Establishment of Hairy Root Transformation System for Evaluating Stress-Tolerant Gene in Jojoba
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
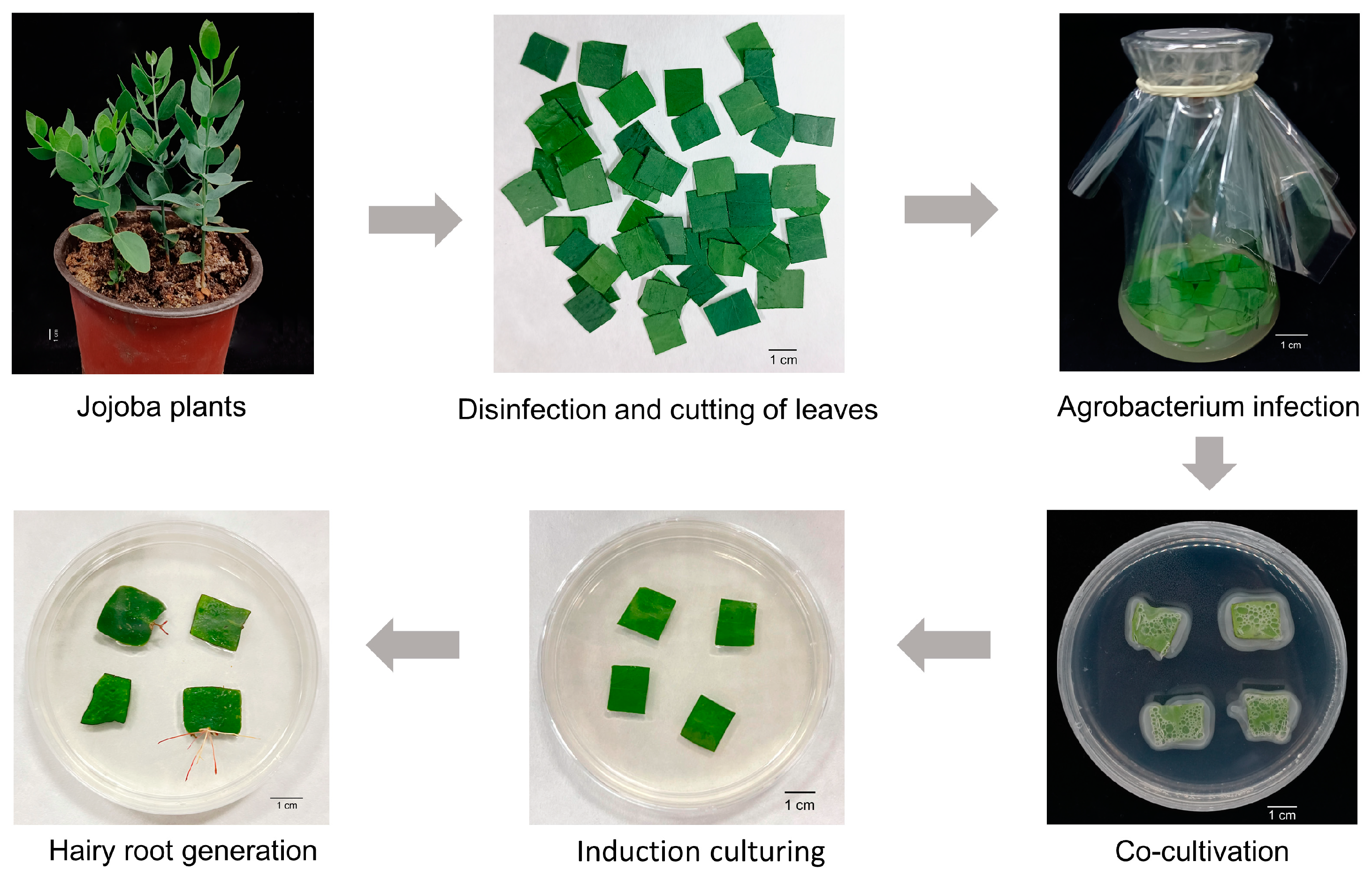
2.2. Establishment of Hairy Root Transformation System Using “Soaking Co-Culture Method” Under Sterile Conditions
2.3. Optimization of Hairy Root Transformation System Using “Soaking Co-Culture Method”
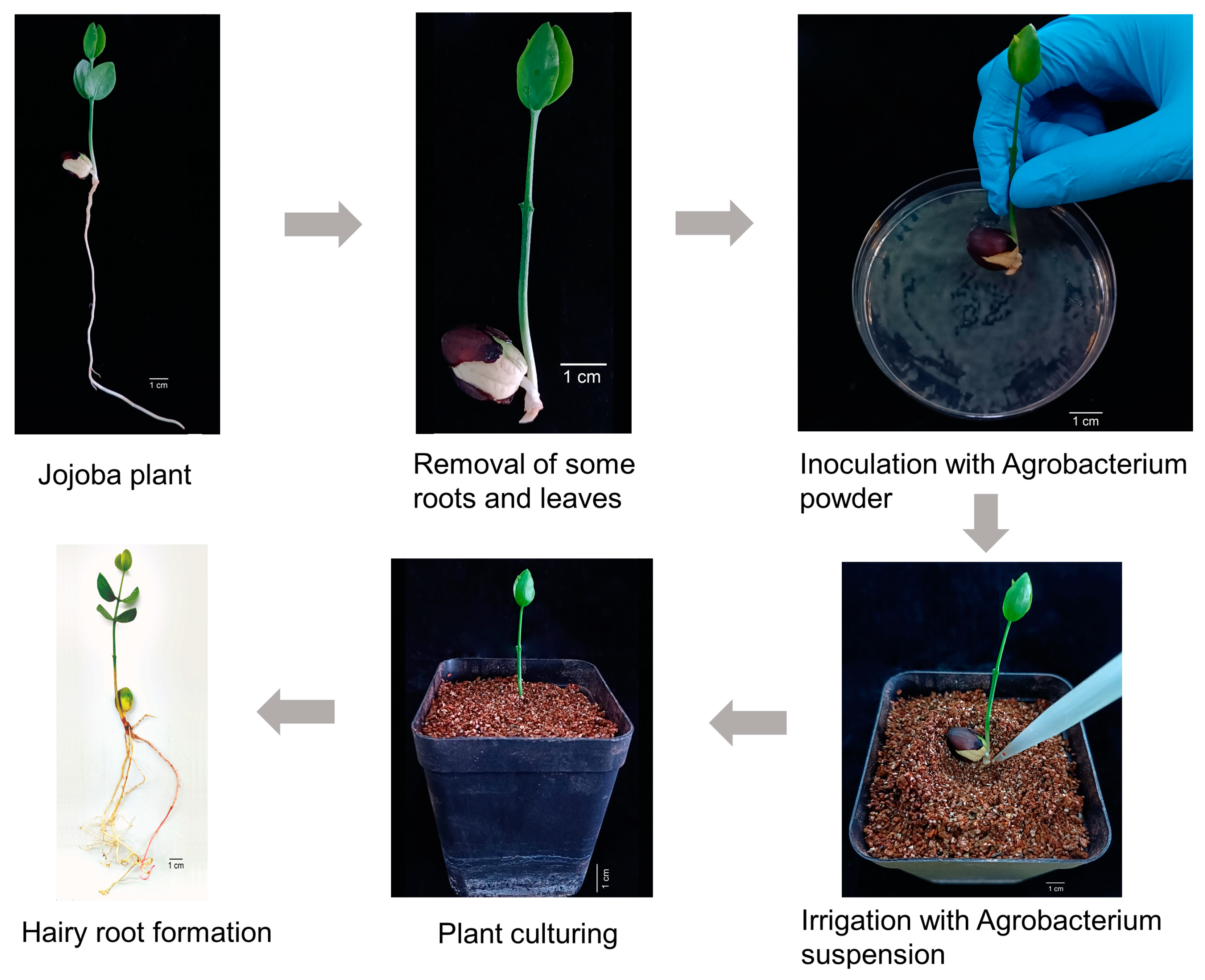
2.4. Induction of Hairy Roots in Jojoba Using “Wrapping Co-Culture Method” Under Non-Sterile Conditions
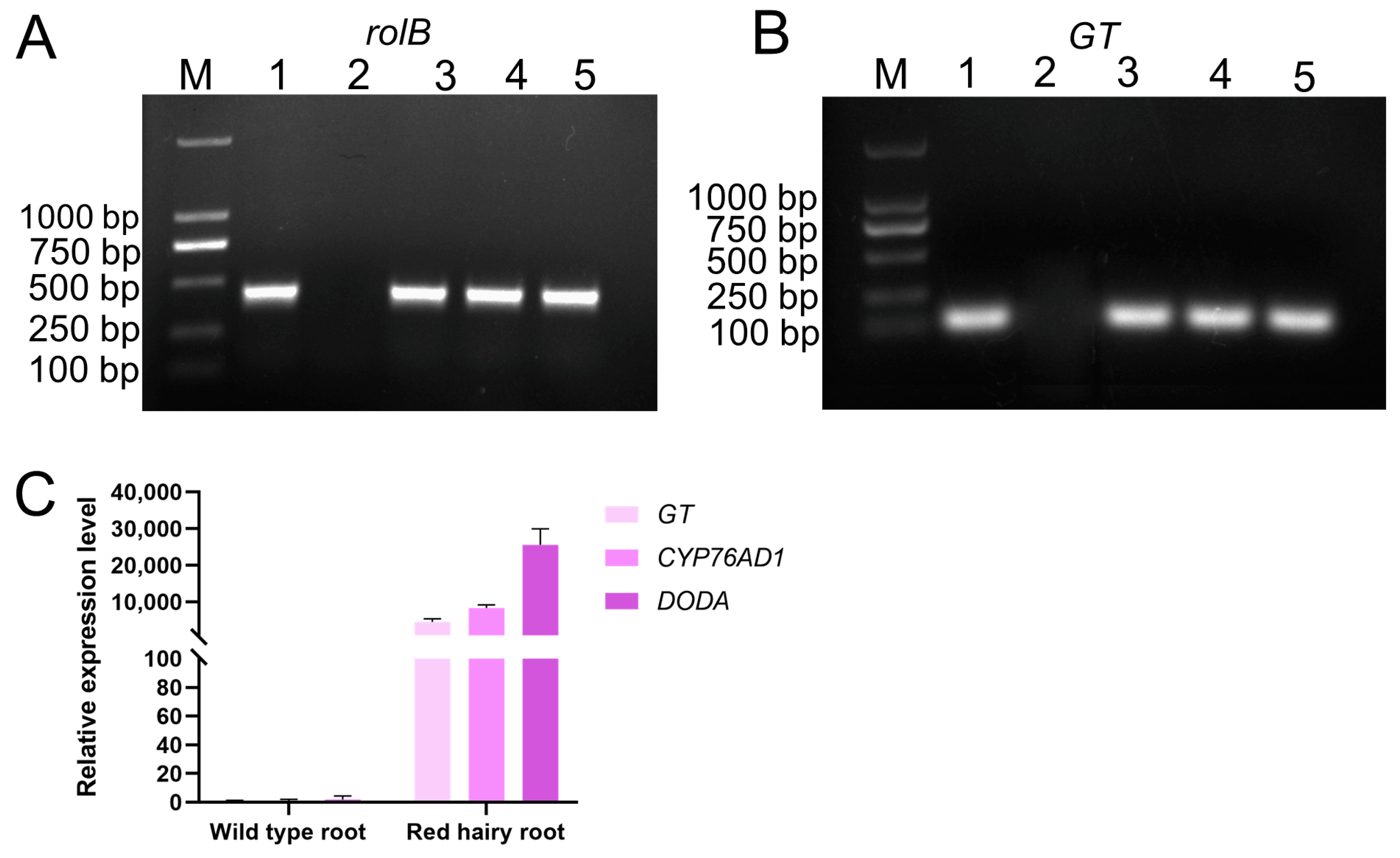
2.5. Genomic DNA PCR Analysis of rolB and GT Genes
2.6. qRT-PCR Analysis of Transgenic Hairy Roots
