Abstract
Pentatricopeptide repeat (PPR) proteins, with tandem 30–40 amino acids, were characterized as one kind of nucleus coding protein. They have been demonstrated to play important roles in RNA editing, plant growth and development, and plant immunity. Although the PPR gene family has been characterized in some plant species, less is known about this family in peanut, especially their functions in response to Ralstonia solanacearum. In this study, we performed a genome-wide analysis to identify PPR genes and their functions in resistance to R. solanacearum. Here, 389, 481, and 1079 PPR genes were identified from Arachis duranensis, Arachis ipaensis, and Arachis hypogaea, respectively. Allopolyploidization was the main reason for the increased number of the AhPPR members. Gene duplication brought about 367 pairs of homologous genes of PPRs in A. hypogaea. Whole-genome replication, tandem repeats, scattered repeats, and unconnected repeats constituted the replication types. The substitution rates of nonsynonymous (Ka) versus synonymous (Ks) of all homologous pairs were less than 1.0, suggesting that the homologous AhPPRs underwent intense purifying selection pressure and remained conserved in both structure and function. RNA-seq and RT-qPCR analyses showed that AhPPR598 gene was highly expressed in the aerial part of peanut and involved in response to R. solanacearum. The transient expression of AhPPR598 in Nicotiana benthamiana induced the HR-mediated cell death, up-regulated expression of resistant marker genes, and enhanced the resistance to R. solanacearum, suggesting AhPPR598 was a positive regulator of immunity by regulating the JA and SA pathways. These results provide a new understanding of the origin, distribution, and evolution of the AhPPR gene family and potential gene resources for peanut-resistant breeding.
1. Introduction
Pentatricopeptide repeat proteins (PPR proteins), as one of the largest gene families in land plants, are nucleus-encoded proteins with an array of 2–30 tandem repeats of a degenerate 35-amino-acid repeat motif (PPR motif) [1]. PPR motifs have three types, including P motif with the typical 35-amino-acid PPR repeat, L motif with 35–36-amino-acid, and S motif with 31-amino-acid [2]. According to the types of motifs and their distribution, PPR proteins are classified into two subfamilies: the P subfamily is characterized by P motifs adjacent to each other without gaps, and the PLS subfamily consists of PPR motifs interspaced by L motif and S motif. There are four highly conserved domains in the carboxyl terminally of the PLS subfamily. Based on the types of domains, the PLS subfamily is further divided into five subgroups: PLS, E1, E2, E+, and DYW [3]. PPR proteins have been reported to be widely distributed in various plants [4]. There were 441 members in Arabidopsis thaliana, 491 in rice, and 456 in maize [5,6,7]. Although the genome of several peanuts has been sequenced [8,9], detailed information about PPR proteins remains largely unknown.
PPR proteins, mainly localized in mitochondria and/or chloroplasts, play important roles in various post-transcriptional events, including RNA-editing [10], RNA-splicing [11], and RNA-processing [12]. In addition, PPR proteins play important roles in plant growth and development [13]. Mutants of PPR proteins lead to pollen abortion, seed development defects, and growth retardation in plants [13]. An increasing number of studies have demonstrated that PPR proteins also participate in plant response to biotic and abiotic stresses [2]. Eleven PPR proteins are validated to be associated with the response to biotic or abiotic stresses in A. thaliana. PPR40, a mitochondrial PPR protein, provides a signaling connection for mitochondrial electron transport in A. thaliana [14]. The knockout mutants of PPR40 result in increased reactive oxygen species (ROS) accumulation, lipid peroxidation, and superoxide dismutase activity. PGN is a mitochondria-localized PPR protein; the pgn mutant of A. thaliana results in susceptibility to necrotrophic fungal pathogens as well as hypersensitivity to abscisic acid (ABA), glucose, and salinity [15]. The PPR proteins of A. thaliana, MED11/LOI1 [16], ABO5 [17], AHG11 [18], SLG1 [19], SLO2 [20], PPR96 [21], and POCO1 [22] were also demonstrated to be involved in responses to various biotic or abiotic stresses. In rice, the inactivation of the chloroplast-located PPR protein WSL displays enhanced sensitivity to sugar, salt, and ABA [23]. The silencing of the PPR gene PPS1 enhanced sensibility to abiotic stress and resulted in the remarkable accumulation of ROS in rice [24]. OsNBL3 is a rice mitochondrion-localized PPR protein; its mutant exhibited spontaneous cell death response and H2O2 accumulation, and it displayed enhanced resistance to the fungal and bacterial pathogens Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae [25]. However, the study of the function of PPR proteins in peanut is scarce, especially for peanut responses to R. solanacearum.
Peanut is among the most important oil crop, grown in more than 100 countries around the world. It is often consumed in the form of high-quality cooking oil, edible nuts, peanut butter, and confectioneries [26]. However, bacterial wilt (BW), a bacterial soil-borne disease caused by R. solanacearum, is seriously harming the yield and quality of peanut. Generally, BW causes a 50–100% yield loss in peanut production [27]. More than 200 plant species from 54 families can be attacked by R. solanacearum, such as peanut (A. hypogaea), tomato (Solanum lycopersicum), potato (Solanum tuberosum), pepper (Capsicum annuum), and tobacco (N. benthamiana), causing very serious economic losses in the world every year [27]. To avoid infection by pathogens, plants have formed two-layer innate immune system during evolution, the so-called pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [28]. The pattern-recognition receptors (PRRs) in plant cell surface that recognize the pathogen-associated molecular patterns (PAMPs) from pathogen triggers PTI and the intracellular R protein that recognizes type III effectors (T3Es) of pathogen both activate ETI. Both of them induce the downstream defense responses, including hypersensitive response (HR), an outburst of reactive oxygen species (ROS), the activation of mitogen-activated protein kinases (MAPKs), phytohormone accumulation, and the transcriptional activation of defense-related genes [29,30,31]. Although more than 150 resistance (R) genes have been identified in plants thus far, the R genes of BW are still rarely reported [32]. In A. thaliana, RRS1-R/RPS4 and ERECTA act as R genes and are involved in resistance to BW [33]. AhRLK1, AhRRS5, and AhGLK1b have been demonstrated as being BW resistance-related genes in peanut [34,35,36]. Transgenic tobacco, separately overexpressing the three genes, increased the tolerance to R. solanacearum. A genome-wide analysis showed that peanut defensins and WRKY genes were involved in the response to R. solanacearum [37,38]. The transient expression of AhDef2.2 in N. benthamiana and peanut leaves enhanced resistance to R. solanacearum [37].
