Different Nutritional Regimes in a Tomato Soilless System Affect the Bacterial Communities with Consequences on the Crop Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Setup
2.2. Sampling for Microbial Analyses
2.3. DNA Extraction and Quantitation
2.4. Quantitative Real-Time PCR
2.5. PCR-ARISA
2.6. Chemical Analyses
2.7. Statistical Analyses
3. Results
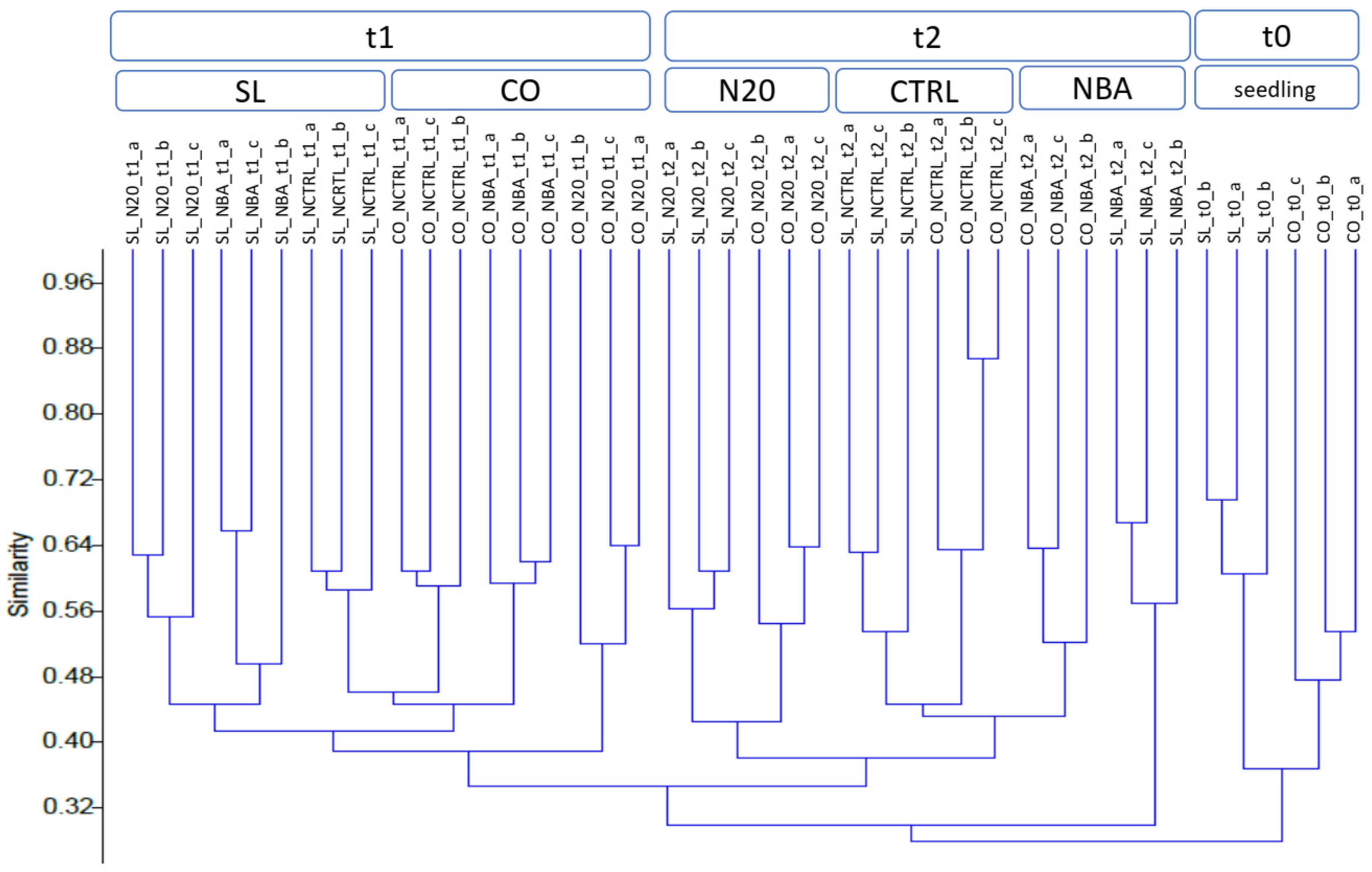
3.1. Bacterial Communities of the Root-Substrate System
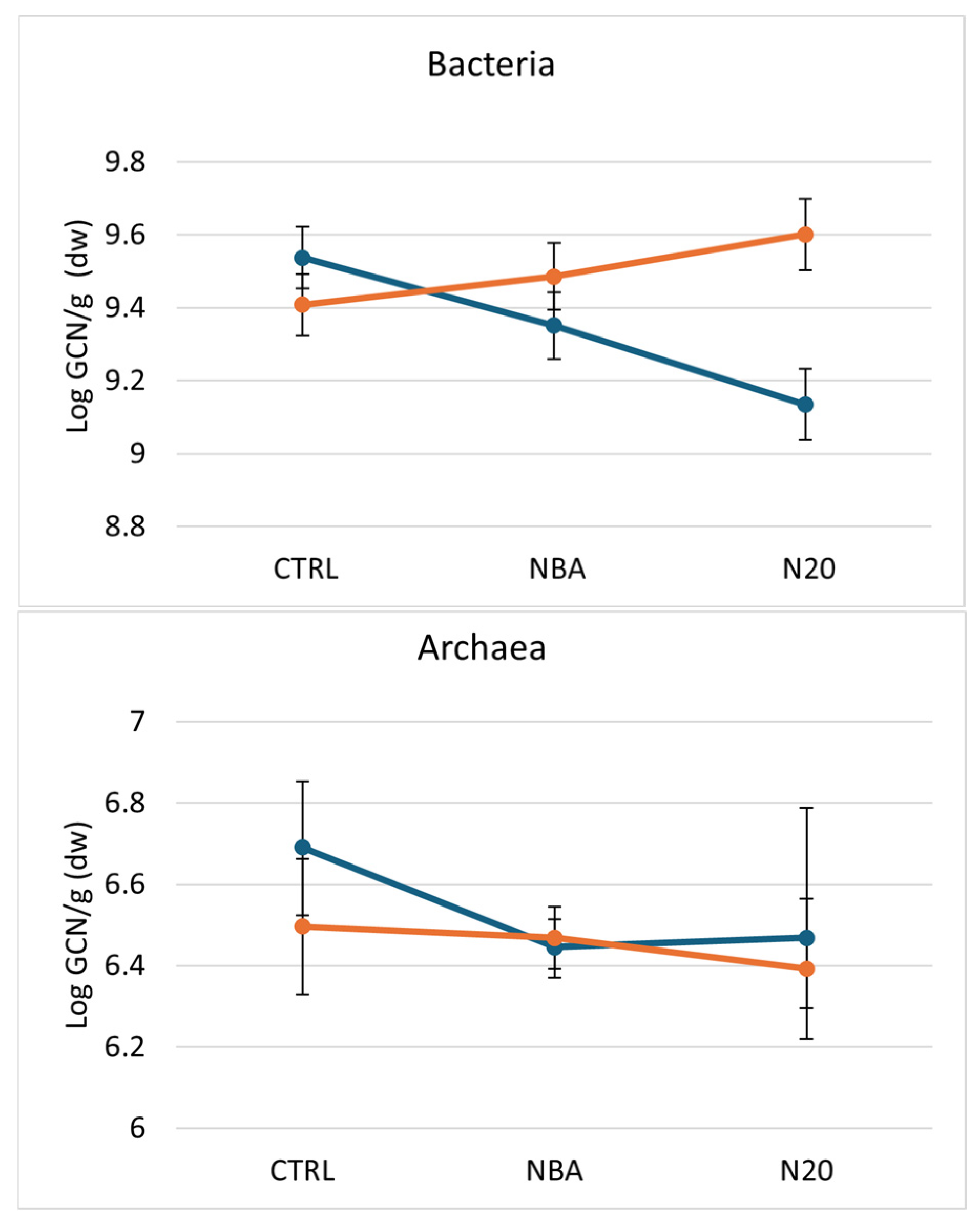
3.2. Quantification of Bacteria, Archaea and Nitrogen Cycle-Associated Microbial Groups
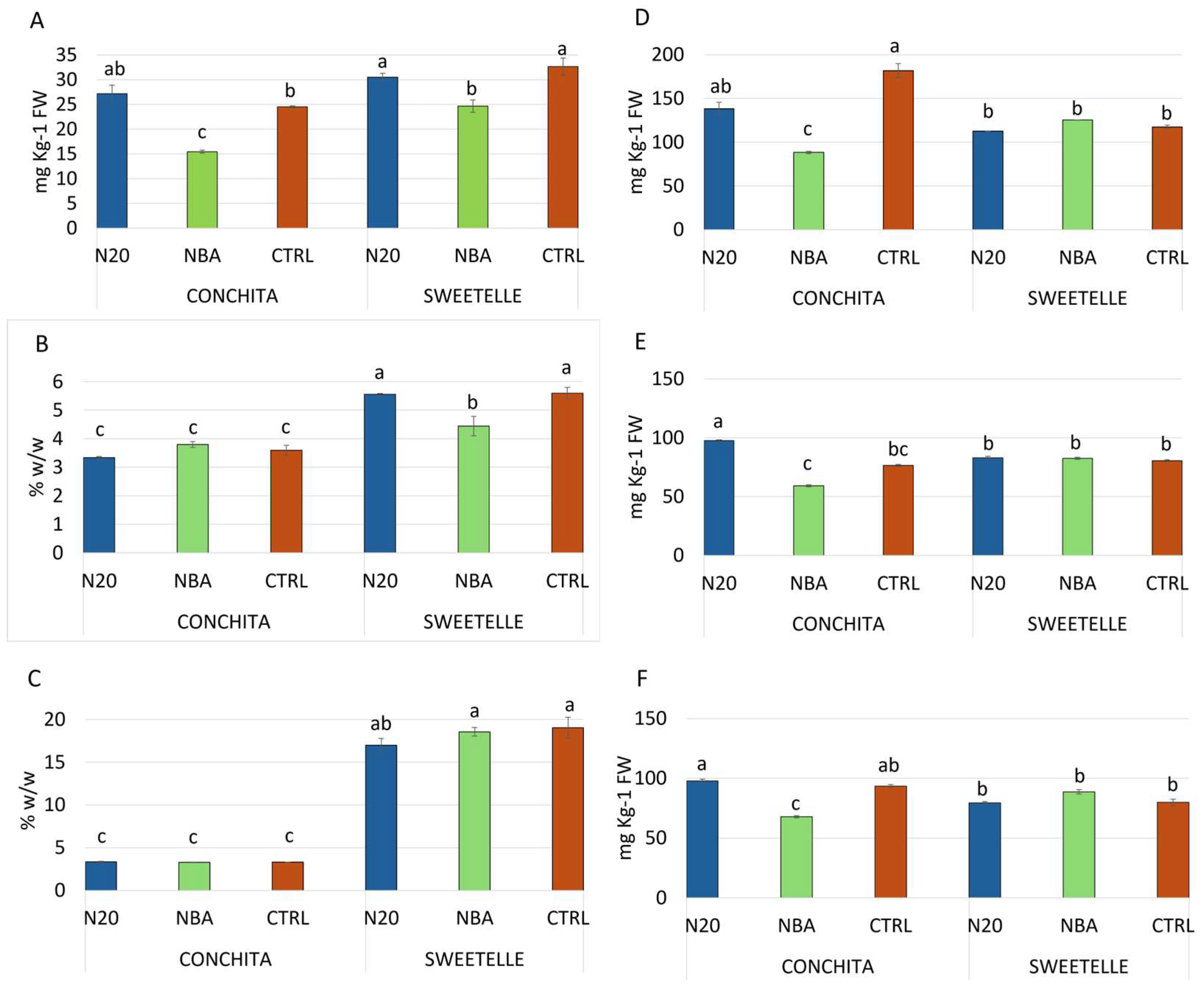
3.3. Tomato Production Quality Attributes
4. Discussion
4.1. Tomato Varieties
4.2. Qualitative Parameters of Tomato Production
4.3. Bacterial Diversity
4.4. Bacterial, Archaeal and N-Cycling Bacterial Populations
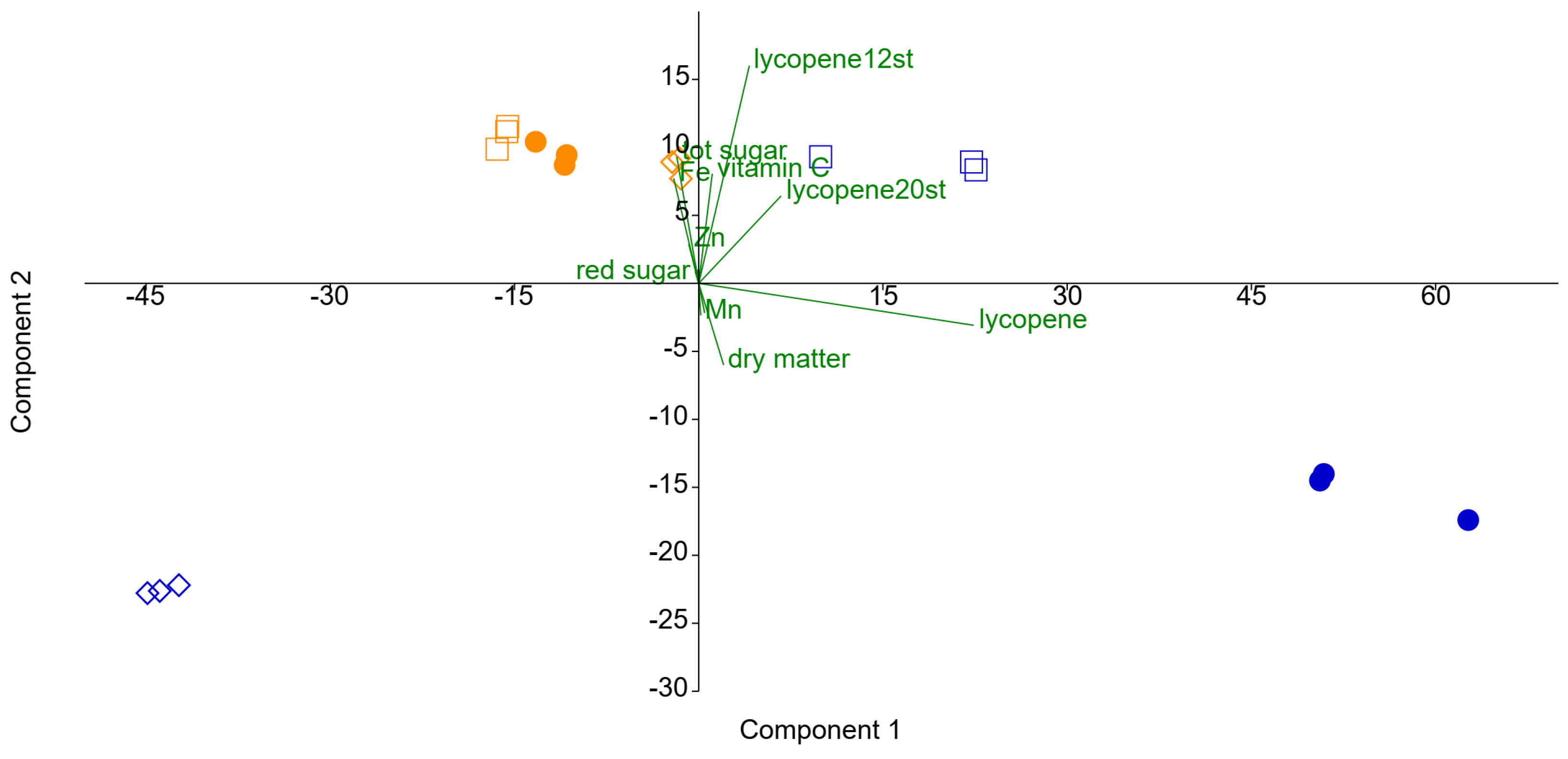
4.5. Correlations of Bacterial Communities with Tomato Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Good Agricultural Practices for Greenhouse Vegetable Crops; Soil. Cult. FAO Plant Prod. Prot. Pap. No. 217; Baudoin, W., Nono-Womdim, R., Lutaladio, N., Hodder, A., Eds.; 2013; Volume 217, pp. 303–354. [Google Scholar]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2020, 10, 1384. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms 2023, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Rosadi, R.A.B.; Senge, M.; Suhandy, D.; Tusi, A. The Effect of EC Levels of Nutrient Solution on the Growth, Yield, and Quality of Tomatoes (Solanum Lycopersicum) under the Hydroponic System. J. Agric. Eng. Biotechnol. 2014, 2, 7–12. [Google Scholar] [CrossRef]
