Evaluation of Herbal Anticoccidials on Growth Performance in Experimentally Infected Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding
- E. ecervulina HP 500–650 oocyst;
- E. maxima CP 200–260 oocyst;
- E. maxima MFP 100–130 oocyst;
- E. mitis HP 1000–1300 oocyst;
- E. tenella HP 500–650 oocyst.
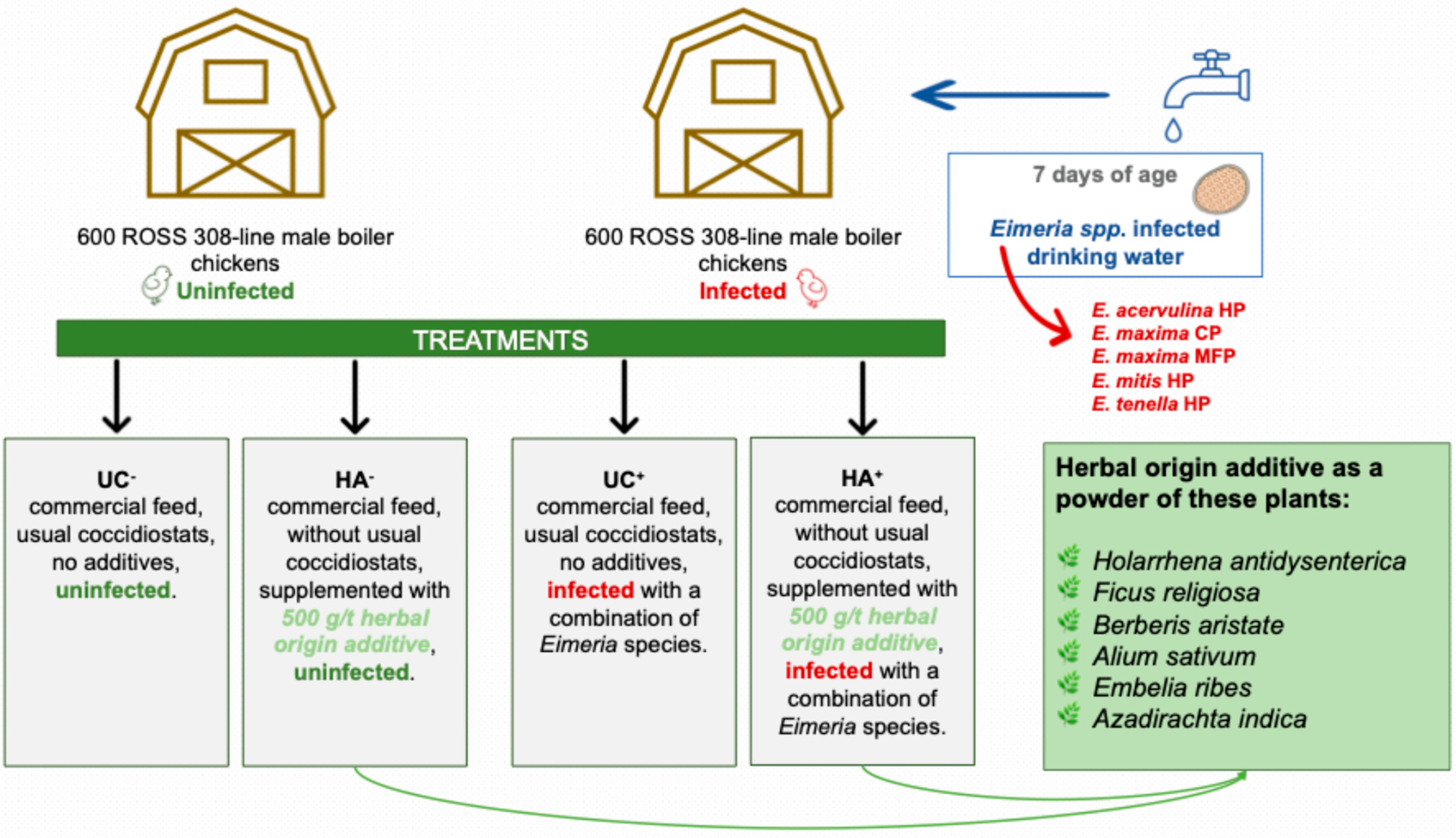
2.2. Growth and Slaughter Performance
2.3. Litter Oocyst Analysis
2.4. Lesion Scores
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Slaughter Performance
3.3. Litter Dry Matter and Oocyst Count
3.4. Lesion Scores
4. Discussion
4.1. Growth and Slaughter Performance
4.2. Litter Dry Matter and Oocyst Count
4.3. Lessions Scores
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bera, A.K.; Bhattacharya, D.; Pan, D.; Dhara, A.; Kumar, S.; Das, S.K. Evaluation of Economic Losses due to Coccidiosis in Poultry Industry in India. Agric. Econ. Res. Rev. 2010, 23, 91–96. [Google Scholar]
- Györke, A.; Kalmár, Z.; Pop, L.M.; Şuteu, O.L. The economic impact of infection with Eimeria spp. in broiler farms from Romania. Rev. Bras. Zootec. 2016, 45, 273–280. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Gjevre, A.G.; Skjerve, E.; Kaldhusdal, M. survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. 2008, 37, 333–341. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Adeleke, M.A. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. 2018, 42, 483–493. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shehata, A.M.; Arif, M.; Paswan, V.K.; Batiha, G.E.; Khafaga, A.F.; Elbestawy, A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: A review. Environ. Sci. Pollut. Res. 2021, 28, 4989–5004. [Google Scholar] [CrossRef]
- Swelum, A.A.; Elbestawy, A.R.; El-Saadony, M.T.; Hussein, E.O.S.; Alhotan, R.; Suliman, G.M.; Taha, A.E.; Ba-Awadh, H.; El-Tarabily, K.A.; Abd El-Hack, M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: An updated overview. Poult. Sci. 2021, 100, 101039. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.U.; El-Hack, M.E.A.; Hassan, F.; El-Saadony, M.T.; Khafaga, A.F.; Batiha, G.E.; Yehia, N.; Elnesr, S.S.; Alagawany, M.; El-Tarabily, K.A.; et al. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021, 100, 101143. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 Fatty Acids in Poultry Nutrition: Effect on Production Performance and Health. Animals 2019, 9, 573. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Farahat, M.; Attia, G.; Alagawany, M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021, 100, 101266. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Kuo, T.; Wu, Y.; Yang, W. Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. Evid. Based Complement. Altern. Med. 2016, 2016, 2657981. [Google Scholar] [CrossRef] [PubMed]
- Puvaca, N.; Stanacev, V.; Glamocic, D.; Levic, J.; Peric, L.; Milic, D. Beneficial effects of phytoadditives in broiler nutrition. World’s Poult. Sci. J. 2013, 69, 27–34. [Google Scholar] [CrossRef]