In this study, we first performed a genome-wide analysis to identify PPR genes in A. duranensis, A. ipaensis, and A. hypogaea, respectively. The characteristics of PPR genes were explored, including intron-exon organization, chromosomal location, types of PPR motifs, and phylogenetic relationships. With a focus on the involvement of PPR genes in peanut responses to R. solanacearum, we also examined their expression patterns through an RNA-seq analysis from the peanut leaves with R. solanacearum infection [39]. The function of AhPPR598 was further investigated owing to its significantly up-regulated expression by R. solanacearum infection in resistant peanut leaves. The transient overexpression of AhPPR598 enhanced the tolerance of N. benthamiana to R. solanacearum. These results will contribute to the understanding of PPR genes’ distribution and functions in peanut, and also improve our understanding of the relationship between PPR genes and peanut BW resistance.
2. Materials and Methods
2.1. Plant Materials
N. benthamiana seeds were germinated in a pot with a soil mix. Two weeks later, one seedling was transferred into one pot and grown in a greenhouse at 28 ± 2 °C under a 16 h/8 h (light/dark) photoperiod. Five- to six-week-old plants were used for transient expression. Peanuts were cultivated according to our previous methods [40]. Two weeks later, different tissues of peanut were collected for RNA extraction.
2.2. Bacterial Materials
Escherichia coli DH5α, Agrobacterium tumefaciens GV3101, and R. solanacearum HA4-1 [41] were used in this study. DH5α cells were cultured on Luria–Bertani (LB) solid plates (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, 15 g/L agar, 1 mL/L kanamycin (50 mg/mL)) at 37 °C. GV3101 cells were cultured on Luria–Bertani (LB) solid plates (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, 15 g/L agar, 1 mL/L kanamycin (50 mg/mL), 1 mL/L rifampicin (50 mg/mL)) at 28 °C. HA4-1 cells were cultured according to our previous study [41].
2.3. Identification and Physicochemical Characterization Analysis
The genomes of the cultivated species (A. hypogaea cv., Shitouqi) (http://peanutgr.fafu.edu.cn/index.php, accessed on December 2017.) and the wild species (A. duranensis and A. ipaensis) (https://peanutbase.org/home, accessed on March 2016.) were downloaded. The PPR motifs ‘PF13041’, ‘PF12854’, ‘PF01535’, ‘PF13812’, ‘PF20431’, ‘PF17177’, ‘PF14432’, and ‘PF20430’ from the Pfam 36.0 (http://pfam.xfam.org/) database were used to perform BlastP searches against protein sequences on the A. hypogaea, A. duranensis, and A. ipaensis genomic databases. After redundant sequences were removed, the no-redundant PPR proteins with more than 2 PPR motifs were taken into further analysis. These PPR proteins were classified as P or PLS (E1, E2, E+, and DYW) by the HMM search program from the HMMER package 3.3.2 (https://docs.csc.fi/apps/hmmer/). The molecular weight (MW) and isoelectric point (IP) of the PPR proteins were predicted in Ensembl genomes and ProtComp 9.0 (http://linux1.softberry.com/).
2.4. Chromosome Localization and Collinearity Analysis
The GFF3 files were downloaded from the genome database to obtain the length information and location information of the PPR genes. The updated TBtools software (TBtools v2.031) [42] was employed to analyze the gene structures and chromosomal localization. The Multiple Collinearity Scan toolkit MCScanX 0.8 (http://chibba.pgml.uga.edu/mcscan2/) was used to analyze the collinearity relationship of AhPPR within A. hypogaea genome and that of homologous proteins between A. duranensis and A. ipaensis genomes. The results were visualized by using TBtools. The ratio of Ka/Ks between AhPPR members was calculated using KaKs_Calculator2.0 software [43].
2.5. Gene Cloning and Vector Construction
The full sequence of AhPPR genes was obtained from our transcript data of peanut cultivars infected by R. solanacearum [39], and SnapGene 4.1.8 was used to design the cloned primers (Table S6). The first-strand cDNA of peanut cultivar, which was infected with R. solanacearum, was chosen as the amplification template to perform PCR. The PCR product was ligated to the PC-2300-35S-eGFP vector and was then transformed into E. coli DH5α. The PCR products of PC2300-35S-AhPPR244/AhPPR598-eGFP were recovered for gel detection and sent to Guangzhou Tianyihuiyuan Biotechnology Co., Ltd. (Guangzhou, China) for sequencing. The accurate PC2300-AhPPR244/AhPPR598-eGFP vectors were transformed into A. tumefaciens GV3101 for transient expression analysis.
2.6. Transient Expression and R. solanacearum Inoculation
A. tumefaciens cells with PC2300-35S-AhPPR244/AhPPR598-eGFP constructs were grown overnight in LB liquid medium with appropriate antibiotics at 28 °C. Bacterial cells were centrifuged and resuspended in infiltration buffer (10 mM MES, 10 mM MgSO4, 200 mM acetosyringone, pH 5.6). The OD600 was adjusted to 0.1. Test and control A. tumefaciens were infiltrated into one leaf of N. benthamiana. Two different leaves of one N. benthamiana plant were employed. N. benthamiana was cultured in greenhouse and subsequently followed for further analysis at different timepoints. R. solanacearum cells were prepared according the previous study [40]. N. benthamiana was injected with R. solanacearum at 48 h after infiltration with A. tumefaciens.
2.7. Electrolyte Leakage Measurement and Trypan Blue and DAB Staining
Six leaves of three N. benthamiana plants were used for electrolyte leakage measurement. Six leaf discs per leaf were excised with a puncher of 6 mm diameter. The procedure was performed as described previously in detail [39].
Six leaves were selected to perform trypan blue staining. The leaves were boiled for 2 min in trypan blue solution and placed overnight in the dark at 28 °C. The stained leaves were transferred into chloral hydrate solution (1.25 g/mL) for decolorating with 25 °C and 50 r/min. The liquid was changed every 3 h until the leaves were completely colorless; then, the photos were taken. These leaves could be preserved in ethanol absolute for a long time.
Six leaves were used for the histochemical analysis of 3, 3′-diaminobenzidine (DAB). The treated leaves were vacuumized for 2 min at 0.8 MPa in the DAB staining solution and placed overnight at 28 °C. These leaves were transferred into 90% ethanol to boil until completely colorless. These leaves could be preserved in ethanol absolute for a long time.
2.8. Total RNA Extraction and Expression Profiles
The leaf samples were collected from transient-transformed N. benthamiana plants or via R. solanacearum inoculation at 0, 24, 48, 72, and 96 h after infiltration. Different tissues of peanut were collected from cultivar Zhongkaihua 1. Total RNA was extracted using HiPure Plant RNA Mini Kit B (Magen Biotechnology, Guangzhou, China) based on the protocol. The RNA of peanut tissues and N. benthamiana leaves was reverse-transcribed to synthesize first-strand cDNA for RT-qPCR, referring to the instructional manual of EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotechnology, Beijing, China). RT-qPCR analysis was performed according to a previous study [39]. The immune-related marker genes included HR marker genes NbH1N1 and NbHsr203J, PTI marker genes NbWrky22 and NbPTI5, JA signal pathway genes NbOPR3 and NbLOX, and SA signal pathway genes NbPR1 and NbPR2. NbEF1a and Ahactin were used as internal reference genes, respectively. The primers for RT-qPCR are listed in Table S6.
