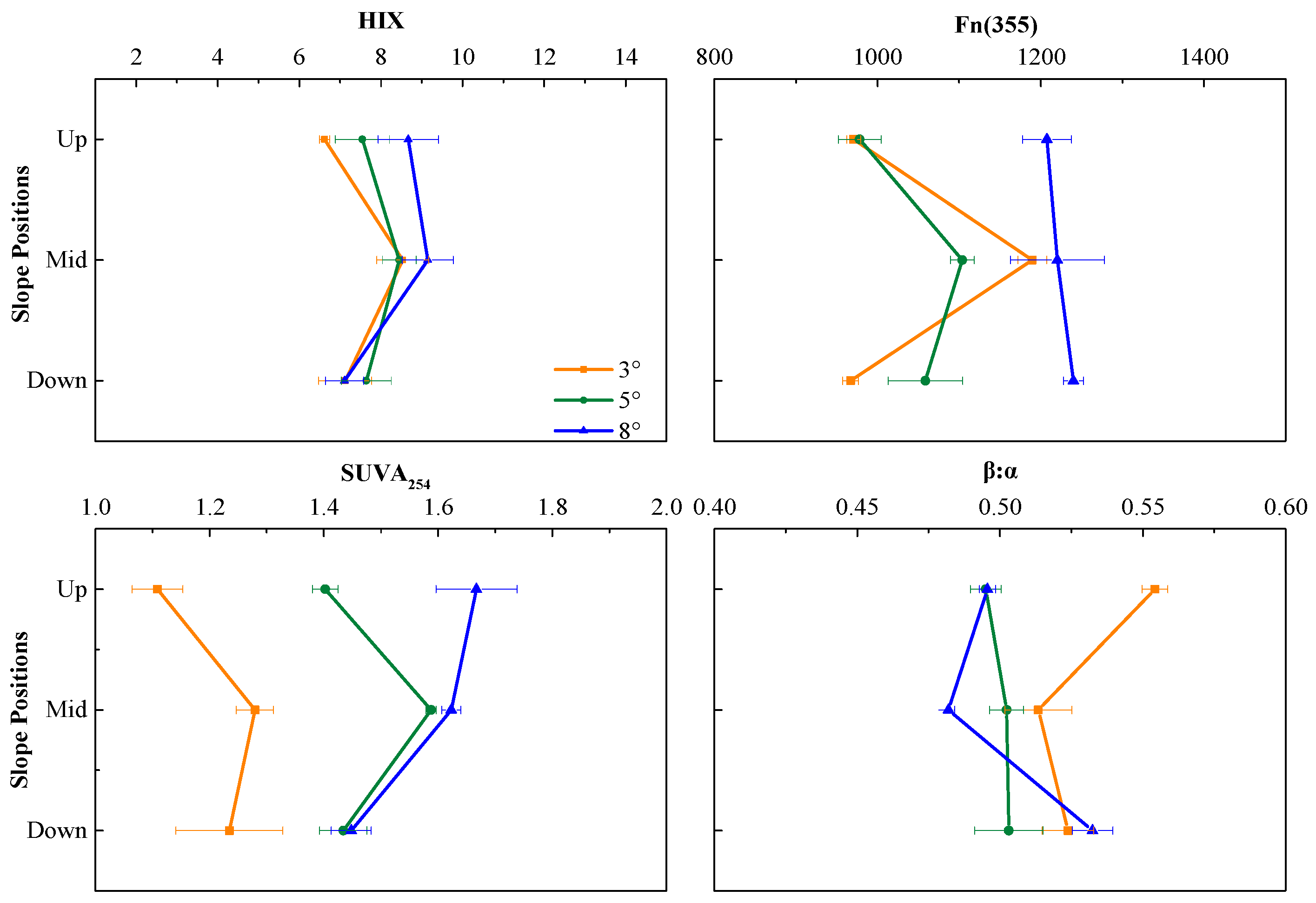
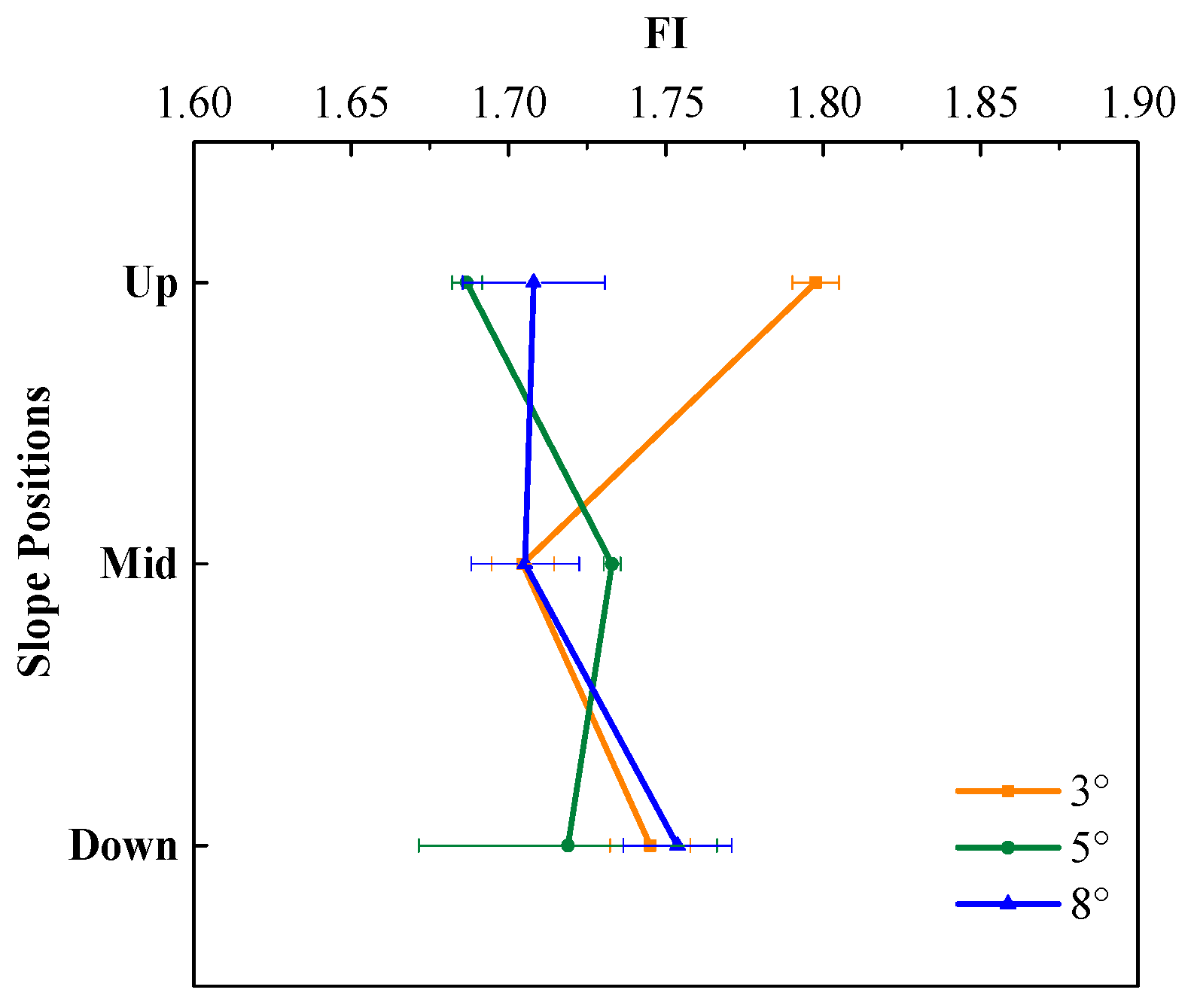
3.2. DOM Bioavailability
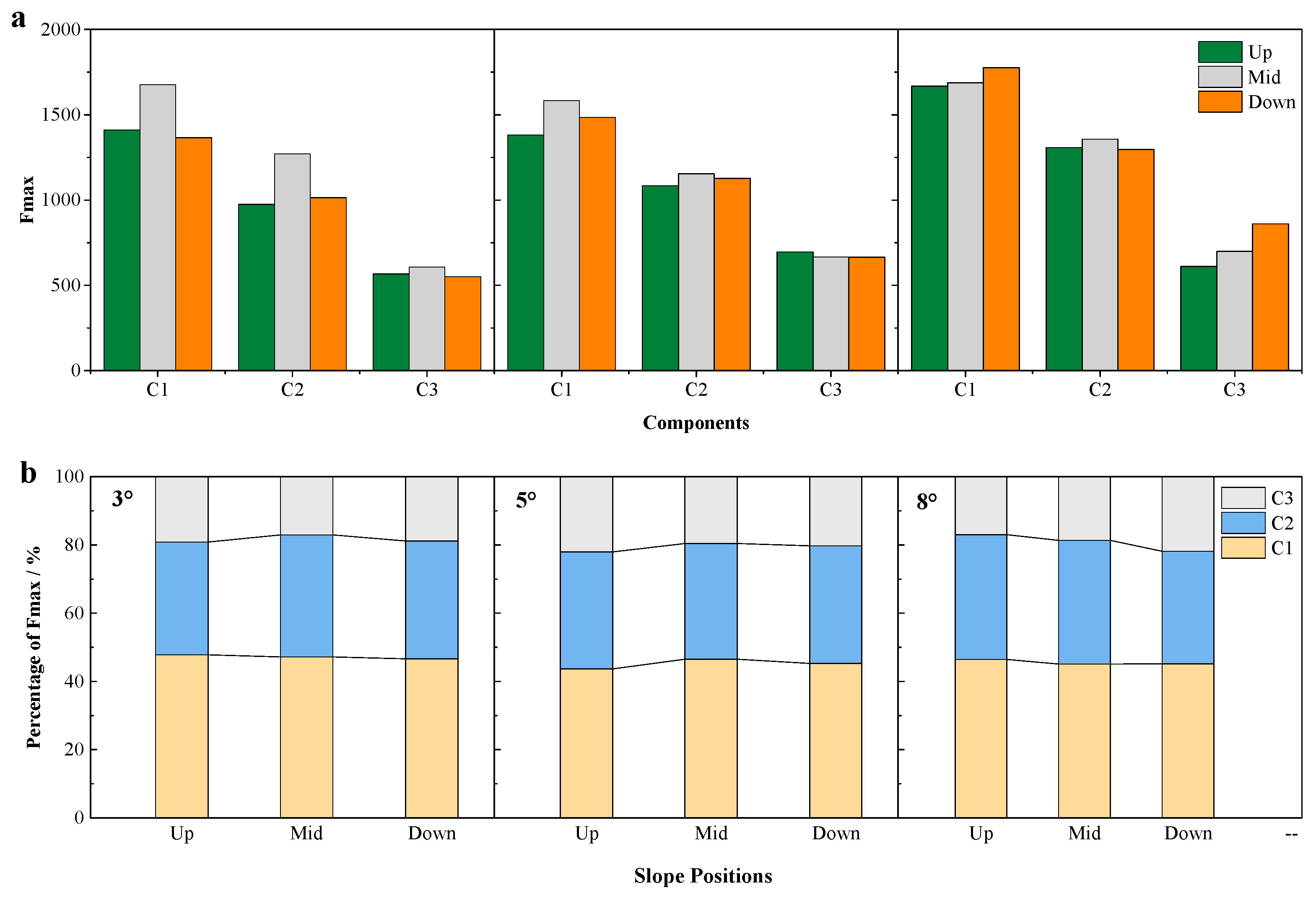
3.3. DOM Source Analysis
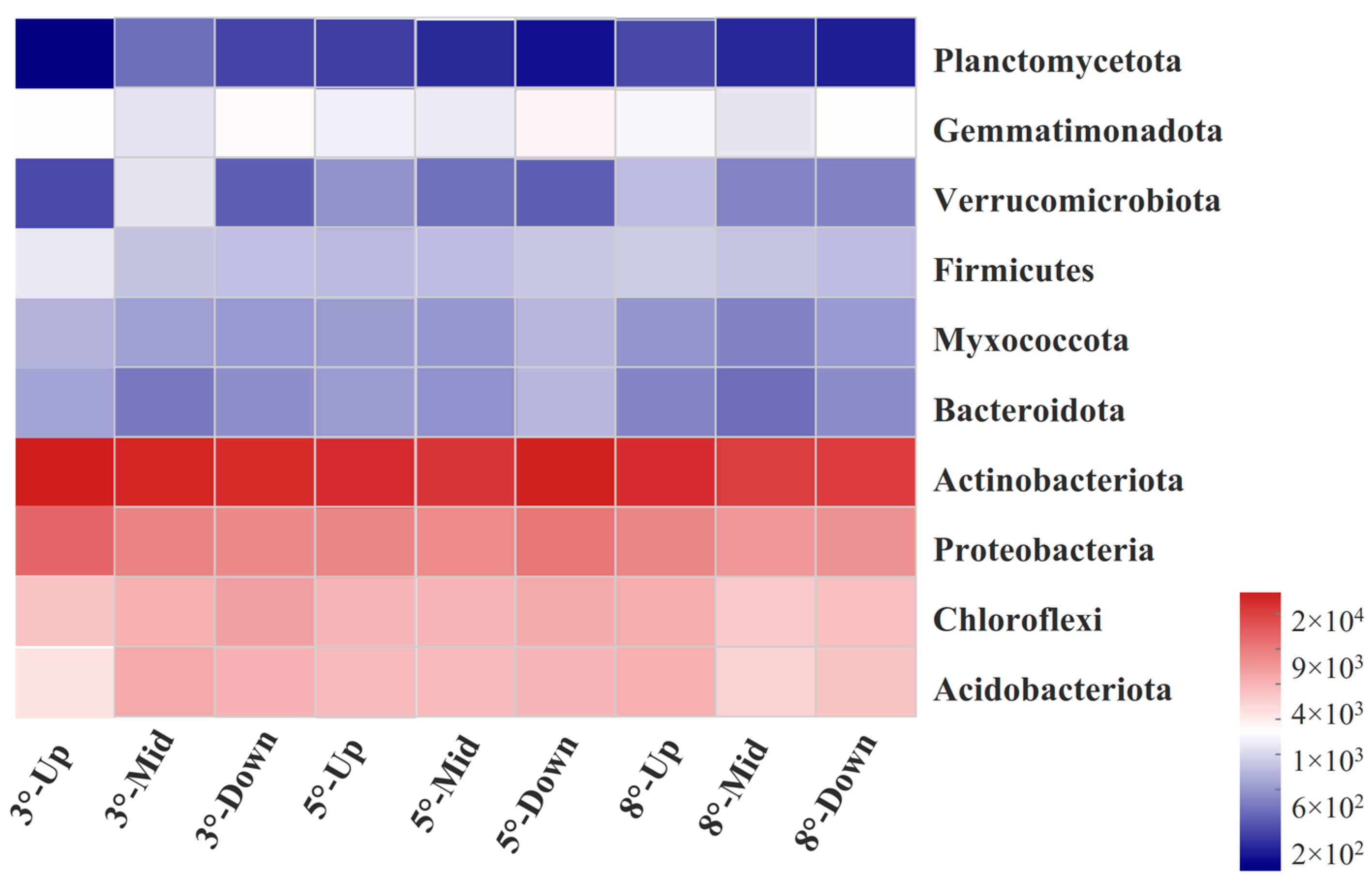
3.4. Soil Bacterial Community of Different Slopes
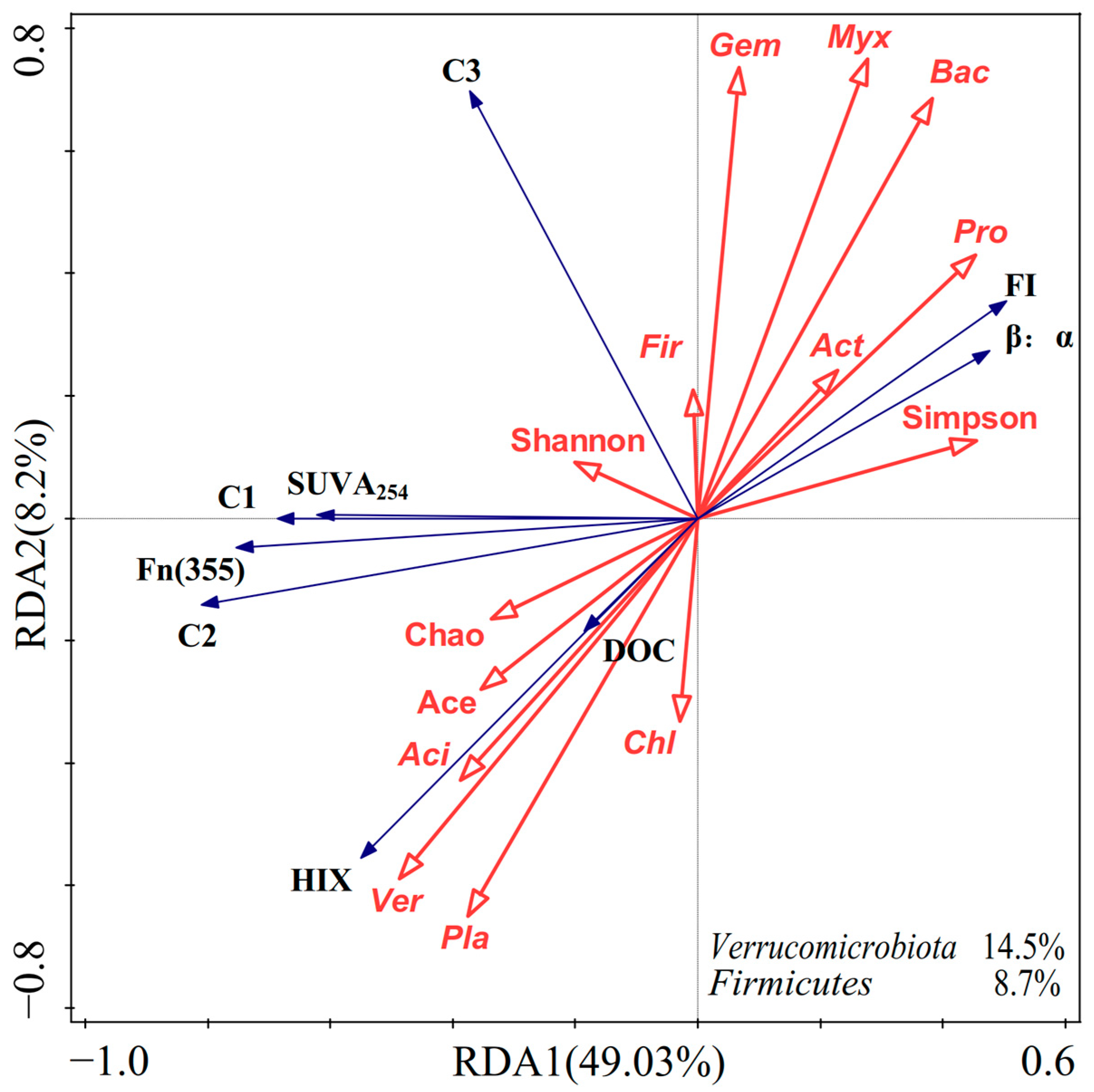
3.5. Relationship between Bacterial Community and DOM
4. Discussion
4.1. Content and Composition of DOM Vary with the Slope Gradients
4.2. Bacteria Affected the Slope Distribution of DOM
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, J.; Zheng, F.; Wang, B. Using 137Cs technique to investigate the spatial distribution of erosion and deposition regimes for a small catchment in the black soil region, Northeast China. Catena 2014, 123, 243–251. [Google Scholar] [CrossRef]
- Lal, R. Accelerated Soil erosion as a source of atmospheric CO2. Soil Tillage Res. 2018, 188, 35–40. [Google Scholar] [CrossRef]
- Jin, K.; Cornelis, W.M.; Schiette, W.; Lu, J.J.; Buysse, T.; Baert, G.; Wu, H.J.; Yao, Y.; Cai, D.X.; Jin, J.Y.; et al. Redistribution and loss of soil organic carbon by overland flow under various soil management practices on the Chinese Loess Plateau. Soil Use Manag. 2010, 24, 181–191. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Guggenberger, G.; Kaiser, K. Dissolved organic matter in soil: Challenging the paradigm of sorptive preservation. Geoderma 2003, 113, 293–310. [Google Scholar] [CrossRef]
- Ramos, M.; Martínez-Casasnovas, J. Nutrient losses by runoff in vineyards of the Mediterranean Alt Penedès region (NE Spain). Agric. Ecosyst. Environ. 2006, 113, 356–363. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Nie, X.; Huang, M.; Wang, D.; Xiao, H.; Liu, C.; Peng, H.; Jiang, J.; Zeng, G. The role of dissolved organic matter in soil organic carbon stability under water erosion. Ecol. Indic. 2019, 102, 724–733. [Google Scholar] [CrossRef]
