Phenotypic Diversity of Quinoa Landraces Cultivated in the Ecuadorian Andean Region: In Situ Conservation and Detection of Promising Accessions for Breeding Programs
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Agro-Morphological Evaluation
2.3. Selection Index
2.4. Data Analysis
3. Results
3.1. Diversity in Quantitative Traits
3.2. Diversity in Qualitative Traits
3.3. Cluster Analysis Based on Phenotypic Characters
3.4. Selection Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ain, Q.T.; Siddique, K.; Bawazeer, S.; Ali, I.; Mazhar, M.; Rasool, R.; Mubeen, B.; Ullah, F.; Unar, A.; Jafar, T.H. Adaptive mechanisms in quinoa for coping in stressful environments. PeerJ 2022, 11, 31–32. [Google Scholar] [CrossRef]
- FAO; Future Smart Food. Rediscovering Hidden Treasures of Neglected and Underutilized Species for Zero Hunger in Asia, Executive Summary; Food & Agriculture Organization: Bangkok, Tailandia, 2018; p. 36. [Google Scholar]
- Salazar, J.; De Lourdes Torres, M.; Gutierrez, B.; Torres, A.F. Molecular characterization of Ecuadorian quinoa (Chenopodium quinoa Willd.) diversity: Implications for conservation and breeding. Euphytica 2019, 215, 60. [Google Scholar] [CrossRef]
- Yugcha, T. Zonificación Potencial del Cultivo de la Quinua en el Callejón Interandino del Ecuador; Ministerio de Agricultura y Ganadería (MAG): Quito, Ecuador, 1996; p. 10.
- Peralta, E.; Mazón, N. La Quinua en Ecuador. Capítulo 5.3. En Estado del Arte de la Quinua en el Mundo en 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO (Santiago de Chile) y CIRAD: Montpillier, Francia, 2014; pp. 462–746. [Google Scholar]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Risi, J.C.; Galwey, N.W. The Chenopodium grains of the Andes: Inca crops for modern agriculture. Adv. Appl. Biol. 1984, 10, 145–216. [Google Scholar]
- Bazile, D.; Jacobsen, S.E.; Verniau, A. The global expansion of quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Tapia, C.; Peralta, E.; Mazón, N. Colección núcleo de quinua (Chenopodium quinoa Willd.) del Banco de Germoplasma del INIAP, Ecuador. Axioma 2015, 12, 5–9. [Google Scholar]
- Gandarillas, H.; Nieto, C.; Castillo, R. Razas de Quinua en Ecuador. Boletín Técnico No.67. Estación Experimental Santa Catalina; Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 1989; p. 22. [Google Scholar]
- Tapia, E.; Rosas, A. Agrobiodiversidad en La Encañada. Sistematización de las Experiencias de Conservación in Situ de los Recursos Fitogenéticos, Cajamarca; Condesan, Aspaderuc, CIP, GTZ: Cajamarca, Perú, 1998; p. 26. [Google Scholar]
- INABIO (Instituto Nacional de Biodiversidad). Metas AICHI. 13: Diversidad Genética Mantenida. 2019. Available online: http://inabio.biodiversidad.gob.ec/2019/01/30/13-diversidad-genetica-mantenida/ (accessed on 15 July 2022).
- DENAREF (Departamento Nacional de Recursos Fitogenéticos); Banco Nacional de Germoplasma. Estación Experimental Santa Catalina; INIAP: Quito, Ecuador, 2016; p. 34. [Google Scholar]
- Peralta, E.; Mazón, N.; Murillo, Á.; Villacrés, E.; Rivera, M. Catalogó de Variedades Mejoradas de Granos Andino: Chocho, Quinua, Amaranto, Sangorache, Para la Sierra Ecuatoriana. Publicación Miscelánea No. 151. Tercera Edición. Programa Nacional de Leguminosas y Granos Andinos; Estación Experimental Santa Catalina INIAP: Quito, Ecuador, 2013; p. 63. [Google Scholar]
- Monteros, A. Rendimientos de Quinua en el Ecuador. In Dirección de Análisis y Procesamiento de la Información Coordinación General del Sistema de Información Nacional Ministerio de Agricultura; Ganadería, Acuacultura y Pesca: Quito, Ecuador, 2016; p. 9. [Google Scholar]
- El-Harty, E.H.; Ghazy, A.; Alateeq, T.K.; Al-Faifi, S.A.; Khan, M.A.; Afzal, M.; Alghamdi, S.S.; Migdadi, H.M. Morphological and Molecular Characterization of Quinoa Genotypes. Agriculture 2021, 11, 286. [Google Scholar] [CrossRef]
- Manjarres-Hernández, E.H.; Arias-Moreno, D.M.; Morillo, A.C.; Ojeda-Pérez, Z.Z.; Cárdenas-Chaparro, A. Phenotypic Characterization of Quinoa (Chenopodium quinoa Willd.) for the Selection of Promising Materials for Breeding Programs. Plants 2021, 10, 1339. [Google Scholar] [CrossRef]
- Romero, M.; Mujica, A.; Pineda, E.; Ccamapaza, Y.; Zavalla, N. Genetic identity based on simple sequence repeat (SSR) markers for Quinoa (Chenopodium quinoa Willd.). Cienc. Inv. Agric. 2019, 46, 166–178. [Google Scholar] [CrossRef]
- Mazón, N.; Rivera, M.; Peralta, E.; Estrella, J.; Tapia, C. Catálogo del Banco de Germoplasma de Quinua (Chenopodium quinoa Willd.); de INIAP-Ecuador: Quito, Ecuador, 2001; p. 97. [Google Scholar]
- Peralta, E. Amaranto y Ataco: Preguntas y Respuestas. Boletín Divulgativo No. 359. Programa Nacional de Leguminosas y Granos Andinos. Estación Experimental Santa Catalina; INIAP: Quito, Ecuador, 2009; p. 8. [Google Scholar]
- Bioversity International; FAO; PROINPA; INIAF; FIDA. Descriptores Para Quinua (Chenopodium quinoa Willd.) y Sus Parientes Silvestres; Bioversity International: Roma, Italy; Organización de las Naciones Unidas para la Agricultura y la Alimentación: Roma, Italy; Fundación PROINPA: La Paz, Bolivia; Instituto Nacional de Innovación Agropecuaria y Forestal: La Paz, Bolivia; Fondo Internacional de Desarrollo Agrícola: Roma, Italy, 2013; p. 52. [Google Scholar]
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Gómez, L.; Aguilar, E. Guía de Cultivo de la Quinua; Organización de las Naciones Unidas para la Alimentación y la Agricultura, Universidad Nacional Agraria La Molina, Programa de Investigación y Proyección Social de Cereales y Granos Nativos Facultad de Agronomía: Lima, Peru, 2016; p. 130. [Google Scholar]
- Delgado, A.I.; Palacios, J.H.; Betancourt, C. Evaluation of 16 genotypes of sweet quinoa (Chenopodium quinoa Willd.) in the municipality of Iles, Nariño (Colombia). Agron. Colomb. 2009, 27, 159–167. [Google Scholar]
- INFOSTAT. Grupo InfoStat, Versión 2020; Software Estadístico; Universidad Nacional de Córdoba (FCA): Córdoba, Argentina, 2020; Available online: http://www.infostat.com.ar (accessed on 15 April 2020).