From the Peanut Genome Resource (PGR) website (http://peanutgr.fafu.edu.cn/), the expression data of the represent AhPPR genes in different tissues were obtained. Transcriptome data were downloaded from NCBI under BioProject ID: PRJNA861998. The Log2 (FPKM) of each gene and sample was calculated to analyze the expression of AhPPRs. Results were visualized using TBtools.
2.9. Statistical Analysis
Statistical analyses were performed and graphs were created by using the GraphPad Prism 8.0 software.
3. Results
3.1. Identification and Characterization of the PPR Gene Family
With the completion of the peanut genome sequencing (http://peanutgr.fafu.edu.cn/inde), it was convenient to obtain all PPR genes in peanut by searching for the PPR motif sequences with BlastP. A total of 1079 PPR genes were identified in the genome of peanut (Figure 1a), named AhPPR1 to AhPPR1079, based on their physical locations on chromosomes (Table S1). Similarly, 389 and 481 PPR genes were identified in A. duranensis and A. ipaensis, respectively (Figure 1b,c). According to the protein structures, the 1079 peanut PPR genes contained 495 P subgroup members and 584 PLS subgroup members, including 251 E and 280 DYW subgroups (Figure 1a; Table S2). There were 176 and 262 P subgroup members, as well as 213 and 219 PLS subgroup members in A. duranensis and A. ipaensis, respectively (Figure 1b,c; Table S2). There were 58 E and 123 DYW subgroups in A. duranensis, and 104 E and 84 DYW sub-families in A. ipaensis (Figure 1b,c; Table S2). Detailed information for all the aforementioned PPR proteins, including gene ID, chromosome location, molecular weight (MW), and isoelectric point, are listed in Table S1. The number of PPR genes in peanut was higher than the 389 in A. duranensis (176 P-type and 213 PLS-type), 481 in A. ipaensis (262 P-type and 219 PLS-type), 491 in rice (246 P-type and 245 PLS-type) [6], 450 in Arabidopsis (251 P-type and 199 PLS-type) [5], 471 in tomato (233 P-type and 238 PLS-type) [44], 105 in moss (89 P-type and 16 PLS-type) [45], and 422 in watermelon (197 P-type and 219 PLS-type) [46] (Figure 1e).
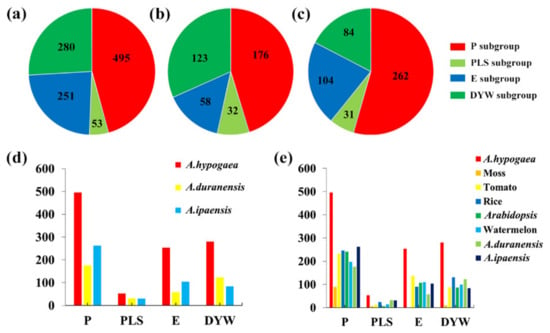
Figure 1.
The number of the PPR gene subgroups in different plant species. (a–c) Distribution of different subgroups in A. hypogaea, A. duranensis, and A. ipaensis, respectively. (d) Number of different subgroups in A. hypogaea, A. duranensis, and A. ipaensis, respectively. (e) Number of different subgroups in the represent species. P: P subgroup; PLS: PLS subgroup; E: E subgroup; DYW: DYW subgroup.
3.2. Chromosome Distribution and Gene Structure Analysis
To reveal the gene structures, we analyzed the exon–intron organization of PPR genes. It showed that the majority of PPR genes consisted of more than one intron in the peanut (Figure 2a). The peanut PPR genes were 36% without introns, 25% with 1 intron, 34% with 2–5 introns, and only 4% with more than 6 introns (Figure 2a). In two diploid species, the PPR gene structures were generally similar. The PPR genes were 25% without intron and 39% with 2–5 introns in A. duranensis and A. ipaensis (Figure 2b,c). The ratios of PPR genes with more than six introns were 13% and 11% in A. duranensis and A. ipaensis, respectively (Figure 2b,c). The PPR genes were 23% with one intron in A. duranensis, while they were 25% in A. ipaensis (Figure 2b,c).
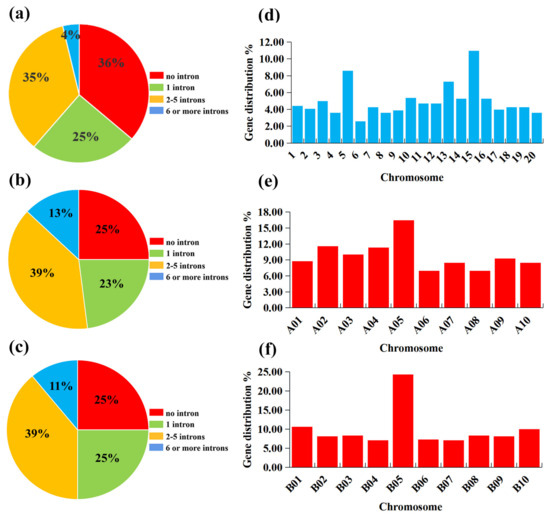
Figure 2.
The intron numbers and distribution on chromosomes of PPR genes in A. hypogaea, A. duranensis, and A. ipaensis. (a–c) Number of introns in A. hypogaea, A. duranensis, and A. ipaensis, respectively. (d–f) PPR genes distribution on the chromosome in A. hypogaea, A. duranensis, and A. ipaensis, respectively.
According to the annotated genomic locations, it was found that these PPR genes were widely distributed among the 20 chromosomes of peanut unevenly. Chromosomes 15 and 6 possessed the most and least PPR genes, respectively, which were 119 (10.98%) and 28 (2.58%) (Figure 2d). In two diploid species, the distribution of PPR genes on the chromosomes was also uneven. There were the most PPR genes on chromosome 5 in A. duranensis, including 64 genes (16.45%) (Figure 2e). Chromosomes 6 and 8 possessed the least number of PPR genes, both containing 27 (6.94%) PPR genes (Figure 2e). In A. ipaensis, chromosome 5 contained the highest number of PPR genes, including 117 (24.32%) genes (Figure 2f). Chromosomes 4 and 7 possessed the lowest number of PPR genes, both containing 34 (7.07%) PPR genes (Figure 2f).