- Cui, M.; Cai, Q.; Zhu, A.; Fan, H. Soil erosion along a long slope in the gentle hilly areas of black soil region in Northeast China. J. Geogr. Sci. 2007, 17, 375–383. [Google Scholar] [CrossRef]
- Hu, W.; Zhai, X.; Du, S.; Zhang, X. Impacts of Slope and Longitudinal Ridge on Soil Organic Carbon Dynamics in the Typical Mollisols Sloping Farmland (China). Eurasian Soil Sci. 2021, 54, 951–963. [Google Scholar] [CrossRef]
- Pang, H.; Chen, Y.; He, J.; Guo, D.; Pan, X.; Ma, Y.; Qu, F.; Nan, J. Cation exchange resin-induced hydrolysis for improving biodegradability of waste activated sludge: Characterization of dissolved organic matters and microbial community. Bioresour. Technol. 2020, 302, 122870. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Zhang, W.; Lin, L.; Wang, L.; Niu, L.; Zhang, H.; Wang, P.; Wang, C. Response of bacterial community in composition and function to the various DOM at river confluences in the urban area. Water Res. 2020, 169, 115293. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Mccann, K.; Setälä, H.; De Ruiter, P.C. Top-down is bottom-up, Does predation in the rhizosphere regulate aboveground dynamics? Ecology 2003, 84, 846–857. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, F.; Gonsior, M.; Li, Y.; Wang, Y.; He, C.; Cai, R.; Xu, J.; Wang, Y.; Xu, D.; et al. Correspondence between DOM molecules and microbial community in a subtropical coastal estuary on a spatiotemporal scale. Environ. Int. 2021, 154, 106558. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Jeppesen, E.; Wang, L.; Tan, H.; Zhang, J. Linking heterotrophic bacterioplankton community composition to the optical dynamics of dissolved organic matter in a large eutrophic Chinese lake. Sci. Total Environ. 2019, 679, 136–147. [Google Scholar] [CrossRef]
- Kirchman, D.L.; Dittel, A.I.; Findlay, S.E.G.; Fischer, D. Changes in bacterial activity and community structure in response to dissolved organic matter in the Hudson River, New York. Aquat. Microb. Ecol. 2004, 35, 243–257. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J.; Chen, Y.; Wu, J.; Wang, Y. Linkage between water soluble organic matter and bacterial community in sediment from a shallow, eutrophic lake, Lake Chaohu, China. J. Environ. Sci. 2020, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, H.; Peng, Y.; Zhang, Y.; Sun, Q. Characteristics and significance of dissolved organic matter in river sediments of extremely water-deficient basins: A Beiyun River case study. J. Clean. Prod. 2020, 277, 123063. [Google Scholar] [CrossRef]
- Traving, S.J.; Rowe, O.; Jakobsen, N.M.; Sørensen, H.; Dinasquet, J.; Stedmon, C.A.; Andersson, A.; Riemann, L. The Effect of Increased Loads of Dissolved Organic Matter on Estuarine Microbial Community Composition and Function. Front. Microbiol. 2017, 8, 351. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Kuhn, N.J.; Wang, Q.; Zhang, X. Effect of ridge tillage, no-tillage, and conventional tillage on soil temperature, water use, and crop performance in cold and semi-arid areas in Northeast China. Aust. J. Soil Res. 2010, 48, 737–744. [Google Scholar] [CrossRef]