- Bravo, D.; Pirgozliev, V.; Rose, S.P. A mixture of carvacrol, cinnamaldehyde, and Capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim. Sci. 2014, 92, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, C.A. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance. Trop. Anim. Health Prod. 2020, 52, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkader, A.M.; Labib, S.; Taha, T.F.; Althobaiti, F.; Aldhahrani, A.; Salem, H.M.; Saad, A.; Ibrahim, F.M. Phytogenic compounds from avocado (Persea americana L.) extracts; antioxidant activity, amylase inhibitory activity, therapeutic potential of type 2 diabetes. Saudi J. Biol. Sci. 2022, 29, 1428–1433. [Google Scholar] [CrossRef]
- Ashour, E.A.; Farsi, R.M.; Alaidaroos, B.A.; Abdel-Moneim, A.E.; El-Saadony, M.T.; Osman, A.O.; Abou Sayed-Ahmed, E.T.; Albaqami, N.M.; Shafi, M.E.; Taha, A.E.; et al. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021, 20, 1357–1372. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Oregano: A Feed Additive with Functional Properties. In Therapeutic Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 179–208. [Google Scholar]
- Abbas, R.Z.; Colwell, D.D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. World’s Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Bozkurt, M.; Ege, G.; Aysul, N.; Akşit, H.; Tüzün, A.E.; Küçükyılmaz, K.; Borum, A.E.; Uygun, M.; Akşit, D.; Aypak, S.; et al. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016, 95, 1858–1868. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, D.; Xue, W.; Yao, X.; Li, S. Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure–activity relationships. Life Sci. 2012, 90, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Selamat, J.; Perumal, V. Phytochemistry, Pharmacological Properties, and Recent Applications of Ficus benghalensis and Ficus religiosa. Plants 2021, 10, 2749. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.; Sahu, J.; Koul, S.; Kosha, R.L. Detailed pharmacognostical studies on Berberis aristata DC plant. Anc. Sci. Life 2013, 32, 234. [Google Scholar] [CrossRef]
- Jikah, A.N.; Edo, G.I. Mechanisms of action by sulphur compounds in Allium sativum. A review. Pharmacol. Res. Mod. Chin. Med. 2023, 9, 100323. [Google Scholar] [CrossRef]
- Rini Vijayan, K.P.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Kostadinovic, L.; Popovic, S.; Pelic, D.L.; Cabarkapa, I.; Duragic, O.; Levi, J. Medicinal plants as natural alternative to coccidial synthetic drugs in broiler chicken production. J. Agron. Technol. Eng. Manag. 2019, 2, 325–334. [Google Scholar]