3.3. Gene Duplication on the Expansion of PPR Genes
Gene duplication plays an important regulatory role in plant evolution, expansion of gene families, and crop domestication. To reveal the evolution of the PPR gene family after the formation of the allotetraploid peanut, the replication events of PPR genes were investigated. A collinearity analysis showed a total of 367 pairs of homologous genes of PPR genes in peanut (Figure 3a; Table S3). Further analysis revealed that 1079 PPR genes were derived from gene replication. Among them, 515 were derived from whole-genome replication, 379 from tandem repeats, 181 from scattered repeats, and 4 from unconnected repeats (Table S4). This suggested that the expansion of the AhPPRs was formed mainly through genome-wide replication. Collinearity analysis between the allotetraploid peanut and the two diploid species showed that they had a large number of homologous PPR genes. In peanut, 389 PPR genes were collinear with A. duranensis, and 445 PPR genes were collinear with A. ipaensis (Figure 3b). This indicated that peanut evolved from the two diploid species. To explore the selection pressure of AhPPRs, the ratio of Ka/Ks was subsequently calculated. The results showed that the Ka/Ks ratios of all homologous pairs were almost always less than 1.0 (Table S5). This suggested that the homologous AhPPRs underwent intense purifying selection pressure and remained conserved in both structure and function in peanut.
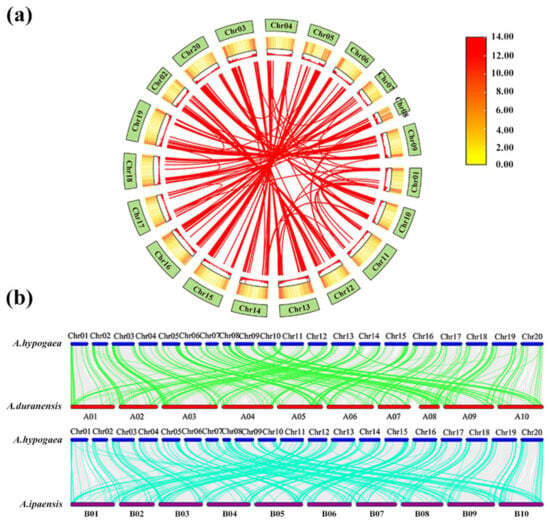
Figure 3.
Collinearity analysis of PPR genes in A. hypogaea, A. duranensis, and A. ipaensis. (a) Chromosome localization and duplication events of AhPPR genes. The red lines connect collinear PPR gene pairs. (b) Collinearity analysis of PPR genes between A. hypogaea and two other species. Homologous PPR gene pairs between species are linked by green and blue lines.
3.4. Expression Patterns of AhPPRs in Peanut Response to R. solanacearum
To explore the possible biological functions of AhPPRs in peanut response to R. solanacearum, their expression profiles were analyzed by using our previous RNA-seq data [39]. It was obvious that these AhPPR genes had different expression patterns in response to R. solanacearum. To identify the BW resistant-related AhPPRs, we paid more attention to the AhPPRs that were highly expressed in resistant peanut and slightly expressed in susceptible peanut (Figure 4a). Ten AhPPR genes, AhPPR244, AhPPR332, AhPPR387, AhPPR551, AhPPR580, AhPPR598, AhPPR687, AhPPR803, AhPPR925, and AhPPR1017, were identified based on the criteria. AhPPR244 and AhPPR598 had significantly up-regulated expression at 36 and 72 hpi, AhPPR687 at 24 and 36 hpi, and AhPPR1017 at 24 hpi, in resistant peanut (Figure 4a). The remaining six AhPPRs had significantly up-regulated expression at 36 hpi in resistant peanut (Figure 4a). Furthermore, we investigated the expression levels of the ten AhPPR genes in different tissues of peanut by using publicly available transcriptome datasets [8]. It was found that AhPPRs showed diverse expression patterns among tissues. AhPPR332 and AhPPR925 were highly expressed in the root and stem (Figure 4b).
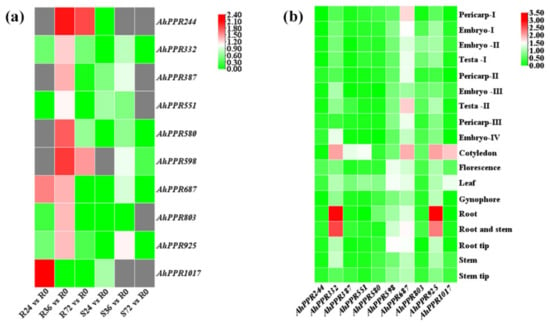
Figure 4.
Heat map of expression profiles of AhPPR genes. (a) Expression profiles represent AhPPR genes in the leaves of resistant and susceptible peanuts at 0, 24, 36, 48, and 72 hpi with R. solanacearum. R0, R24, R36, and R72 represent the leaves of resistant peanut after inoculation at 0, 24, 36, 48, and 72 h, respectively. S0, S24, S36, and S72 represent the leaves of susceptible peanut after inoculation at 0, 24, 36, 48, and 72 h, respectively. (b) Expression profiles represent AhPPR genes from 18 peanut tissues. The expression abundance of each gene is indicated by the color bar: red means higher expression; green represents lower expression.
3.5. Transient Overexpression of AhPPR598 Caused Tobacco Leaf Necrosis and Defense Response
To further analyze the function of these 10 AhPPR genes, we tried to clone them from the leaves of resistant peanut infected with R. solanacearum. Regrettably, only five AhPPR genes, AhPPR244, AhPPR387, AhPPR551, AhPPR589, and AhPPR803, were successfully cloned. To verity their functions in plant cells, we transiently overexpressed the recombinant plasmid pPC2300-35S-AhPPRs (35S::AhPPRs) and the empty vector pPC2300-35S (35S::00) into N. benthamiana leaves through Agrobacterium transformation. Compared with control leaves agroinfiltrated with an empty binary vector, the leaf necrosis was evident 48 h post agroinfiltration (hpa) in the AhPPR598 overexpression leaves, followed by AhPPR244 (Figure 5a,b). The other three genes did not cause leaf necrosis. The high expression of AhPPR598 could be observed at 48 and 72 hpa in the 35S::AhPPR598 leaves of N. benthamiana, indicating the successful expression of AhPPR598 in tobacco leaves (Figure 5c). The leaf necrosis caused by AhPPR598 was also demonstrated through trypan blue staining and electrolyte leakage detection (Figure 5b,d). These results indicated that AhPPR598 could induce cell death in N. benthamiana leaves. Diaminobenzidine (DAB) staining showed that the degree of browning of tobacco leaves with the AhPPR598 gene differed significantly from the empty control, which indicated that AhPPR598 induced ROS accumulation and may contribute to the defense response. Considering that AhPPR598 is a potential gene contributing to disease resistance in peanut, we analyzed the expression pattern of AhPPR598 by qPCR in different peanut tissues. The result showed that the expression level of AhPPR598 was high in the aerial part including the flower, leaf, and stem, which displayed some differences with the aforementioned publicly available transcriptome data (Figure 5b).