- Jiang, T.; Kaal, J.; Liang, J.; Zhang, Y.; Wei, S.; Wang, D.; Green, N.W. Composition of dissolved organic matter (DOM) from periodically submerged soils in the Three Gorges Reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci. Total Environ. 2017, 603, 461–471. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Yang, L.; Cao, C.; Li, X. Variation in quantity and chemical composition of soil dissolved organic matter in a peri-urban critical zone observatory watershed in Eastern China. Sci. Total Environ. 2019, 688, 622–631. [Google Scholar] [CrossRef]
- Santos, P.S.; Santos, E.B.; Duarte, A.C. First spectroscopic study on the structural features of dissolved organic matter isolated from rainwater in different seasons. Sci. Total Environ. 2012, 426, 172–179. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating binding characteristics of cadmium and copper to DOM derived from compost and rice straw using EEM-PARAFAC combined with two-dimensional FTIR correlation analyses. J. Hazard. Mater. 2018, 344, 539–548. [Google Scholar] [CrossRef]
- Rodríguez-Vidal, F.J.; García-Valverde, M.; Ortega-Azabache, B.; González-Martínez, Á.; Bellido-Fernández, A. Characterization of urban and industrial wastewaters using excitation-emission matrix (EEM) fluorescence: Searching for specific fingerprints. J. Environ. Manag. 2020, 263, 110396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Kung, H.-T.; Ghulam, A.; Trumbo, A.L.; Yang, J.; Ren, Y.; Jing, Y. Evaluation and estimation of surface water quality in an arid region based on EEM-PARAFAC and 3D fluorescence spectral index: A case study of the Ebinur Lake Watershed, China. CATENA 2017, 155, 62–74. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Li, W.-T.; Chen, S.-Y.; Xu, Z.