- Tsiouris, V.; Giannenas, I.; Bonos, E.; Papadopoulos, E.; Stylianaki, I.; Sidiropoulou, E.; Lazari, D.; Tzora, A.; Ganguly, B.; Georgopoulou, I. Efficacy of a Dietary Polyherbal Formula on the Performance and Gut Health in Broiler Chicks after Experimental Infection with Eimeria spp. Pathogens 2021, 10, 524. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, A.; Srinivasan, B.P.; Chayhan, A.; Dubey, K. Cardioprotective effects of Ficus religiosa in neonatal streptozotocin-induced diabetic cardiomyopathy in rats. Biomed. Aging Pathol. 2014, 4, 53–58. [Google Scholar] [CrossRef]
- Mumtaz, S.; Akhtar, M.; Awais, M.M.; Anwar, M.I. Evaluation of immunomodulatory, growth promoting and protective effects of Ficus religiosa against coccidiosis in broilers. Pak. J. Agric. Sci. 2021, 58, 219–228. [Google Scholar]
- Aviagen. Ross 308 AP: Ross Broiler Management Handbook; 1118-AVNR-032; Aviagen Group: Huntsville, AL, USA, 2018; pp. 80–105. [Google Scholar]
- Close, B.; Banister, K.; Baumans Bernoth, E.M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; Morton, D.; et al. Recommendations foreuthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Khan, M.A.; Shoma, J.F.; Rahman, T.; Rahmatullah, M. Holarrhena antidysenterica (Linn.) Wall. (Apocynaceae)—A Plant for Gastrointestinal Disorders. EC Gastroenterol. Dig. Syst. 2018, 5, 437–443. [Google Scholar]
- Singh, D.; Singh, B.; Goel, R.K. Traditional uses, phytochemistry and pharmacology of Ficus religiosa: A review. J. Ethnopharmacol. 2011, 134, 565–583. [Google Scholar] [CrossRef]
- Malik, T.A.; Kamili, A.N.; Chishti, M.Z.; Ahad, S.; Tantry, M.A.; Hussain, P.R.; Johri, R.K. Breaking the resistance of Escherichia coli: Antimicrobial activity of Berberis lycium Royle. Microb. Pathog. 2017, 102, 12–20. [Google Scholar] [CrossRef]
- Foroutan-Rad, M.; Tappeh, K.H.; Khademvatan, S. Antileishmanial and Immunomodulatory Activity of Allium sativum (Garlic) A Review. J. Evid. Based Complement. Altern. Med. 2017, 22, 141–155. [Google Scholar] [CrossRef]
- Pathak, K.; Chhabra, M.B. Medicinal plants as alternative to anthelmintics for livestock: An overview with particular reference to Indian subcontinent. Indian J. Anim. Sci. 2014, 84, 335–349. [Google Scholar] [CrossRef]
- Nawaz, Z.; Rashid, N.; Abbasi, B.H.A.; Naveed, A.; Khan, D. Evaluating the Use of Neem (Azadirachta indica) Leaves Powder as A Growth Promoter in Broiler Chicken. Pak Euro J. Med. Life Sci. 2023, 6, 63–70. [Google Scholar]
- Alcicek, A.; Bozkurt, M.; Cabuk, M. The effect of an essential oil combination derived from selected herbs growing wild in Turkey on broiler performance. S. Afr. J. Anim. Sci. 2003, 33, 89–94. [Google Scholar] [CrossRef]
- Kucukyilmaz, K.; Cinar, M.; Bozkurt, M.; Catli, A.U. Effect of dietary mannan oligosaccharide with or without oregano essential oil and hop extract supplementation on the performance and slaughter characteristics of male broilers. S. Afr. J. Anim. Sci. 2009, 39, 223–232. [Google Scholar]