- Fisher, R.A. The Use of Multiple Measurements in Taxonomy Problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Taiyun, W.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix (version 0.84). Statistician 2017, 56, e24. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 2020, 1, 337–354. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Cochran, W.G. Some methods of strengthening the common X2 tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Pearson, K. On the X2 test of goodness of fit. Biometrika 1922, 14, 186–191. [Google Scholar] [CrossRef]
- Cramér, H. Mathematical Methods of Statistics; Princeton University Press: Princeton, NJ, USA, 1946. [Google Scholar]
- Gower, J.A. Comparison of some methods of cluster analysis. Biometrics 1967, 23, 623–627. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy System (ver. 2.2); Exeter Publishing, Ltd.: Setaukert, NY, USA, 2008. [Google Scholar]
- Nguyen, D.C.; Tran, D.S.; Tran, T.T.H.; Ohsawa, R.; Yoshioka, Y. Genetic diversity of leafy amaranth (Amaranthus tricolor L.) resources in Vietnam. Breed. Sci. 2019, 69, 640–650. [Google Scholar] [CrossRef]
- Nyasulu, M.; Sefasi, A.; Chimzinga, S.; Maliro, M. Agromophological characterisation of Amaranth Accessions from Malawi. Am. J. Plant Sci. 2021, 12, 1528–1542. [Google Scholar] [CrossRef]
- De Santis, G.; Ronga, D.; Caradonia, F.; D Ambrosio, D.; Troisi, J.; Rascio, A.; Fragasso, M.; Pecchioni, N.; Rinaldi, M. Evaluation of two groups of quinoa (Chenopodium quinoa Willd.) accessions with different seed colours for adaptation to the Mediterranean environment. Crop. Pasture Sci. 2018, 69, 1264–1275. [Google Scholar] [CrossRef]
- Maliro, M.F.A.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary Studies of the Performance of Quinoa (Chenopodium quinoa Willd.) Genotypes under Irrigated and Rainfed Conditions of Central Malawi. Front. Plant Sci. 2017, 8, 1268014. [Google Scholar] [CrossRef]
- Mhada, M.; Jellen, E.N.; Jacobsen, S.E.; Benlhabib, O. Diversity Analysis of a Quinoa (Chenopodium quinoa Willd.) Germplasm during Two Seasons. Int. J. Agric. Biol. Eng. 2014, 8, 273–276. [Google Scholar] [CrossRef]
- Morillo, A.C.; Manjarres-Hernández, E.H.; Morillo, Y. Evaluación morfoagronómica de 19 materiales de Chenopodium quinoa en el Departamento de Boyacá. Biotecnol. Sect. Agropecu. Agroind. 2020, 8, 84–96. [Google Scholar]