-X.; Li, Y.; Shuang, C.-D.; Li, A.-M. Characterization of Dissolved Organic Matter in Municipal Wastewater Using Fluorescence PARAFAC Analysis and Chromatography Multi-Excitation/Emission Scan: A Comparative Study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, J.; Kong, F.; Li, Y.; Li, M.; Dong, Y.; Xi, M. Identification of source apportionment and its spatial variability of dissolved organic matter in Dagu River-Jiaozhou Bay estuary based on the isotope and fluorescence spectroscopy analysis. Ecol. Indic. 2019, 102, 528–537. [Google Scholar] [CrossRef]
- Barker, J.D.; Sharp, M.J.; Turner, R.J. Using synchronous fluorescence spectroscopy and principal components analysis to monitor dissolved organic matter dynamics in a glacier system. Hydrol. Process. 2010, 23, 1487–1500. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef]
- Jacinthe, P.A.; Lal, R. A mass balance approach to assess carbon dioxide evolution during erosional events. Land Degrad. Dev. 2011, 12, 329–339. [Google Scholar] [CrossRef]
- Novara, A.; Minacapilli, M.; Santoro, A.; Rodrigo-Comino, J.; Carrubba, A.; Sarno, M.; Venezia, G.; Gristina, L. Real cover crops contribution to soil organic carbon sequestration in sloping vineyard. Sci. Total Environ. 2019, 652, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Berhe, A.A.; Kleber, M. Erosion, deposition, and the persistence of soil organic matter: Mechanistic considerations and problems with terminology. Earth Surf. Process. Landf. 2013, 38, 908–912. [Google Scholar] [CrossRef]
- Wang, J.-G.; Li, Z.-X.; Cai, C.-F.; Yang, W.; Ma, R.-M.; Zhang, G.-B. Effects of stability, transport distance and two hydraulic parameters on aggregate abrasion of Ultisols in overland flow. Soil Tillage Res. 2013, 126, 134–142. [Google Scholar] [CrossRef]