- Wallace, R.J.; Oleszek, W.; Franz, C.; Hahn, I.; Baser, K.H.C.; Mathe, A.; Teichmann, K. Dietary plant bioactives for poultry health and productivity. Br. Poult. Sci. 2010, 51, 461–487. [Google Scholar] [CrossRef]
- Arczewska-Włosek, A.; Świątkiewicz, S. Improved performance due to dietary supplementation with selected herbal extracts of broiler chickens infected with Eimeria spp. J. Anim. Feed Sci. 2013, 22, 257–263. [Google Scholar] [CrossRef]
- Bozkurt, M.; Selek, N.; Küçükyilmaz, K.; Eren, H.; Güven, E.; Çatli, A.U.; Çinar, M. Effects of dietary supplementation with a herbal extract on the performance of broilers infected with a mixture of Eimeria species. Br. Poult. Sci. 2012, 53, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Habibi, H.; Firouzi, S.; Nili, H.; Razavi, M.; Asadi, S.L.; Daneshi, S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: In vitro and in vivo study. J. Parasit. Dis. 2016, 40, 401–407. [Google Scholar] [CrossRef]
- Orengo, J.; Buendía, A.J.; Ruiz-Ibáñez, M.R.; Madrid, J.; Del Río, L.; Catalá-Gregori, P.; García, V.; Hernández, F. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet. Parasitol. 2012, 185, 158–163. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices? A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef]
- Ramezani, F.; Shekarabi, S.P.H.; Mehrgan, M.S.; Foroudi, F.; Islami, H.R. Supplementation of Siberian sturgeon (Acipenser baerii) diet with barberry (Berberis vulgaris) fruit extract: Growth performance, hemato-biochemical parameters, digestive enzyme activity, and growth-related gene expression. Aquaculture 2021, 540, 736750. [Google Scholar] [CrossRef]
- Martland, M.F. Ulcerative dermatitis dm broiler chickens: The effects of wet litter. Avian Pathol. 1985, 14, 353–364. [Google Scholar] [CrossRef]
- Wheeler, E.F.; Casey, K.D.; Gates, R.S.; Xin, H.; Topper, P.A.; Yi, L. Ammonia emissions from USA broiler chicken barns managed with new bedding, built-up litter, or acid-treated litter. In Proceedings of the Livestock Environment VIII, Iguassu Falls, Brazil, 31 August–4 September 2008; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2009; p. 4. [Google Scholar]
- Dehau, T.; Cherlet, M.; Croubels, S.; van Immerseel, F.; Goossens, E. A High Dose of Dietary Berberine Improves Gut Wall Morphology, Despite an Expansion of Enterobacteriaceae and a Reduction in Beneficial Microbiota in Broiler Chickens. mSystems 2023, 8, e0123922. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, J.-B.; Park, H.-J.; Cho, S.-M.; Park, Y.-J.; Sin, J.-I. Biological Properties of Different Types and Parts of the Dandelions: Comparisons of Anti-Oxidative, Immune Cell Proliferative and Tumor Cell Growth Inhibitory Activities. Prev. Nutr. Food Sci. 2005, 10, 172. [Google Scholar] [CrossRef]
- Anwar, M.I.; Muhammad, F.; Awais, M.M.; Akhtar, M. A review of β-glucans as a growth promoter and antibiotic alternative against enteric pathogens in poultry. World’s Poult. Sci. J. 2017, 73, 651–661. [Google Scholar] [CrossRef]