- Morillo, A.C.; Manjarres-Hernández, E.H.; Reyes, W.L.; Morillo, Y. Intrapopulation phenotypic variation in Piartal (Chenopodium quinoa Willd.) from the Departament of Boyacá, Colombia. Afr. J. Agric. Res. 2020, 16, 1195–1203. [Google Scholar] [CrossRef]
- Morillo, A.C.; Manjarres-Hernández, E.H.; Morillo, Y. Phenotypic diversity of agromorphological characteristics of quinoa (Chenopodium quinoa Willd.) germplasm in Colombia. Sci. Agric. 2022, 79, 4. [Google Scholar] [CrossRef]
- Fuentes, F.F.; Bhargava, A. Morphological analysis of quinoa germplasm grown under lowland desert conditions. J. Agron. Crop. Sci. 2011, 197, 124–134. [Google Scholar] [CrossRef]
- Infante, I.; Albesiano, S.; Arrienta, L.; Gomez, N. Morphological characterization of varieties of Chenopodium quinoa cultivated in the department of Boyacá, Colombia. Rev. U.D.C.A. Actual. Divulg. Cient. 2018, 21, 329–339. [Google Scholar]
- Bonifacio, A.; Aroni, G.; Villca, M. Catálogo Etnobotánico de la Quinua Real; PROINPA: Cochabamba, Bolivia, 2012; p. 63. [Google Scholar]
- Monteros, C.; Nieto, C.; Caicedo, C.; Rivera, M.; Vimos, C.; INIAP-ALEGRÍA. Primera Variedad Mejorada de Amaranto Para la Sierra Ecuatoriana. Boletín Divulgativo No.246. Programa de Cultivos Andinos. Estación Experimental Santa Catalina; INIAP: Quito, Ecuador, 1994; p. 24. [Google Scholar]
- Peralta, E.; Mazón, N.; Murillo, Á.; Rivera, M.; Rodríguez, D.; Lomas, L.; Monar, C. Manual Agrícola de Granos Andinos: Chocho, Quinua, Amaranto y Ataco. Cultivos, variedades y costos de producción, 3rd ed.; INIAP: Quito, Ecuador, 2012; p. 68. [Google Scholar]
- McLean-Rodríguez, F.D.; Costich, D.E.; Camacho-Villa, T.C.; Pé, M.E.; Dell´Acqua, M. Genetic diversity and selection signatures in maize landraces compared across 50 years of in situ and ex situ conservation. Heredity 2021, 126, 913–928. [Google Scholar] [CrossRef]
- Curti, R.N.; Andrade, A.J.; Bramardi, S.; Velásquez, B.; Bertero, H.D. Ecogeographic structure of phenotypic diversity in cultivated populations of quinoa from Northwest Argentina. Ann. Appl. Biol. 2012, 160, 114–125. [Google Scholar] [CrossRef]
- Nieto, C.; Castillo, R.; Peralta, E. Guía Para la Producción de Semilla de Quinua. Boletín Divulgativo No. 186. Estación Experimental Santa Catalina; INIAP: Quito, Ecuador, 1986; p. 120. [Google Scholar]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
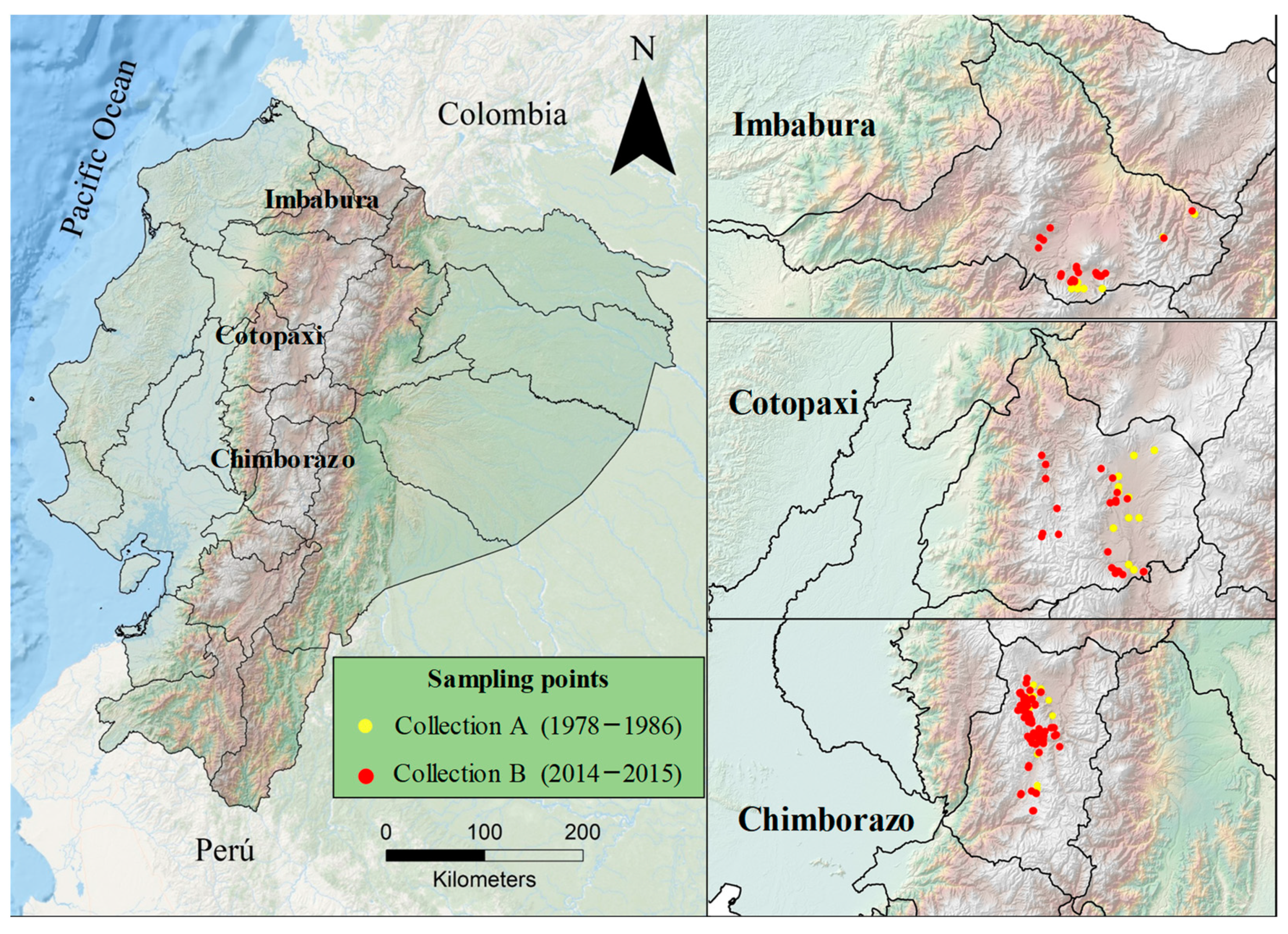
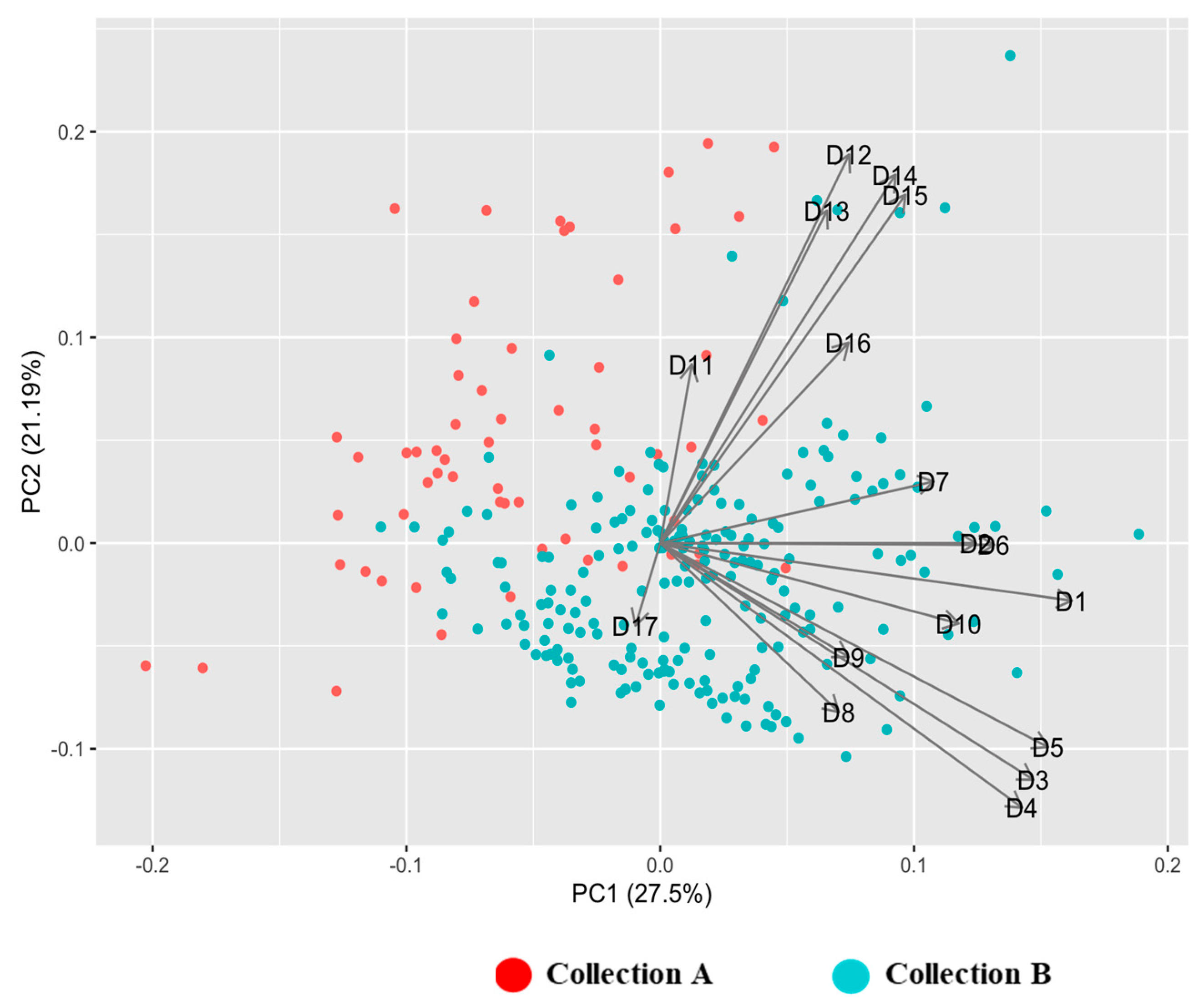