- Osuna-Canizalez, F.J.; Ramírez-Rojas, S.; Canul-Kú, J. Seasonal Variation of Greenhouse Soilless Tomato Productivity in Warm Climate with Different Substrates and Water Quality, in Open and Closed System. Acta Hortic. 2012, 947, 93–99. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A Targeted Management of the Nutrient Solution in a Soilless Tomato Crop According to Plant Needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Kaya, S.; Caturano, E.; Tuzel, Y.; Okur, N.; Leonardi, C. Response of Tomato Plants to Organic Nutrition in Soilless Culture. J. Food Agric. Environ. 2008, 6, 303–305. [Google Scholar]
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and Physiological Responses of Tomato Plants Grown in Different Soilless Culture Systems with Saline Water under Greenhouse Conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in Antioxidant Content of Tomato Fruits in Response to Cultivar and Nutrient Solution Composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Perneel, M.; Van Labeke, M.C.; Reheul, D.; Boon, N. Molecular insights on the functional microbial community from organic and mineral growing media and its interaction with agrobacterium rhizogenes. Commun. Agric. Appl. Biol. Sci. 2014, 79, 345–356. [Google Scholar]
- Vargas, P.; Bosmans, L.; Van Calenberge, B.; Van Kerckhove, S.; Lievens, B.; Rediers, H. Bacterial Community Dynamics of Tomato Hydroponic Greenhouses Infested with Hairy Root Disease. FEMS Microbiol. Ecol. 2021, 97, fiab153. [Google Scholar] [CrossRef]
- Anzalone, A.; Mosca, A.; Dimaria, G.; Nicotra, D.; Tessitori, M.; Privitera, G.F.; Pulvirenti, A.; Leonardi, C.; Catara, V. Soil and Soilless Tomato Cultivation Promote Different Microbial Communities That Provide New Models for Future Crop Interventions. Int. J. Mol. Sci. 2022, 23, 8820. [Google Scholar] [CrossRef] [PubMed]