- Rizwan, H.M. Evaluation of Avena sativa Derived Arabinoxylans as Native Biological Response Modifiers in Broilers. Int. J. Agric. Biol. 2017, 19, 834–840. [Google Scholar] [CrossRef]
- Khaliq, K.; Akhtar, M.; Awais, M.M.; Anwar, M.I. Evaluation of immunotherapeutic effects of aloe vera polysaccharides against coccidiosis in chicken. Veteriner Fakultesi Dergisi 2017, 23, 895–901. [Google Scholar]
- Ullah, M.I.; Akhtar, M.; Awais, M.M.; Anwar, M.I.; Khaliq, K. Evaluation of immunostimulatory and immunotherapeutic effects of tropical mushroom (Lentinus edodes) against eimeriasis in chicken. Trop. Anim. Health Prod. 2018, 50, 97–104. [Google Scholar] [CrossRef]
- Allen, P.C.; Fetterer, R.H. Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry. Clin. Microbiol. Rev. 2002, 15, 58–65. [Google Scholar] [CrossRef]
- Ware, M.W.; Augustine, S.A.J.; Erisman, D.O.; See, M.J.; Wymer, L.; Hayes, S.L.; Dubey, J.P.; Villegas, E.N. Determining UV Inactivation of Toxoplasma gondii Oocysts by Using Cell Culture and a Mouse Bioassay. Appl. Environ. Microbiol. 2010, 76, 5140–5147. [Google Scholar] [CrossRef] [PubMed]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Téllez, G.; Hargis, B.M. Coccidiosis: Recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. Res. Rep. 2014, 5, 23–34. [Google Scholar]
- Akhtar, M.; Tariq, A.F.; Awais, M.M.; Iqbal, Z.; Muhammad, F.; Shahid, M.; Hiszczynska-Sawicka, E. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohydr. Polym. 2012, 90, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.H.; Elasbali, A.M.; Alanazi, M.K.; El Azab, E.F. Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules 2023, 28, 8045. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Ghafouri, S.A.; Ghaniei, A.; Tamannaei, A.E.T.; Sadr, S.; Charbgoo, A.; Ghiassi, S.; Abuali, M. Evaluation of therapeutic effects of an herbal mixture (Echinacea purpurea and Glycyrrhiza glabra) for treatment of clinical coccidiosis in broilers. Vet. Med. Sci. 2023, 9, 829–836. [Google Scholar] [CrossRef] [PubMed]
- El-Khtam, A.; Shata, A.; El-Hewaity, M.H. Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria species in broilers. Int. J. Basic Appl. Sci. 2014, 3, 349–356. [Google Scholar]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
| Period | ||||
|---|---|---|---|---|
| Ingredient (%) 1,2,3 | Pre-Starter | Starter | Grower | Finisher |
| Soybean meal | 37.84 | 34.12 | 26.99 | 22.26 |
| Wheat | 28.72 | 27.07 | 26.63 | 28.48 |
| Maize | 25.00 | 25.00 | 25.00 | 20.00 |
| Triticale | - | 5.00 | 10.00 | 15.00 |
| Rapeseed cake | - | - | 2.00 | 4.00 |
| Vegetable oil | 3.58 | 4.67 | 5.60 | 6.53 |
| Limestone | 1.55 | 1.38 | 1.23 | 1.21 |
| Monocalcium phosphate | 1.28 | 0.98 | 0.72 | 0.65 |
| Lysine sulphate | 0.45 | 0.31 | 0.38 | 0.44 |
| Methionine | 0.40 | 0.34 | 0.31 | 0.29 |