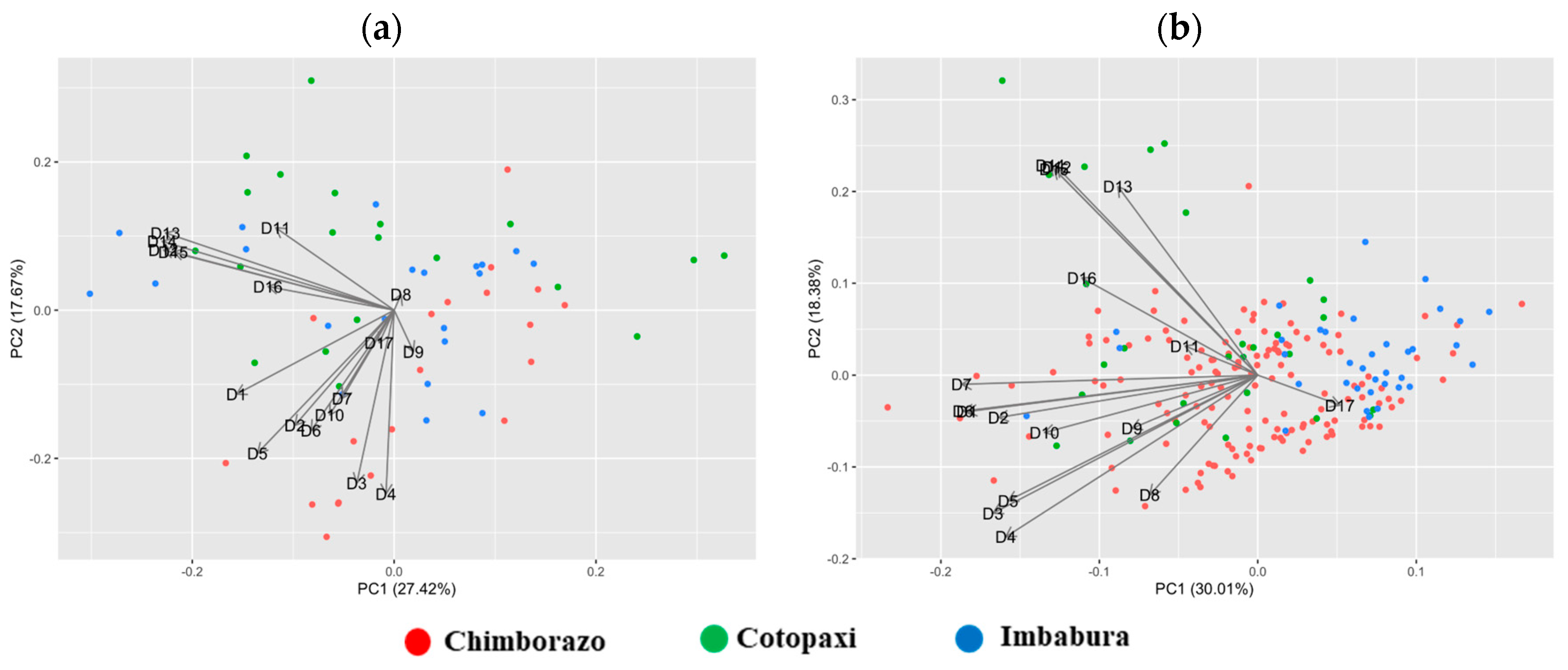

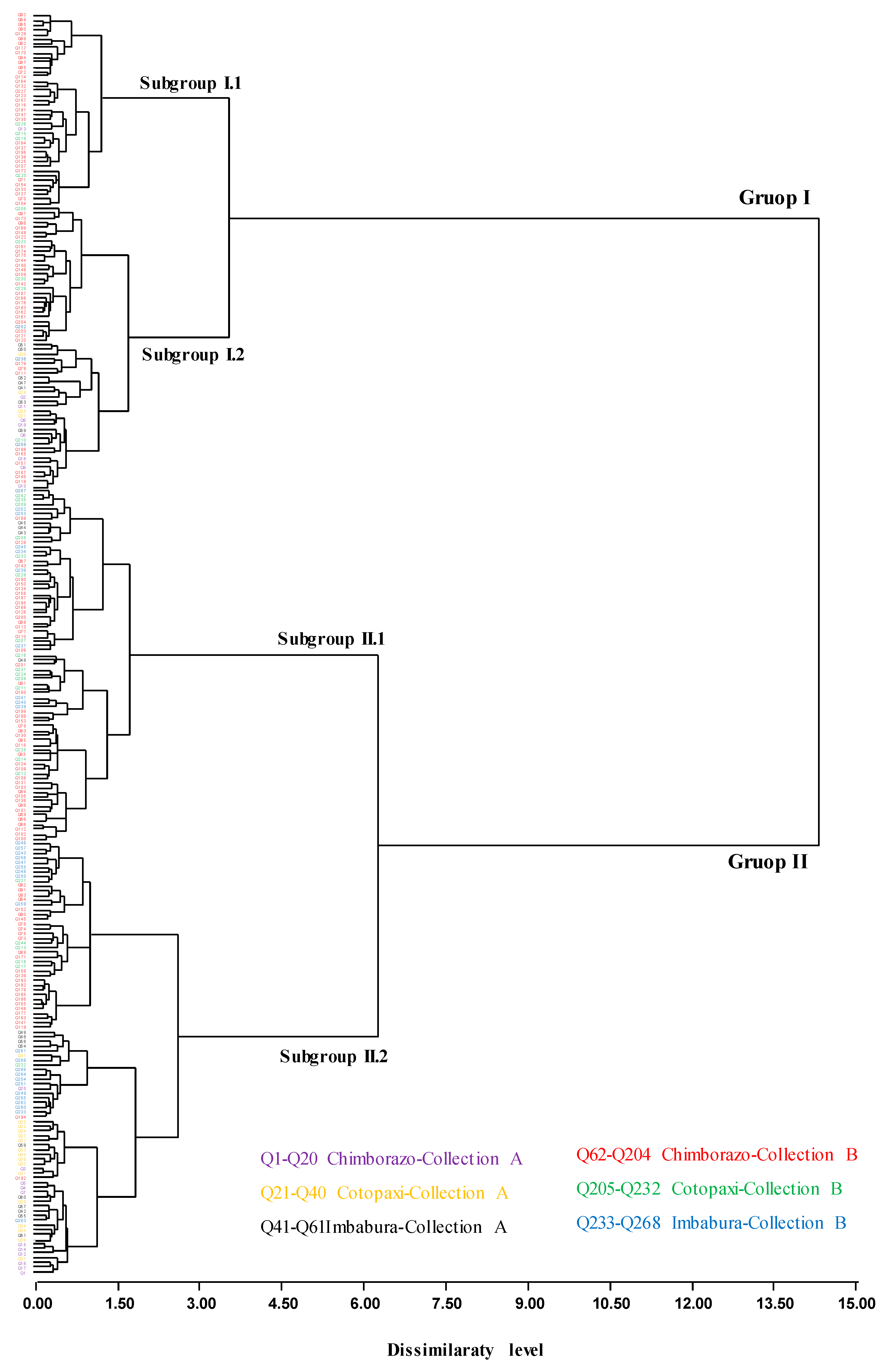
| Quantitative Descriptors (Unit of Measure) | Qualitative Descriptors |
|---|---|
| D1-Plant height (cm) | D18-Type of growth |
| D2-Main stem diameter (cm) | D19-Growth habit |
| D3-Petiole length (cm) | D20-Main stem shape |
| D4-Maximum leaf length (cm) | D21-Main stem colour |
| D5-Maximum leaf width (cm) | D22-Presence of pigmented axils |
| D6-Panicle length (cm) | D23-Presence of striae |
| D7-Panicle width (cm) | D24-Striae colour |
| D8-Grain width (mm) | D25-Presence of branching |
| D9-1000-grain weight (g) | D26-Leaf shape |
| D10-Seed yield per plant (g) | D27-Leaf margin |
| D11-Days to emergence (#) | D28-Number of teeth on leaf blade |
| D12-Number of days to floral bud formation (#) | D29-Petiole colour |
| D13-Number of days to start of flowering (#) | D30-Leaf lamina colour |
| D14-Number of days to 50% of flowering (#) | D31-Leaf granules colour |
| D15-Number of days to end of flowering (#) | D32-Panicle colour at flowering |
| D16-Number of days to 50% physiological maturity (#) | D33-Panicle colour at physiological maturity |
| D17-Harvest index | D34-Panicle shape |
| D35-Panicle density | |
| D36-Dehiscence degree | |
| D37-Pericarp colour | |
| D38-Episperm colour | |
| D39-Grain shape | |
| D40-Presence of saponin * |
| Code—Descriptor | Collection A | Collection B | Collection A (Means/Province) | Collection B (Means/Province) | Collection A vs. Collection B (p-Values/Province) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV (%) | Mean | CV (%) | p-Value | Chimborazo | Cotopaxi | Imbabura | p-Value | Chimborazo | Cotopaxi | Imbabura | p-Value | Chimborazo | Cotopaxi | Imbabura | |
| D1—plant height | 151.84 | 15.60 | 176.48 | 10.27 | <0.0001 | 154.65 | 152.20 | 148.81 | 0.7364 | 177.62 A | 186.96 B | 163.81 C | <0.0001 | <0.0001 | <0.0001 | 0.0025 |
| D2—main stem diameter | 1.18 | 15.67 | 1.25 | 14.46 | 0.0462 | 1.13 | 1.13 | 1.31 | 0.1770 | 1.23 | 1.33 | 1.24 | 0.0518 | 0.0198 | 0.0002 | 0.1532 |
| D3—petiole length | 3.05 | 28.37 | 4.89 | 13.37 | <0.0001 | 3.79 B | 2.57 A | 2.79 A | <0.0001 | 5.05 B | 4.87 B | 4.23 A | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D4—maximum leaf length | 5.67 | 20.59 | 7.93 | 11.09 | <0.0001 | 6.78 B | 5.08 A | 5.17 A | <0.0001 | 8.18 B | 7.56 A | 7.22 A | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D5—maximum leaf width | 4.05 | 23.01 | 6.53 | 15.58 | <0.0001 | 4.19 | 3.75 | 4.21 | 0.2067 | 6.91 C | 6.18 B | 5.33 A | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D6—panicle length | 62.90 | 20.17 | 65.42 | 17.00 | 0.1330 | 66.58 | 62.67 | 59.62 | 0.2159 | 65.44 AB | 69.48 B | 62.18 A | 0.0326 | 0.6776 | 0.0623 | 0.3813 |