- Cytryn, E.; Levkovitch, I.; Negreanu, Y.; Dowd, S.; Frenk, S.; Silber, A. Impact of Short-Term Acidification on Nitrification and Nitrifying Bacterial Community Dynamics in Soilless Cultivation Media. Appl. Environ. Microbiol. 2012, 78, 6576–6582. [Google Scholar] [CrossRef]
- Grunert, O.; Robles-Aguilar, A.A.; Hernandez-Sanabria, E.; Schrey, S.D.; Reheul, D.; Van Labeke, M.C.; Vlaeminck, S.E.; Vandekerckhove, T.G.L.; Mysara, M.; Monsieurs, P.; et al. Tomato Plants Rather than Fertilizers Drive Microbial Community Structure in Horticultural Growing Media. Sci. Rep. 2019, 4, 9561. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of Extreme Root Restriction on the Nutritional and Flavor Quality, and Sucrose Metabolism of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Tóth, E.; Csambalik, L.; Biró, B.; Gere, A.; Koren, D.; Kotroczó, Z.; Szalai, Z. Are the Nutritional Properties of Organic Tomatoes Altered by Single and Combined Microbial Soil Inoculants?: A Multiperspective Approach. J. Plant Growth Regul. 2024, 43, 3718–3728. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O Emissions from Biochar-Amended Soil to the Structure and Function of the N-Cycling Microbial Community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Bustin, S.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Sorlini, C.; Corselli, C.; Zanardini, E.; Daffonchio, D. Comparison of Different Primer Sets for Use in Automated Ribosomal Intergenic Spacer Analysis of Complex Bacterial Communities Comparison of Different Primer Sets for Use in Automated Ribosomal Intergenic Spacer Analysis of Complex Bacterial Communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Chandraju, S.; Venkatesh, R.; Chidan Kumar, C.S.; Ajay Kumar, B. Estimation of Reducing Sugar by Acid Hydrolysis of Sunflower Helianthus annuus) Husk by Standard Methods. Agric. Sci. 2016, 07, 322–325. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package For Education And Data Analysis. Palaeontol. Electron. 2001, 4, 1–10. [Google Scholar]
- Tsaniklidis, G.; Delis, C.; Nikoloudakis, N.; Katinakis, P.; Aivalakis, G. Low Temperature Storage Affects the Ascorbic Acid Metabolism of Cherry Tomato Fruits. Plant Physiol. Biochem. 2014, 84, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dziugieł, T.; Wadas, W. Effect of Plant Biostimulants on Macronutrient Content in Early Crop Potato Tubers. Agronomy 2020, 10, 1202. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Cwalina-Ambroziak, B.; Głosek, M.; Sienkiewicz, S. Effect of Biostimulators on Yield and Selected Chemical Properties of Potato Tubers. J. Elem. 2015, 20, 757–768. [Google Scholar] [CrossRef][Green Version]
- Głosek-Sobieraj, M.; Wierzbowska, J.; Cwalina-Ambroziak, B.; Waśkiewicz, A. Protein and Sugar Content of Tubers in Potato Plants Treated with Biostimulants. J. Plant Prot. Res. 2023, 62, 370–384. [Google Scholar] [CrossRef]
- Szwejda-Grzybowska, J.; Ropelewska, E.; Wrzodak, A.; Sabat, T. The Influence of Natural Preparations on the Chemical Composition, Flesh Structure and Sensory Quality of Pepper Fruit in Organic Greenhouse Cultivation. Food Control 2024, 155, 110088. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Gómez, H.A.G.; Seabra Junior, S.; Maraschin, M.; Tecchio, M.A.; Borges, C.V. Functional and Nutraceutical Compounds of Tomatoes as Affected by Agronomic Practices, Postharvest Management, and Processing Methods: A Mini Review. Front. Nutr. 2022, 9, 868492. [Google Scholar] [CrossRef]