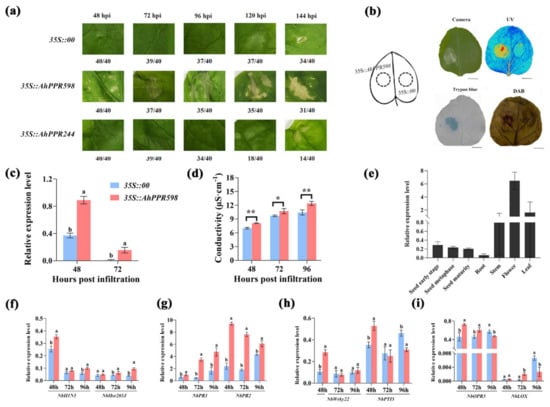
Figure 5.
Function and expression analyses of AhPPR598. (a) Phenotype analysis of the tobacco leaves agroinfiltrated by Agrobacterium with control (35S::00) and experimental group (35S::AhPPR598 and 35S::AhPPR244) at 48, 72, 96, 120, and 144 h. The numbers on the bottom indicate the agroinfiltrated leaf number. (b) Phenotype, trypan blue, and DAB staining analyses of the tobacco leaves agroinfiltrated by Agrobacterium with control (35S::00) and experimental group (35S::AhPPR598) at 72 hpa. Cell death was monitored using visible light (Camera), UV light (UV), and trypan blue, the darker color indicates the more cell death. ROS was detected using DAB staining, the darker color represents the more ROS accumulation. Bar = 5 cm. (c) The expression levels of AhPPR598 in the tobacco leaves agroinfiltrated by Agrobacterium with 35S::00 and 35S::AhPPR598 at 48 and 72 hpa. (d) The electrolyte leakage in the tobacco leaves agroinfiltrated by Agrobacterium with 35S::00 and 35S::AhPPR598. The asterisks indicate significant differences between 35S::00 and 35S::AhPPR598 according to Student’s t-test (mean ± SE, * p < 0.05, ** p < 0.001). (e) Expression analysis of AhPPR598 in different peanut tissues. Error bars represent standard errors, n = 3. (f–i) Expression analysis of immune marker genes in the tobacco leaves agroinfiltrated by 35S::00 and 35S::AhPPR598 at 48, 72, and 96 hpa. HR marker genes: NbH1N1 and NbHsr203J; SA signal pathway genes: NbPR1 and NbPR2; PTI marker genes: NbWrky22 and NbPTI5. JA signal pathway genes: NbOPR3 and NbLOX. Different lowercases mean significant differences according to ANOVA (mean ± SE, p < 0.05) in (c,f–i).
To confirm the contribution of AhPPR598 to disease resistance, we transiently overexpressed 35S::AhPPR598 and 35S::00 into N. benthamiana leaves. Then, we detected the expression of immune-related marker genes in the N. benthamiana leaves overexpressing AhPPR598. Compared with the 35S::00, the transient overexpression of AhPPR598 induced the expression of HR-, PTI-, SA-, and JA-related marker genes. The HR marker genes NbH1N1 (48 and 96 hpi) and NbHsr203J (72 and 96 hpi) were induced to yield up-regulated expression in the 35S::AhPPR598 tobacco leaves (Figure 5f). This result indicated that the leaf necrosis was an HR response. The PTI-related genes NbWrky22 and NbPTI5 were induced to yield up-regulated expression at 48 hpi (Figure 5h). The SA signaling-pathway-related genes NbPR1 and NbPR2 had significantly up-regulated expression at all timepoints (Figure 5g). The JA signaling-pathway-related gene NbOPR3 was induced to yield up-regulated expression at 48 and 72 hpi, while NbLOX at 72 hpi (Figure 5i). Overall, the expression analysis suggested that AhPPR598 positively regulates immunity through SA and JA pathways.
3.6. Transient Overexpression of AhPPR598 Positively Regulated the Resistance to R. solanacearum
Further, to analyze the BW resistance function of AhPPR598, we inoculated R. solanacearum in the N. benthamiana leaves overexpressing 35S::AhPPR598 and 35S::00, subsequently evaluated leaf necrosis, and detected the expression of immune-related marker genes. Compared with the empty control, the cell death was lower in the leaves overexpressing AhPPR598, which was also demonstrated via electrolyte leakage detection (Figure 6a,c). This suggested that AhPPR598 partly counteracted the virulence of R. solanacearum and rescued some leaf cells. The HR marker gene NbH1N1 and NbHsr203J were induced to yield up-regulated expression at 24 and 48 hpi in the 35S::AhPPR598 tobacco leaves with R. solanacearum (Figure 6d). The PTI-related gene NbWrky22 was induced to yield up-regulated expression at 48 hpi, and NbPTI5 at 24 hpi (Figure 6e). The SA signaling pathway-related gene NbPR2 had significantly up-regulated expression at 24 hpi (Figure 6f). The JA signaling pathway-related gene NbOPR3 was induced to yield up-regulated expression at 24 and 48 hpi (Figure 6g). Therefore, our results demonstrated that AhPPR598 increases the tobacco resistance to R. solanacearum.
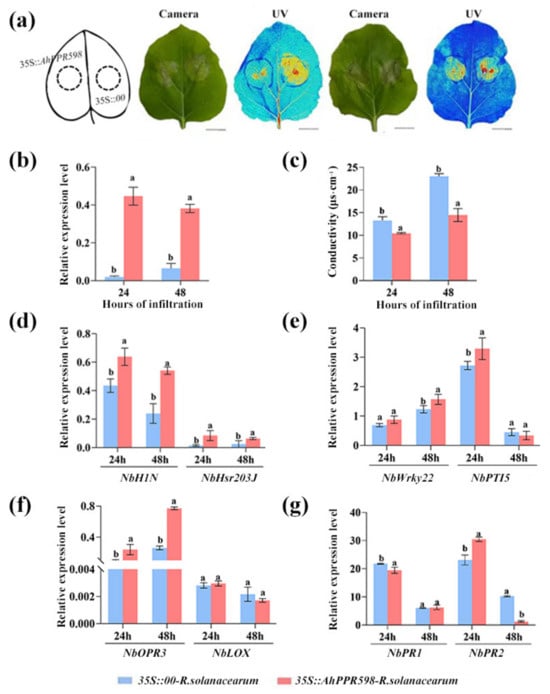
Figure 6.
Analysis of the resistance function of AhPPR598 to R. solanacearum in N. benthamiana leaves. (a) Phenotype analysis of the tobacco leaves after inoculation with R. solanacearum for 24 and 48 h (from left to right). Cell death was monitored via visible light (Camera) and UV light (UV), the darker color indicates the more cell death. Bar = 5 cm. (b) The expression levels of AhPPR598 in the tobacco leaves agroinfiltrated by Agrobacterium with 35S::00 and 35S::AhPPR598 at 24 and 48 hpi. (c) The electrolyte leakage in the tobacco leaves agroinfiltrated by Agrobacterium with 35S::00 and 35S::AhPPR598 and inoculated with R. solanacearum at 24 and 48 hpi. (d–g) Expression analysis of immune marker genes in the tobacco leaves agroinfiltrated by 35S::00 and 35S::AhPPR598 and inoculated with R. solanacearum at 48, 72, and 96 hpi. Different lowercases mean significant differences according to ANOVA (mean ± SE, p < 0.05) in (b–g).