| D7—panicle width | 14.65 | 66.48 | 13.65 | 40.58 | 0.6682 | 14.32 | 10.71 | 17.02 | 0.0944 | 13.16 A | 17.00 B | 13.01 A | 0.0023 | 0.3938 | 0.0002 | 0.1056 |
| D8—grain width | 1.92 | 5.39 | 1.99 | 4.92 | <0.0001 | 1.92 | 1.94 | 1.90 | 0.5061 | 2.00 B | 1.99 B | 1.93 A | 0.0006 | 0.0007 | 0.0244 | 0.2552 |
| D9—1000-grain weight | 2.74 | 9.83 | 2.90 | 11.35 | 0.0007 | 2.84 | 2.67 | 2.70 | 0.1058 | 2.95 B | 2.93 B | 2.67 A | <0.0001 | 0.1846 | 0.0007 | 0.6073 |
| D10—seed yield per plant | 16.65 | 48.99 | 22.01 | 40.86 | <0.0001 | 17.91 | 13.72 | 18.24 | 0.1458 | 23.20 B | 22.00 AB | 17.33 A | 0.0020 | 0.0141 | 0.0018 | 0.6678 |
| D11—days to emergence | 7.30 | 30.09 | 7.92 | 19.59 | 0.0135 | 7.00 A | 8.75 B | 8.00 AB | 0.0638 | 7.00 | 7.75 | 7.78 | 0.0076 | 0.5973 | 0.1983 | 0.7304 |
| D12—days to floral bud formation | 73.14 | 11.65 | 76.11 | 8.26 | 0.0029 | 74.15 | 78.05 | 76.14 | 0.3864 | 72.83 A | 77.25 B | 71.22 A | 0.0002 | 0.2882 | 0.7815 | 0.0131 |
| D13—days to start of flowering | 83.50 | 10.84 | 84.97 | 12.71 | 0.3289 | 81.40 A | 88.70 B | 84.81 AB | 0.0404 | 83.15 A | 88.93 B | 80.64 A | 0.0059 | 0.4863 | 0.9462 | 0.0341 |
| D14—days to 50% of flowering | 93.73 | 10.70 | 94.52 | 9.66 | 0.5583 | 90.70 | 96.95 | 95.95 | 0.1309 | 93.01 A | 102.43 B | 89.83 A | <0.0001 | 0.1801 | 0.1373 | 0.0165 |
| D15—days to end of flowering | 105.98 | 9.99 | 106.25 | 7.43 | 0.8335 | 105.00 | 105.9 | 107.00 | 0.8369 | 105.70 A | 114.25 B | 102.19 A | <0.0001 | 0.5711 | 0.0522 | 0.0296 |
| D16—days to 50% physiological maturity | 181.50 | 11.35 | 182.39 | 9.35 | 0.7326 | 176.15 A | 173.15 A | 197.14 B | <0.0001 | 184.25 B | 181.39 B | 170.67 A | <0.0001 | 0.0308 | 0.1509 | <0.0001 |
| D17—harvest index | 0.54 | 30.94 | 0.56 | 21.01 | 0.1589 | 0.62 B | 0.56 B | 0.44 A | 0.0011 | 0.59 B | 0.53 B | 0.49 A | <0.0001 | 0.2226 | 0.5098 | 0.1585 |
| Code—Descriptor | State | Collection A | Total | Collection B | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Chimborazo | Cotopaxi | Imbabura | Frequencies | Chimborazo | Cotopaxi | Imbabura | Frequencies | ||