- Schiettecatte, W.; Gabriels, D.; Cornelis, W.M.; Hofman, G. Enrichment of Organic Carbon in Sediment Transport by Interrill and Rill Erosion Processes. Soil Sci. Soc. Am. J. 2008, 72, 50–55. [Google Scholar] [CrossRef]
- Tan, W.; Jia, Y.; Huang, C.; Zhang, H.; Li, D.; Zhao, X.; Wang, G.; Jiang, J.; Xi, B. Increased suppression of methane production by humic substances in response to warming in anoxic environments. J. Environ. Manag. 2018, 206, 602–606. [Google Scholar] [CrossRef]
- Yu, G.-H.; Wu, M.-J.; Wei, G.-R.; Luo, Y.-H.; Ran, W.; Wang, B.-R.; Zhang, J.; Shen, Q.-R. Binding of Organic Ligands with Al(III) in Dissolved Organic Matter from Soil: Implications for Soil Organic Carbon Storage. Environ. Sci. Technol. 2012, 46, 6102–6109. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Zhuo, J.-F.; Guo, W.-D.; Deng, X.; Zhang, Z.-Y.; Xu, J.; Huang, L.-F. Fluorescence excitation-emission matrix spectroscopy of CDOM from Yundang Lagoon and its indication for organic pollution. Spectrosc. Spectr. Anal. 2010, 30, 1539–1544. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- I Kuznetsov, S.; A Dubinina, G.; A Lapteva, N. Biology of Oligotrophic Bacteria. Annu. Rev. Microbiol. 1979, 33, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Wu, Y.; Zhang, L.; Cheng, J.; Wei, G.; Lin, Y. Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci. Total Environ. 