4. Discussion
As a large gene family, the PPR protein family has been identified in many species that have been sequenced [5,6,7,44,45,46]. The PPR genes have been reported to function in various biological processes in plants and play important roles in plant response to microbial pathogens [2,13]. No studies have been performed to demonstrate the potential link between PPR genes and resistance to bacterial wilt caused by R. solanacearum in peanut. In the present study, genome-wide analyses of PPR genes were performed in A. hypogaea, A. duranensis, and A. ipaensis. The number of PPR genes was higher in peanut than the sum of that in A. duranensis and A. ipaensis (Figure 1). There was a large number of homologous PPR genes between the cultivated species and the wild species (Figure 3b). The reasons for these might be that the allotetraploid peanut (AABB, 2n = 40) evolved from the two diploid species and was formed through natural allopolyploidization between A. duranensis (AA, 2n = 20) and A. ipaensis (BB, 2n = 20). Gene duplication, mainly including segmental duplication or whole-genome duplication and tandem duplication, played crucial roles in the expansion of a gene family [47]. There were 367 pairs of homologous genes of PPRs in peanut (Figure 3a). Among the AhPPRs, the replication types consisted of whole-genome replication, tandem repeats, scattered repeats, and unconnected repeats (Table S4). The Ka/Ks ratios of all homologous pairs were less than 1.0 in peanut, suggesting that the homologous AhPPRs underwent intensive purifying selection pressure and remained conserved in both structure and function (Table S5).
The spatial expression is closely related to the function of PPR genes. In Arabidopsis, eleven AtPPR genes had been shown to respond to biotic or abiotic stresses. The expression of AtPPR96 was induced in responses to salt, abscisic acid (ABA), and oxidative stress, which could alter transcription levels of several stress-responsive genes under abiotic stress treatments [21]. The rice OsV4 gene, encoding a novel PPR protein, was mostly expressed in young leaves [48]. The transcript levels of some ribosomal components and plastid-encoded polymerase-dependent genes were dramatically decreased in the OsV4 mutants grown at 20 °C. In this study, we investigated the expression patterns of AhPPR genes. AhPPR332 and AhPPR925 were highly expressed in the root and stem (Figure 4b), and AhPPR244 and AhPPR598 were highly expressed in the resistant peanut A165 under the stress of R. solanacearum (Figure 4a). Real-time quantitative PCR confirmed that AhPPR598 was highly expressed in the aerial part including the stem (Figure 5e). In addition, the transient expression of AhPPR598 induced HR-associated cell death, ROS accumulation, and PTI-related marker genes up-regulation in N. benthamiana leaves (Figure 5). These results implied that AhPPR598 was involved in the defense response to pathogens.
BW, caused by R. solanacearum, is a bacterial soil-borne disease that seriously threatens the peanut industry. However, little is known about the mechanisms of peanut resistance to BW, and no R genes conferring resistance to BW have been identified. Here, we found that AhPPR genes could increase the resistance to R. solanacearum in N. benthamiana. PPR proteins have been demonstrated to be a regulator of resistance to pathogen infection in plants [2]. AtRTP7, a P-type PPR protein, mediates plant immunity to a broad spectrum of pathogens by modulating the mitochondrial oxidative burst in Arabidopsis [49]. OsNBL3, a rice mitochondrion-localized PPR protein, regulated enhanced resistance to the fungal and bacterial pathogens [24]. In our study, the transient overexpression of AhPPR598 in tobacco caused HR and H2O2 accumulation (Figure 5b), which was consistent with the LRR-RLK (leucine-rich repeat receptor-like protein kinases) genes AhRRS5 and AhRLK1 [33,34]. Moreover, AhPPR598 could significantly increase the immune-related marker genes and decrease the cell death caused by R. solanacearum in N. benthamiana (Figure 5 and Figure 6). Our results were consistent with those obtained in the study of AhRRS5 and AhRLK1 function, where HR-, JA-, and SA-signaling pathway-related genes were all induced and significantly up-regulated in tobacco leaves overexpressing AhRRS5 or AhRLK1 genes [34,35]. The expression of defensin AhDef2.2 gene was induced by SA, MeJA, ABA, and R. solanacearum infection in resistant peanut [37]. Overexpression of AhDef2.2 in N. benthamiana and peanut leaves increased resistance to R. solanacearum, suggesting that it acts as a positive regulator in response to BW infection [37]. SA and JA, as the well-known hormones associated with plant–pathogen interaction, play positive or negative regulator roles in plant immunity [50]. SA is usually associated with R gene-mediated disease resistance, which is accompanied by the expression of several PR genes [51,52,53]. Although SA and JA pathways are usually antagonistic, their synergistic interactions have also been elucidated in defense response to pathogens [54,55,56]. These lines of evidence demonstrated that the AhPPR598 gene is a positive regulatory gene to regulate the resistance to R. solanacearum.
5. Conclusions
In this study, 389, 481, and 1079 PPR genes were identified in A. duranensis, A. ipaensis, and A. hypogaea, respectively. The origin, distribution, and evolution of the AhPPR gene family were systematically analyzed. The transient expression of AhPPR598 in N. benthamiana induced the up-regulated expression of resistant marker genes and enhanced the resistant to R. solanacearum, suggesting that AhPPR598 is a positive regulator of immunity by means of mediating the JA and SA pathways. Comprehensively summarizing all the results in the present study, a proposed model was formulated for AhPPR598-mediated regulation in the N. benthamiana response to R. solanacearum infection, which depicted that the transient overexpression of the AhPPR598 gene in N. benthamiana could induce the HR-associated cell death, active PTI, and increased resistance to R. solanacearum. However, more evidence is needed to validate the model. In the following study, we will construct the overexpressed AhPPR598 lines in Arabidopsis and peanut to verify its function in resistance to R. solanacearum, and to detect the content of JA and SA in the transgenic lines. In conclusion, our findings describe a positive regulated mechanism of AhPPR598 in resistance to R. solanacearum and provide certain candidate genes with potential applications in molecular breeding to improve peanut disease resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14020195/s1, Table S1: PPR genes in Arachis hypogaea, Arachis duranensis, and Arachis ipaensis. Table S2: PPR subgroup in Arachis hypogaea, Arachis duranensis, and Arachis ipaensis. Table S3: The homologous genes of PPRs in Arachis hypogaea. Table S4: PPR gene replication in Arachis hypogaea. Table S5: Homologous gene pairs and Ka/Ks ratios among AhPPR genes. Table S6: All primes used in the study.