| D19—growth habit | 1 simple | 20 | 20 | 21 | 61 | 138 | 28 | 32 | 198 |
| 2 branched to bottom third | - | - | - | - | 2 | - | - | 2 | |
| 3 branched to second third | - | - | - | - | 3 | - | 4 | 7 | |
| D20—main stem shape | 1 cylindrical | 20 | 20 | 21 | 61 | 46 | 1 | - | 47 |
| 2 angular | - | - | - | - | 97 | 27 | 36 | 160 | |
| D21—main stem colour | 2 purple | 8 | 4 | 7 | 19 | 67 | 10 | 1 | 78 |
| 4 pink | 5 | 3 | 3 | 11 | 1 | - | 4 | 5 | |
| 5 yellow | 7 | 13 | 11 | 31 | 75 | 18 | 31 | 124 | |
| D22—presence of pigmented axils | 0 absent | 6 | 16 | 14 | 36 | 8 | 15 | 33 | 56 |
| 1 present | 5 | - | - | 5 | 68 | 4 | - | 72 | |
| 2 undetermined | 9 | 4 | 7 | 20 | 67 | 9 | 3 | 79 | |
| D24—striae colour | 1 green | 13 | 19 | 15 | 47 | 68 | 19 | 34 | 121 |
| 2 yellow | 7 | 1 | 2 | 10 | 75 | 9 | 2 | 86 | |
| 4 purple | - | - | 4 | 4 | - | - | - | - | |
| D25—presence of branching | 0 absent | 19 | 17 | 12 | 48 | 108 | 25 | 29 | 162 |
| 1 present | 1 | 3 | 9 | 13 | 35 | 3 | 7 | 45 | |
| D26—leaf shape | 1 rhomboidal | 19 | 19 | 14 | 52 | 61 | 12 | 24 | 97 |
| 2 triangular | 1 | 1 | 7 | 9 | 82 | 16 | 12 | 110 | |
| D28—number of teeth on leaf blade | 5 seven to twelve teeth | 19 | 19 | 14 | 52 | 59 | 10 | 23 | 92 |
| 7 more than twelve teeth | 1 | 1 | 7 | 9 | 84 | 18 | 13 | 115 | |
| D29—petiole colour | 1 green | 12 | 17 | 16 | 45 | 77 | 10 | 34 | 129 |
| 2 green-red (ridged/variegated) | 8 | 3 | 5 | 16 | 66 | 18 | 2 | 78 | |
| D30—leaf lamina colour | 1 green | 12 | 17 | 16 | 45 | 77 | 10 | 34 | 129 |
| 2 green-red (ridge/variegated) | 8 | 3 | 5 | 16 | 66 | 18 | 2 | 78 | |
| D32—panicle colour at flowering | 1 green | 7 | 16 | 9 | 32 | 73 | 14 | 18 | 105 |
| 2 purple | 9 | 3 | 6 | 18 | 68 | 10 | 2 | 80 | |
| 4 mixture (purple and red) | 4 | 1 | 6 | 11 | 2 | 4 | 16 | 22 | |
| D33—panicle colour at physiological maturity | 2 purple | 3 | 3 | 3 | 9 | 56 | 6 | 1 | 63 |
| 4 pink | 6 | - | 1 | 7 | 2 | 2 | 3 | 7 | |
| 5 yellow | 7 | 14 | 8 | 29 | 47 | 10 | 12 | 69 | |
| 12 red and yellow | 4 | 3 | 9 | 16 | 38 | 10 | 20 | 68 | |
| D34—panicle shape | 1 glomerulate | 20 | 18 | 10 | 48 | 123 | 28 | 35 | 186 |
| 2 intermediate | - | 2 | 10 | 12 | 19 | - | 1 | 20 | |
| 3 amarantiform | - | - | 1 | 1 | 1 | - | - | 1 | |
| D35—panicle density | 1 lax | 2 | - | - | 2 | 36 | 14 | 6 | 56 |
| 2 intermediate | 10 | 17 | 17 | 44 | 84 | 13 | 19 | 116 | |
| 3 compact | 8 | 3 | 4 | 15 | 23 | 1 | 11 | 35 | |