- Hashida, S.; Johkan, M.; Kitazaki, K.; Shoji, K.; Goto, F.; Yoshihara, T. Management of Nitrogen Fertilizer Application, Rather than Functional Gene Abundance, Governs Nitrous Oxide Fluxes in Hydroponics with Rockwool. Plant Soil 2014, 374, 715–725. [Google Scholar] [CrossRef]
- Taffner, J.; Bergna, A.; Cernava, T.; Berg, G. Tomato-Associated Archaea Show a Cultivar-Specific Rhizosphere Effect but an Unspecific Transmission by Seeds. Phytobiomes J. 2020, 4, 133–141. [Google Scholar] [CrossRef]
- Edaroyati, P.; Wahab, M.; Che-othman, M.H.; Al-Tawaha, A.R.M. Managing Nitrogen Toxicity in the Soilless Culture System: A Review. J. Trop. Plant Physiol. 2024, 15, 11–26. [Google Scholar]
- Lin, W.; Li, Q.Z.; Zhou, W.; Yang, R.; Zhang, D.; Wang, H.; Li, Y.; Qi, Z.; Li, Y. Insights into Production and Consumption Processes of Nitrous Oxide Emitted from Soilless Culture Systems by Dual Isotopocule Plot and Functional Genes. Sci. Total Environ. 2023, 856, 159046. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sempere, M.; Guivernau, M.; Caceres, R.; Biel, C.; Noguerol, J.; Viñas, M. Effect of Fertigation with Struvite and Ammonium Nitrate on Substrate Microbiota and N2O Emissions in a Tomato Crop on Soilless Culture System. Agronomy 2024, 14, 119. [Google Scholar] [CrossRef]
- Lorenc, F.; Pešková, V.; Modlinger, R.; Podrázský, V.; Baláš, M.; Kleinová, D. Effect of Bio-Algeen® Preparation on Growth and Mycorrhizal Characteristics of Norway Spruce Seedlings. J. For. Sci. 2016, 62, 285–291. [Google Scholar] [CrossRef]