| Sodium chloride | 0.19 | 0.19 | 0.19 | 0.18 |
| Sodium sulphate | 0.15 | 0.15 | 0.15 | 0.17 |
| Threonine | 0.15 | 0.10 | 0.11 | 0.11 |
| Guar gum | 0.15 | 0.15 | 0.15 | 0.20 |
| Mycotoxin binder | 0.10 | 0.10 | 0.10 | 0.10 |
| Mineral premix | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix | 0.24 | 0.24 | 0.24 | 0.23 |
| Nutrient absorption enhancer | 0.05 | 0.05 | 0.05 | 0.05 |
| Coccidiostats (chemical-based on UC− and UC+; herbal origin on HA− and HA+) | 0.05 | 0.05 | 0.05 | - |
| Calculated analysis, % (unless stated otherwise) | ||||
| ME (MJ/kg) | 12.57 | 12.99 | 13.42 | 13.66 |
| Crude protein | 23.00 | 21.50 | 19.50 | 18.50 |
| Crude fat | 5.57 | 6.65 | 7.69 | 8.63 |
| Crude fibre | 2.62 | 2.55 | 2.64 | 2.78 |
| Crude ash | 6.75 | 6.10 | 5.48 | 5.25 |
| Calcium | 0.97 | 0.85 | 0.75 | 0.73 |
| Phosphorus | 0.70 | 0.62 | 0.55 | 0.53 |
| Sodium | 0.16 | 0.16 | 0.16 | 0.16 |
| Magnesium | 0.18 | 0.17 | 0.16 | 0.16 |
| Potassium | 1.03 | 0.97 | 0.86 | 0.80 |
| Chlorine | 0.17 | 0.17 | 0.17 | 0.17 |
| Lysine | 1.42 | 1.26 | 1.15 | 1.10 |
| Methionine | 0.71 | 0.63 | 0.58 | 0.55 |
| Methionine + Cysteine | 1.06 | 0.96 | 0.90 | 0.85 |
| Tryptophan | 0.29 | 0.27 | 0.24 | 0.23 |
| Item 1 (g) | Period 2 | Group 3,4,5 | SEM 6 | p-Value | |||
|---|---|---|---|---|---|---|---|
| UC− | UC+ | HA− | HA+ | ||||
| BW | |||||||
| 1 d | 48.88 | 48.87 | 48.87 | 48.88 | 0.02 | 0.997 | |
| 10 d | 317.04 a | 358.19 b | 261.69 c | 319.28 a | 7.68 | 0.000 | |
| 21 d | 1125.50 a | 1117.31 a | 972.26 b | 1018.26 c | 14.62 | 0.000 | |
| 35 d | 2660.92 a | 2615.65 ab | 2507.60 c | 2543.17 bc | 17.99 | 0.004 | |
| ADG | |||||||
| 1–10 d | 34.33 a | 39.77 b | 29.77 c | 33.63 a | 0.85 | 0.000 | |
| 11–21 d | 73.90 a | 67.19 ab | 61.38 b | 68.11 ab | 1.60 | 0.038 | |
| 22–35 d | 107.13 a | 100.40 ab | 95.25 b | 107.14 a | 1.85 | 0.048 | |
| Item 1 | Period 2 | Treatments 3 | SEM 4 | p-Value | |||
|---|---|---|---|---|---|---|---|
| UC− | UC+ | HA− | HA+ | ||||
| ADFI (g) | 1–35 d | 87.47 | 88.46 | 84.96 | 86.97 | 1.03 | 0.358 |
| FCR (kg/kg) | 1.60 | 1.64 | 1.48 | 1.51 | 0.04 | 0.717 | |
| Liveability (%) | 99.56 | 99.61 | 99.55 | 99.50 | 0.09 | 0.982 | |
| Item (% of BW) 1 | Treatments 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| UC− | UC+ | HA− | HA+ | |||
| Carcass without feathers, head, and legs, with viscera | 80.28 | 80.63 | 79.43 | 82.81 | 1.00 | 0.738 |
| Fully eviscerated carcass, without viscera | 67.86 | 68.62 | 68.21 | 68.18 | 0.91 | 0.994 |
| Thigh muscle (with bone) | 12.14 | 12.41 | 12.13 | 11.74 | 0.26 | 0.879 |
| Drumstick (with bone) | 8.28 | 7.23 | 7.40 | 7.28 | 0.21 | 0.253 |
| Thigh muscle (without bone) | 10.49 | 10.18 | 10.38 | 9.56 | 0.23 | 0.586 |
| Drumstick (without bone) | 6.24 | 5.12 | 5.70 | 5.43 | 0.16 | 0.060 |
| Total breast fillet | 24.74 | 22.48 | 24.29 | 24.40 | 0.43 | 0.217 |