Author Contributions
Y.Y. and X.T. designed the research. Y.Y., D.Y. and Y.W. performed the experiments. D.Y., T.C. and X.D. performed the data analysis and interpretation. J.Y. and H.T. prepared the figures and tables. Y.Y., D.Y. and X.T. wrote the manuscript. Y.Z. and X.W. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (grant no. 32201887, 32071737, and 32301927); the Basic and Applied Basic Research Fund of Guangdong Province (grant no. 2022A1515110018); the Science and Technology Planning Project of Guangzhou (grant no. 2023A04J1434); the Guangdong University Scientific Research Platform and Research Project (grant no. 2023KTSCX052); the Key Laboratory of Green Control of Fruits and Vegetables in South China, Ministry of Agriculture and Rural Affairs/Guangdong University Key Laboratory for Sustainable Control of Fruit and Vegetable Diseases and Pests (grant no. KA21031C5); the Foundation of Guangdong Provincial Department of Education (grant no. 2022ZDJS020); and the Science and Technology Department of Guangdong Province (grant no. KTP20210371). No conflicts of interest exist in the submission of this manuscript, and all authors approve the manuscript for publication.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data analyzed during this study are included in the Supplementary Information Files.
Acknowledgments
We extend our appreciation to the anonymous reviewers for their valuable suggestions to help improve this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Small, I.D.; Peeters, N. The PPR motif—A TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000, 25, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhao, P.; Sun, J.; Zhao, Y.; Zhang, Y.; Yang, Q.; Wang, W.; Chen, Z.; Mai, T.; Zou, Y.; et al. Research Progress of PPR Proteins in RNA Editing, Stress Response, Plant Growth and Development. Front. Genet. 2021, 12, 765580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Gutmann, B.; Zhong, X.; Ye, Y.; Fisher, M.F.; Bai, F.; Castleden, I.; Song, Y.; Song, B.; Huang, J. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016, 85, 532–547. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, N.; Hattori, M.; Andres, C.; Iida, K.; Lurin, C.; Schmitz-Linneweber, C.; Sugita, M.; Small, I. On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 2008, 25, 1120–1128. [Google Scholar] [CrossRef]
- Lurin, C.; Andrés, C.; Aubourg, S.; Bellaoui, M.; Bitton, F.; Bruyère, C.; Caboche, M.; Debast, C.; Gualberto, J.; Hoffmann, B.; et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 2004, 16, 2089–2103. [Google Scholar] [CrossRef]
- Chen, G.; Zou, Y.; Hu, J.; Ding, Y. Genome-wide analysis of the rice PPR gene family and their expression profiles under different stress treatments. BMC Genom. 2018, 19, 720. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.X.; Li, C.; Shi, Y.; Song, Y.; Zhang, D.; Li, Y.; Wang, T. Genome-wide analysis of the pentatricopeptide repeat gene family in different maize genomes and its important role in kernel development. BMC Plant Biol. 2018, 18, 366. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, H.; Yang, M.; Wang, J.; Pandey, M.K.; Zhang, C.; Chang, W.C.; Zhang, L.; Zhang, X.; Tang, R.; et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.M.; Ren, L.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef]
- Hayes, M.L.; Dang, K.N.; Diaz, M.F.; Mulligan, R.M. A conserved glutamate residue in the C-terminal deaminase domain of pentatricopeptide repeat proteins is required for RNA editing activity. J. Biol. Chem. 2015, 290, 10136–10142. [Google Scholar] [CrossRef]
- Ichinose, M.; Tasaki, E.; Sugita, C.; Sugita, M. A PPR-DYW protein is required for splicing of a group II intron of cox1 pre-mRNA in Physcomitrella patens. Plant J. 2012, 70, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, Y.; Wu, M.; Zhu, X.; Teng, X.; Sun, Y.; Zhu, J.; Zhang, Y.; Jing, R.; Lei, J.; et al. The nuclear-localized PPR protein OsNPPR1 is important for mitochondrial function and endosperm development in rice. J. Exp. Bot. 2019, 70, 4705–4720. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Liu, S.; Teng, Q.; Li, S.; Jiang, Y. Functions of PPR Proteins in Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef] [PubMed]
- Zsigmond, L.; Rigó, G.; Szarka, A.; Székely, G.; Otvös, K.; Darula, Z.; Medzihradszky, K.F.; Koncz, C.; Koncz, Z.; Szabados, L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008, 146, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Laluk, K.; Abuqamar, S.; Mengiste, T. The Arabidopsis mitochondria-localized pentatricopeptide repeat protein PGN functions in defense against necrotrophic fungi and abiotic stress tolerance. Plant Physiol. 2011, 156, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Suzuki, M.; Tang, J.; Nagata, N.; Ohyama, K.; Seki, H.; Kiuchi, R.; Kaneko, Y.; Nakazawa, M.; Matsui, M.; et al. Lovastatin insensitive 1, a Novel pentatricopeptide repeat protein, is a potential regulatory factor of isoprenoid biosynthesis in Arabidopsis. Plant Cell Physiol. 2007, 48, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Chen, Z.; Ren, X.; Hong, X.; Gong, Z. ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J. 2010, 63, 749–765. [Google Scholar] [CrossRef]
- Murayama, M.; Hayashi, S.; Nishimura, N.; Ishide, M.; Kobayashi, K.; Yagi, Y.; Asami, T.; Nakamura, T.; Shinozaki, K.; Hirayama, T. Isolation of Arabidopsis ahg11, a weak ABA hypersensitive mutant defective in nad4 RNA editing. J. Exp. Bot. 2012, 63, 5301–5310. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, D. Functional disruption of the pentatricopeptide protein SLG1 affects mitochondrial RNA editing, plant development, and responses to abiotic stresses in Arabidopsis. Plant J. 2012, 70, 432–444. [Google Scholar] [CrossRef]