2.7. Low-Temperature Treatment of Jojoba Seedlings and qRT-PCR Analysis of ScGolS Genes
2.8. Construction of ScGolS1 Gene Expression Vector
2.9. Evaluation of Low-Temperature Tolerance of Plant Tissues Using NBT Staining
2.10. Statistical Analysis
3. Results
3.1. Establishment of Hairy Root Transformation System (Soaking Co-Culture Method) for Jojoba
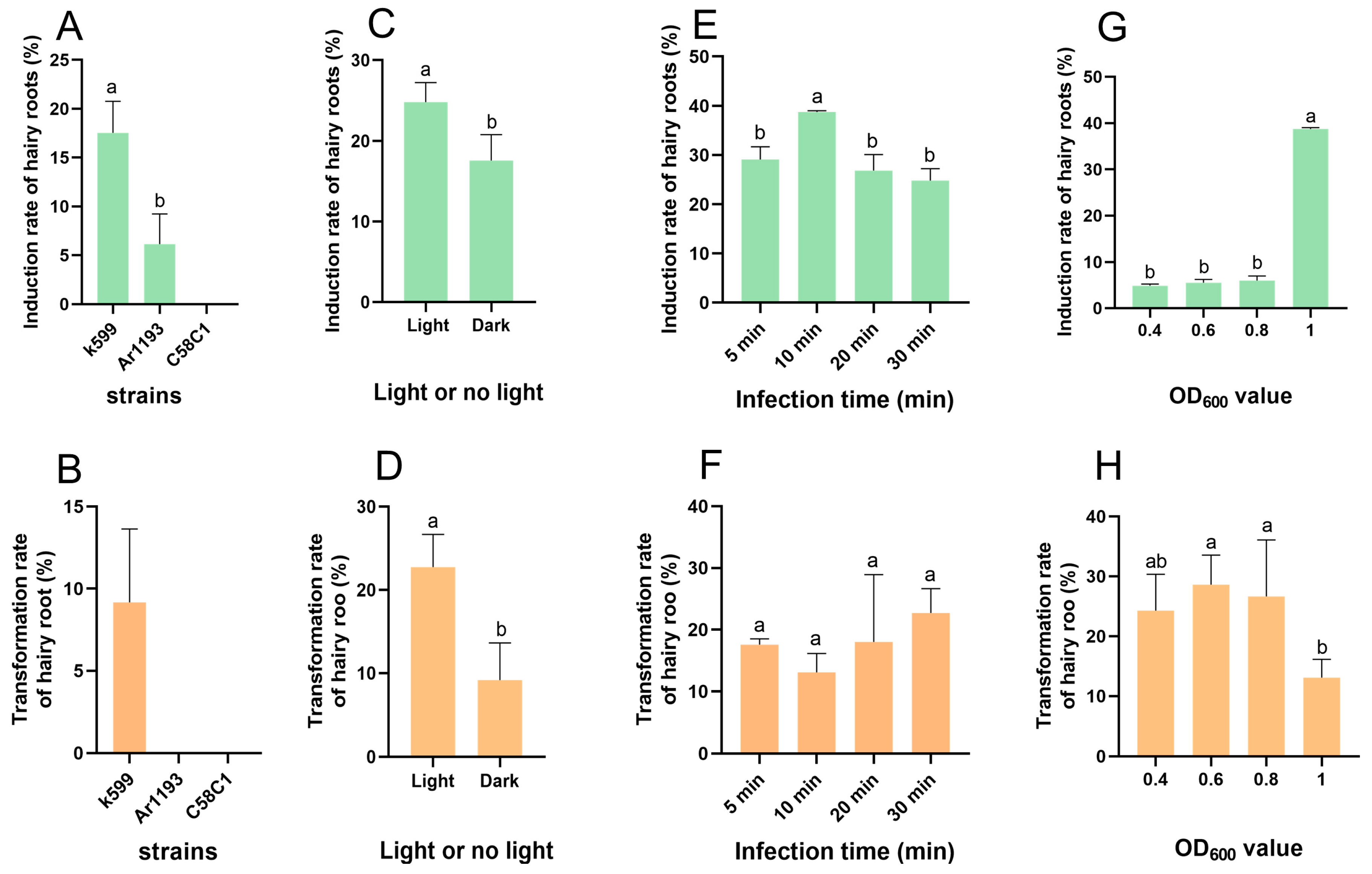
3.2. The Impact of Various Strains of A. rhizogenes on the Induction and Transformation Rates of Hairy Roots in Jojoba
3.3. The Effect of Co-Cultivation-Period Lighting on the Induction Rate and Transformation Rate of Hairy Roots in Jojoba
3.4. The Effect of the Infection Time on the Induction Rate and Transformation Rate of Hairy Roots in Jojoba