| D36—dehiscence degree | 1 light | 14 | 14 | 13 | 41 | 78 | 14 | 24 | 116 |
| 2 regular | 6 | 6 | 8 | 20 | 65 | 14 | 12 | 91 | |
| D37—pericarp colour | 1 cream | 17 | 20 | 17 | 54 | 142 | 28 | 36 | 206 |
| 2 yellow | - | - | - | - | 1 | - | - | 1 | |
| 4 pink | 1 | - | - | 1 | - | - | - | - | |
| 7 coffee | 1 | - | 2 | 3 | - | - | - | - | |
| 10 purple | 1 | - | 2 | 3 | - | - | - | - | |
| D38—episperm colour | 1 transparent | 5 | 10 | 3 | 18 | 9 | 3 | 7 | 19 |
| 2 white | 15 | 10 | 18 | 43 | 134 | 25 | 29 | 188 | |
| D40—presence of saponin | 0 absent | - | - | - | - | 117 | 24 | 29 | 170 |
| 1 present | 20 | 20 | 21 | 61 | 26 | 4 | 7 | 37 | |
| Code—Descriptor | Collection A vs. Collection B | Collection A | Collection B | Chimborazo | Cotopaxi | Imbabura | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X² (p-Value) | X² (p-Value) | X² (p-Value) | X² (p-Value) | X² (p-Value) | X² (p-Value) | |||||||
| D19—growth habit | 2.74 | (0.2536) | 0.03 | (0.9837) | 9.14 | (0.0578) | 0.72 | (0.6972) | 1.33 | (0.2482) | 2.51 | (0.1132) |
| D20—main stem shape | 16.80 | (<0.0001) | 0.03 | (0.9837) | 23.71 | (<0.0001) | 8.96 | (0.0028) | 0.73 | (0.3931) | 3.95 | (0.0469) |
| D21—main stem colour | 20.48 | (<0.0001) | 3.92 | (0.4164) | 34.09 | (<0.0001) | 29.39 | (<0.0001) | 5.19 | (0.0747) | 10.98 | (0.0041) |
| D22—presence of pigmented axils | 25.98 | (<0.0001) | 16.71 | (0.0022) | 27.22 | (<0.0001) | 14.17 | (0.0008) | 4.75 | (0.0928) | 5.73 | (0.0167) |
| D24—striae colour | 24.49 | (<0.0001) | 15.33 | (0.0041) | 119.51 | (<0.0001) | 2.14 | (0.1438) | 5.21 | (0.0224) | 7.97 | (0.0186) |
| D25—presence of branching | 0.01 | (0.9432) | 9.46 | (0.0088) | 27.22 | (<0.0001) | 3.87 | (0.0492) | 0.20 | (0.6580) | 3.60 | (0.0578) |
| D26—leaf shape | 28.12 | (<0.0001) | 8.79 | (0.0123) | 2.74 | (0.2540) | 19.24 | (<0.0001) | 13.87 | (0.0002) | 0.00 | (>0.9999) |
| D28—number of teeth on leaf blade | 31.55 | (<0.0001) | 8.79 | (0.0123) | 6.96 | (0.0307) | 20.31 | (<0.0001) | 17.15 | (<0.0001) | 0.04 | (0.8321) |
| D29—petiole colour | 2.71 | (0.0995) | 3.33 | (0.1895) | 20.24 | (<0.0001) | 0.27 | (0.6046) | 2.53 | (0.1114) | 4.10 | (0.0428) |
| D30—leaf lamina colour | 2.71 | (0.0995) | 3.33 | (0.1895) | 20.24 | (<0.0001) | 0.27 | (0.6046) | 2.53 | (0.1114) | 4.10 | (0.0428) |
| D32—panicle colour at flowering | 3.20 | (0.2017) | 10.70 | (0.0301) | 63.76 | (<0.0001) | 17.44 | (0.0002) | 4.49 | (0.1057) | 6.01 | (0.0494) |