- Zhu, Q.; Dugardeyn, J.; Zhang, C.; Mühlenbock, P.; Eastmond, P.J.; Valcke, R.; De Coninck, B.; Oden, S.; Karampelias, M.; Cammue, B.P.; et al. The Arabidopsis thaliana RNA editing factor SLO2, which affects the mitochondrial electron transport chain, participates in multiple stress and hormone responses. Mol. Plant 2014, 7, 290–310. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhao, J.Y.; Lu, P.P.; Chen, M.; Guo, C.H.; Xu, Z.S.; Ma, Y.Z. The E-Subgroup Pentatricopeptide Repeat Protein Family in Arabidopsis thaliana and Confirmation of the Responsiveness PPR96 to Abiotic Stresses. Front. Plant Sci. 2016, 7, 1825. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Kumar, A.; Kempken, F. Transcriptomic analysis of poco1, a mitochondrial pentatricopeptide repeat protein mutant in Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tan, Z.; Wu, F.; Sheng, P.; Heng, Y.; Wang, X.; Ren, Y.; Wang, J.; Guo, X.; Zhang, X.; et al. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Mol. Plant 2014, 7, 1329–1349. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, Z.; Zou, X.; Xu, Y.; Peng, L.; Hu, J.; Lin, H. Silencing of rice PPR gene PPS1 exhibited enhanced sensibility to abiotic stress and remarkable accumulation of ROS. J. Plant Physiol. 2021, 258–259, 153361. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, X.; Feng, H.; Qi, L.; Yang, J.; Peng, Y.L.; Zhao, W. OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol. J. 2021, 19, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Monyo, E.; Ozias-Akins, P.; Liang, X.; Guimarães, P.; Nigam, S.N.; Upadhyaya, H.D.; Janila, P.; Zhang, X.; Guo, B.; et al. Advances in Arachis genomics for peanut improvement. Biotechnol. Adv. 2012, 30, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Thomma, B.P.; Nürnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Feys, B.J.; Parker, J.E. Interplay of signaling pathways in plant disease resistance. Trends Genet. TIG 2000, 16, 449–455. [Google Scholar] [CrossRef]
- Osuna-Cruz, C.M.; Paytuvi-Gallart, A.; Di Donato, A.; Sundesha, V.; Andolfo, G.; Aiese Cigliano, R.; Sanseverino, W.; Ercolano, M.R. PRGdb 3.0: A comprehensive platform for prediction and analysis of plant disease resistance genes. Nucleic Acids Res. 2018, 46, D1197–D1201. [Google Scholar] [CrossRef] [PubMed]
- Saucet, S.B.; Ma, Y.; Sarris, P.F.; Furzer, O.J.; Sohn, K.H.; Jones, J.D. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat. Commun. 2015, 6, 6338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Zhuang, R.R.; Chen, Y.T.; Deng, Y.; Cai, T.C.; Wang, S.Y.; Liu, Q.Z.; Tang, R.H.; Shan, S.H.; et al. Overexpression of the peanut CLAVATA1-like leucine-rich repeat receptor-like kinase AhRLK1, confers increased resistance to bacterial wilt in tobacco. J. Exp. Bot. 2019, 70, 5407–5421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Cai, T.; Deng, Y.; Zhuang, R.; Zhang, N.; Zeng, Y.; Zheng, Y.; Tang, R.; Pan, R.; et al. Overexpression of a novel peanut NBS-LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Biotechnol. J. 2017, 15, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Chen, H.; Zhang, C.; Khan, S.A.; Gandeka, M.; Xie, D.; Zhuang, W. Ectopic Expression of AhGLK1b (GOLDEN2-like Transcription Factor) in Arabidopsis Confers Dual Resistance to Fungal and Bacterial Pathogens. Genes 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Ren, R.; Ma, X.; Zhao, K.; Qu, C.; Cao, D.; Ma, Q.; Ma, Y.; Gong, F.; Li, Z.; et al. Genome-wide investigation of defensin genes in peanut (Arachis hypogaea L.) reveals AhDef2.2 conferring resistance to bacterial wilt. Crop J. 2021, 8, 809–819. [Google Scholar] [CrossRef]
- Yan, L.; Jin, H.; Raza, A.; Huang, Y.; Gu, D.; Zou, X. WRKY genes provide novel insights into their role against Ralstonia solanacearum infection in cultivated peanut (Arachis hypogaea L.). Front. Plant Sci. 2022, 20, 986673. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Dai, X.; Yang, D.; Wu, Y.; Chen, H.; Zheng, Y.; Zhi, Q.; Wan, X.; Tan, X. Comparative transcriptome analysis revealed molecular mechanisms of peanut leaves responding to Ralstonia solanacearum and its type III secretion system mutant. Front. Microbiol. 2022, 13, 998817. [Google Scholar] [CrossRef]
- Tan, X.; Dai, X.; Chen, T.; Wu, Y.; Yang, D.; Zheng, Y.; Chen, H.; Wan, X.; Yang, Y. Complete Genome Sequence Analysis of Ralstonia solanacearum Strain PeaFJ1 Provides Insights Into Its Strong Virulence in Peanut Plants. Front. Microbiol. 2022, 13, 830900. [Google Scholar] [CrossRef]
- Tan, X.; Qiu, H.; Li, F.; Cheng, D.; Zheng, X.; Wang, B.; Huang, M.; Li, W.; Li, Y.; Sang, K.; et al. Complete Genome Sequence of Sequevar 14M Ralstonia solanacearum Strain HA4-1 Reveals Novel Type III Effectors Acquired Through Horizontal Gene Transfer. Front. Microbiol. 2019, 10, 1893. [Google Scholar] [CrossRef]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists Integrating Various HTS-Data Handling Tools with a User-Friendly Interface; Cold Spring Harbor Laboratory: Laurel Hollow, NY, USA, 2018. [Google Scholar]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0, A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Ding, A.; Li, L.; Qu, X.; Sun, T.; Chen, Y.; Zong, P.; Li, Z.; Gong, D.; Sun, Y. Genome-wide identification and bioinformatic analysis of PPR gene family in tomato. Hereditas 2014, 36, 77–84. [Google Scholar] [PubMed]
- Sugita, M. An Overview of Pentatricopeptide Repeat (PPR) Proteins in the Moss Physcomitrium patens and Their Role in Organellar Gene Expression. Plants 2022, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Subburaj, S.; Tu, L.; Lee, K.; Park, G.S.; Lee, H.; Chun, J.P.; Lim, Y.P.; Park, M.W.; McGregor, C.; Lee, G.J. A Genome-Wide Analysis of the Pentatricopeptide Repeat (PPR) Gene Family and PPR-Derived Markers for Flesh Color in Watermelon (Citrullus lanatus). Genes 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Moore, B.M.; Panchy, N.L.; Meng, F.; Lehti-Shiu, M.D.; Shiu, S.H. Factors Influencing Gene Family Size Variation Among Related Species in a Plant Family, Solanaceae. Genome Biol. Evol. 2018, 10, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Fu, X.; Yang, C.; Tang, X.; Guo, L.; Li, C.; Xu, C.; Luo, K. Genome-wide investigation of pentatricopeptide repeat gene family in poplar and their expression analysis in response to biotic and abiotic stresses. Sci. Rep. 2018, 8, 2817. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zhang, Y.; Niu, L.; Li, W.; Lu, W.; Li, J.; Schafer, P.; Meng, Y.; Shan, W. A mitochondrial RNA processing protein mediates plant immunity to a broad spectrum of pathogens by modulating the mitochondrial oxidative burst. Plant Cell 2022, 34, 2343–2363. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all, Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Yang, D.L.; Yang, Y.; He, Z. Roles of plant hormones and their interplay in rice immunity. Mol Plant. 2013, 6, 675–685. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1998, 1, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Moritz, L.; Pieterse, C.M.; Van Wees, S.C. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front Plant Sci. 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Kyndt, T.; Nzogela, Y.B.; Gheysen, G. Abscisic acid interacts antagonistically with classical defense pathways in rice-migratory nematode interaction. New Phytol. 2012, 196, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).