- Nishi, K.; Akizuki, S.; Toda, T.; Matsuyama, T.; Ida, J. Advanced Light-Tolerant Microalgae-Nitrifying Bacteria Consortia for Stable Ammonia Removal under Strong Light Irradiation Using Light-Shielding Hydrogel. Chemosphere 2022, 297, 134252. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.C.; Reheul, D.; Boon, N. Mineral and Organic Growing Media Have Distinct Community Structure, Stability and Functionality in Soilless Culture Systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- Lee, S.K.; Chiang, M.S.; Hseu, Z.Y.; Kuo, C.H.; Liu, C. Te A Photosynthetic Bacterial Inoculant Exerts Beneficial Effects on the Yield and Quality of Tomato and Affects Bacterial Community Structure in an Organic Field. Front. Microbiol. 2022, 13, 959080. [Google Scholar] [CrossRef]
- Escobar Rodríguez, C.; Novak, J.; Buchholz, F.; Uetz, P.; Bragagna, L.; Gumze, M.; Antonielli, L.; Mitter, B. The Bacterial Microbiome of the Tomato Fruit Is Highly Dependent on the Cultivation Approach and Correlates With Flavor Chemistry. Front. Plant Sci. 2021, 12, 775722. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Takahashi, D.; Uemura, M.; Abadía, J.; López-Millán, A.F.; Rodríguez-Celma, J. Effects of Fe and Mn Deficiencies on the Root Protein Profiles of Tomato (Solanum Lycopersicum) Using Two-Dimensional Electrophoresis and Label-Free Shotgun Analyses. Int. J. Mol. Sci. 2022, 23, 3719. [Google Scholar] [CrossRef]


| Variety | Time | Treatment | Bacteria | Archaea | AOB | NFB |
|---|---|---|---|---|---|---|
| Sweetelle | T1 | CTRL | 9.20 ± 0.23 | 6.47 ± 0.23 | 7.74 ± 0.28 | 6.96 ± 0.20 |
| N20 | 9.35 ± 0.22 | 6.49 ± 0.09 | 7.37 ± 0.23 | 7.06 ± 0.36 | ||
| NBA | 9.39 ± 0.14 | 6.59 ± 0.12 | 8.35 ± 0.17 | 6.87 ± 0.20 | ||
| T2 | CTRL | 9.60 ± 0.11 | 6.52 ± 0.12 | 7.49 ± 0.06 | 7.02 ± 0.27 | |
| N20 | 9.84 ± 0.03 | 6.29 ± 0.19 | 7.49 ± 0.30 | 6.60 ± 0.15 | ||
| NBA | 9.57 ± 0.06 | 6.40 ± 0.16 | 7.30 ± 0.29 | 6.57 ± 0.24 | ||
| Conchita | T1 | CTRL | 9.54 ± 0.17 | 6.77 ± 0.24 | 8.31 ± 0.49 | 7.34 ± 0.15 |