| D33—panicle colour at physiological maturity | 3.48 | (0.0037) | 15.75 | (0.0151) | 26.31 | (0.0002) | 32.39 | (<0.0001) | 6.28 | (0.0989) | 3.25 | (0.3546) |
| D34—panicle shape | 5.47 | (0.0649) | 19.18 | (0.0007) | 7.64 | (0.1058) | 0.14 | (0.2031) | 2.92 | (0.0874) | 19.67 | (0.0001) |
| D35—panicle density | 15.84 | (0.0004) | 9.09 | (0.0590) | 14.76 | (0.0052) | 7.24 | (0.0267) | 14.61 | (0.0007) | 5.83 | (0.0541) |
| D36—dehiscence degree | 2.42 | (0.1194) | 0.41 | (0.8149) | 2.19 | (0.3338) | 1.70 | (0.1917) | 1.92 | (0.1659) | 0.13 | (0.7163) |
| D37—pericarp colour | 24.64 | (0.0001) | 6.29 | (0.3914) | 0.45 | (0.7986) | 21.96 | (0.0002) | 1.33 | (0.2482) | 7.37 | (0.0250) |
| D38—episperm colour | 16.36 | (0.0001) | 6.57 | (0.0374) | 6.06 | (0.0484) | 7.82 | (0.0052) | 9.12 | (0.0025) | 0.24 | (0.6213) |
| D40—presence of saponin | 37.00 | (<0.0001) | 0.03 | (0.9837) | 0.32 | (0.8541) | - | - | 34.24 | (<0.0001) | 34.44 | (<0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, H.; Tapia, C.; Manjarres-Hernández, E.H.; Borja, E.; Naranjo, E.; Martín, J.P. Phenotypic Diversity of Quinoa Landraces Cultivated in the Ecuadorian Andean Region: In Situ Conservation and Detection of Promising Accessions for Breeding Programs. Agriculture 2024, 14, 336. https://doi.org/10.3390/agriculture14030336
Delgado H, Tapia C, Manjarres-Hernández EH, Borja E, Naranjo E, Martín JP. Phenotypic Diversity of Quinoa Landraces Cultivated in the Ecuadorian Andean Region: In Situ Conservation and Detection of Promising Accessions for Breeding Programs. Agriculture. 2024; 14(3):336. https://doi.org/10.3390/agriculture14030336
Chicago/Turabian StyleDelgado, Hipatia, César Tapia, Elsa Helena Manjarres-Hernández, Edwin Borja, Edwin Naranjo, and Juan Pedro Martín. 2024. "Phenotypic Diversity of Quinoa Landraces Cultivated in the Ecuadorian Andean Region: In Situ Conservation and Detection of Promising Accessions for Breeding Programs" Agriculture 14, no. 3: 336. https://doi.org/10.3390/agriculture14030336
APA StyleDelgado, H., Tapia, C., Manjarres-Hernández, E. H., Borja, E., Naranjo, E., & Martín, J. P. (2024). Phenotypic Diversity of Quinoa Landraces Cultivated in the Ecuadorian Andean Region: In Situ Conservation and Detection of Promising Accessions for Breeding Programs. Agriculture, 14(3), 336. https://doi.org/10.3390/agriculture14030336