| Outer breast fillet | 20.61 | 18.50 | 20.35 | 20.44 | 0.40 | 0.199 |
| Inner breast fillet | 4.15 | 3.80 | 3.93 | 3.81 | 0.13 | 0.789 |
| Item 1 | Age 2 | Treatments 3,4 | SEM 5 | p-Value | |||
|---|---|---|---|---|---|---|---|
| UC− | UC+ | HA− | HA+ | ||||
| DM of litter (%) | 14 d | 76.91 | 82.92 | 86.85 | 86.20 | 1.77 | 0.172 |
| 21 d | 75.81 | 78.62 | 76.64 | 76.49 | 0.62 | 0.451 | |
| 35 d | 68.71 a | 76.84 b | 66.97 a | 77.10 b | 1.15 | 0.001 | |
| Oocyst (g/l) | 14 d | 0.00 a | 120.80 b | 173.00 c | 245.60 d | 21.76 | 0.000 |
| 21 d | 0.00 a | 90.00 b | 82.20 b | 107.60 c | 9.71 | 0.000 | |
| 28 d | 2.80 a | 12.40 b | 7.20 c | 8.80 d | 0.82 | 0.000 | |
| Period 1 | 14 d | 21 d | 28 d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 2 | UC− | UC+ | HA− | HA+ | UC− | UC+ | HA− | HA+ | UC− | UC+ | HA− | HA+ |
| Item 3 | ||||||||||||
| Duodenum (with E. acervuline) | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 2 | 2 | 4 | 2 |
| p-value | 0.438 | 0.045 * | 0.372 | |||||||||
| p-value UC−/UC+ | 0.083 | |||||||||||
| p-value UC−/HA− | 0.604 | |||||||||||
| p-value UC−/HA+ | 0.083 | |||||||||||
| p-value HA−/HA+ | 0.030 * | |||||||||||
| Jejunum (with E. maxima) | 1 | 3 | 2 | 2 | 3 | 5 | 4 | 11 | 7 | 8 | 6 | 13 |
| p-value | 0.268 | 0.048 * | 0.049 * | |||||||||
| p-value UC−/UC+ | 0.481 | 0.121 | ||||||||||
| p-value UC−/HA− | 0.452 | 0.762 | ||||||||||
| p-value UC−/HA+ | 0.009 * | 0.003 * | ||||||||||
| p-value HA−/HA+ | 0.015 * | 0.050 * | ||||||||||
| Cecum (with E. tenella) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 3 |
| p-value | 0.415 | 0.415 | 0.466 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilienė, V.; Racevičiūtė-Stupelienė, A.; Murawska, D.; Gesek, M.; Matusevičius, P.; Miknienė, Z.; Nutautaitė, M. Evaluation of Herbal Anticoccidials on Growth Performance in Experimentally Infected Broiler Chickens. Agriculture 2024, 14, 2261. https://doi.org/10.3390/agriculture14122261
Vilienė V, Racevičiūtė-Stupelienė A, Murawska D, Gesek M, Matusevičius P, Miknienė Z, Nutautaitė M. Evaluation of Herbal Anticoccidials on Growth Performance in Experimentally Infected Broiler Chickens. Agriculture. 2024; 14(12):2261. https://doi.org/10.3390/agriculture14122261
Chicago/Turabian StyleVilienė, Vilma, Asta Racevičiūtė-Stupelienė, Daria Murawska, Michał Gesek, Paulius Matusevičius, Zoja Miknienė, and Monika Nutautaitė. 2024. "Evaluation of Herbal Anticoccidials on Growth Performance in Experimentally Infected Broiler Chickens" Agriculture 14, no. 12: 2261. https://doi.org/10.3390/agriculture14122261
APA StyleVilienė, V., Racevičiūtė-Stupelienė, A., Murawska, D., Gesek, M., Matusevičius, P., Miknienė, Z., & Nutautaitė, M. (2024). Evaluation of Herbal Anticoccidials on Growth Performance in Experimentally Infected Broiler Chickens. Agriculture, 14(12), 2261. https://doi.org/10.3390/agriculture14122261