| N20 | 8.91 ± 0.07 | 6.70 ± 0.16 | 7.85 ± 0.20 | 7.06 ± 0.14 | ||
| NBA | 9.17 ± 0.17 | 6.54 ± 0.14 | 8.32 ± 0.31 | 7.19 ± 0.29 | ||
| T2 | CTRL | 9.53 ± 0.09 | 6.61 ± 0.31 | 7.43 ± 0.07 | 6.83 ± 0.13 | |
| N20 | 9.35 ± 0.10 | 6.24 ± 0.27 | 7.01 ± 0.19 | 6.41 ± 0.40 | ||
| NBA | 9.52 ± 0.16 | 6.42 ± 0.23 | 8.37 ± 0.10 | 7.14 ± 0.29 | ||
| p (same) | ||||||
| Time | 0.00011 ** | 0.01104 * | 0.03957 * | 0.00307 * | ||
| Variety | 0.02026 * | 0.25405 | 0.07525 * | 0.1739 | ||
| Nutrition | 0.69021 | 0.1998 | 0.00112 ** | 0.1565 | ||
| Interaction Var./Nut. | 0.00489 ** | 0.5166 | 0.44057 | 0.1215 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneduce, L.; Piergiacomo, F.; Sikorska-Zimny, K. Different Nutritional Regimes in a Tomato Soilless System Affect the Bacterial Communities with Consequences on the Crop Quality. Agriculture 2024, 14, 2254. https://doi.org/10.3390/agriculture14122254
Beneduce L, Piergiacomo F, Sikorska-Zimny K. Different Nutritional Regimes in a Tomato Soilless System Affect the Bacterial Communities with Consequences on the Crop Quality. Agriculture. 2024; 14(12):2254. https://doi.org/10.3390/agriculture14122254
Chicago/Turabian StyleBeneduce, Luciano, Federica Piergiacomo, and Kalina Sikorska-Zimny. 2024. "Different Nutritional Regimes in a Tomato Soilless System Affect the Bacterial Communities with Consequences on the Crop Quality" Agriculture 14, no. 12: 2254. https://doi.org/10.3390/agriculture14122254
APA StyleBeneduce, L., Piergiacomo, F., & Sikorska-Zimny, K. (2024). Different Nutritional Regimes in a Tomato Soilless System Affect the Bacterial Communities with Consequences on the Crop Quality. Agriculture, 14(12), 2254. https://doi.org/10.3390/agriculture14122254