2018, 635, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Brunner, I.; Hagedorn, F.; Bardgett, R.D.; Stierli, B.; Herzog, C.; Chen, X.; Zingg, A.; Graf-Pannatier, E.; Rigling, A.; et al. A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol. Ecol. 2016, 26, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Davinic, M.; Fultz, L.M.; Acosta-Martinez, V.; Calderón, F.J.; Cox, S.B.; Dowd, S.E.; Allen, V.G.; Zak, J.C.; Moore-Kucera, J. Pyrosequencing and mid-infrared spectroscopy reveal distinct aggregate stratification of soil bacterial communities and organic matter composition. Soil Biol. Biochem. 2012, 46, 63–72. [Google Scholar] [CrossRef]
- Tveit, A.; Schwacke, R.; Svenning, M.M.; Urich, T. Organic carbon transformations in high-Arctic peat soils: Key functions and microorganisms. ISME J. 2013, 7, 299–311. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Stevens, S.L.R.; Chan, L.-K.; Bertilsson, S.; del Rio, T.G.; Tringe, S.G.; Malmstrom, R.R.; McMahon, K.D. Ecophysiology of Freshwater Verrucomicrobia Inferred from Metagenome-Assembled Genomes. mSphere 2017, 2, e00277-17. [Google Scholar] [CrossRef] [PubMed]
- A Hug, L.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.-H.; Yang, K.-J. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil 2019, 436, 211–227. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]

| Slope | Positions | Community Diversity | Community Richness | Coverage % | ||
|---|---|---|---|---|---|---|
| Shannon | Simpson | Ace | Chao | |||
| 3° | Up | 6.36 ± 0.09a | 0.0060 ± 0.0007a | 4125.09 ± 103.86ab | 4119.12 ± 155.41b | 97.96 ± 0.08a |
| Mid | 6.43 ± 0.06a | 0.0046 ± 0.0002b | 4526.52 ± 89.53a | 4376.90 ± 70.86a | 97.77 ± 0.06a | |
| Down | 6.41 ± 0.04a | 0.0048 ± 0.0003b | 3844.54 ± 88.53b | 3779.67 ± 138.27c | 98.01 ± 0.08a | |
| 5° | Up | 6.50 ± 0.05a | 0.0047 ± 0.0004a | 4292.00 ± 28.79a | 4276.69 ± 54.35a | 97.59 ± 0.02b |
| Mid | 6.55 ± 0.03a | 0.0043 ± 0.0003a | 4260.00 ± 155.17a | 4256.46 ± 186.33a | 97.50 ± 0.14b | |
| Down | 6.46 ± 0.08a | 0.0047 ± 0.0005a | 4097.66 ± 92.45a | 4070.79 ± 120.16a | 98.03 ± 0.06a | |
| 8° | Up | 6.48 ± 0.05a | 0.0046 ± 0.0004a | 4225.74 ± 51.70a | 4179.96 ± 82.36a | 97.72 ± 0.05a |
| Mid | 6.41 ± 0.06a | 0.0049 ± 0.0005a | 4282.93 ± 505.60a | 4075.77 ± 192.85a | 97.24 ± 0.12b | |
| Down | 6.46 ± 0.03a | 0.0048 ± 0.0003a | 4082.75 ± 48.74a | 4083.34 ± 28.91a | 97.42 ± 0.04ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, S.; Wang, W.; Sun, L.; Li, Y.; Sun, Z.; Gao, Z.; Zhang, J.; Li, Y.; Wei, D. Bacteria Affect the Distribution of Soil-Dissolved Organic Matter on the Slope: A Long-Term Experiment in Black Soil Erosion. Agriculture 2024, 14, 352. https://doi.org/10.3390/agriculture14030352
Cai S, Wang W, Sun L, Li Y, Sun Z, Gao Z, Zhang J, Li Y, Wei D. Bacteria Affect the Distribution of Soil-Dissolved Organic Matter on the Slope: A Long-Term Experiment in Black Soil Erosion. Agriculture. 2024; 14(3):352. https://doi.org/10.3390/agriculture14030352
Chicago/Turabian StyleCai, Shanshan, Wei Wang, Lei Sun, Yumei Li, Zhiling Sun, Zhongchao Gao, Jiuming Zhang, Yan Li, and Dan Wei. 2024. "Bacteria Affect the Distribution of Soil-Dissolved Organic Matter on the Slope: A Long-Term Experiment in Black Soil Erosion" Agriculture 14, no. 3: 352. https://doi.org/10.3390/agriculture14030352
APA StyleCai, S., Wang, W., Sun, L., Li, Y., Sun, Z., Gao, Z., Zhang, J., Li, Y., & Wei, D. (2024). Bacteria Affect the Distribution of Soil-Dissolved Organic Matter on the Slope: A Long-Term Experiment in Black Soil Erosion. Agriculture, 14(3), 352. https://doi.org/10.3390/agriculture14030352