3.5. The Effect of the Bacterial Solution Concentration on the Induction Rate and Transformation Rate of Hairy Roots in Jojoba
3.6. Establishment of Hairy Root Transformation System of Jojoba Without Tissue Culture
3.7. Genetic Transformation and Molecular Identification of ScGolS1 in Jojoba
3.8. Overexpression of ScGolS1 Enhanced Low-Temperature Tolerance of Jojoba
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, F.; Ma, P.; Wu, Y.; Zhou, Y.; Zhang, G. Quantitative proteomic analysis of the response to cold stress in Jojoba, a tropical woody crop. Int. J. Mol. Sci. 2019, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- El-Mallah, M.H.; El-Shami, S.M. Investigation of Liquid Wax Components of Egyptian Jojoba Seeds. J. Oleo Sci. 2009, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.; Halabi, M.F.; AlKhalifah, N.S.; Asanar, S.; Al-Soqeer, A.A.; Attia, M.F. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol. Res. 2017, 50, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-f.; Sun, W.-m.; Li, Z.-q.; Bai, R.-x.; Li, J.-x.; Shi, Z.-h.; Geng, H.; Zheng, Y.; Zhang, J.; Zhang, G.-f. Over-Expression of ScMnSOD, a SOD Gene Derived from Jojoba, Improve Drought Tolerance in Arabidopsis. J. Integr. Agric. 2013, 12, 1722–1730. [Google Scholar] [CrossRef]
- Genaidy, E.A.E.; Atteya, A.K.G.; Adss, I.A.A. Increase the economic value of the jojoba (Simmondsia chinensis) yield using evaluation of distinctive clones grown under the Egyptian environmental conditions. Int. J. Agric. Technol. 2016, 12, 145–165. [Google Scholar]
- Al-Widyan, M.I.; Al-Muhtaseb, M.t.A. Experimental investigation of jojoba as a renewable energy source. Energy Convers. Manag. 2010, 51, 1702–1707. [Google Scholar] [CrossRef]
- Dag, A.; Badichi, S.; Ben-Gal, A.; Perry, A.; Tel-Zur, N.; Ron, Y.; Tietel, Z.; Yermiyahu, U. Optimizing nitrogen application for jojoba under intensive cultivation. Plants 2023, 12, 3132. [Google Scholar] [CrossRef] [PubMed]
- El Sherif, F.; AlDayel, M.; Ismail, M.B.; Alrajeh, H.S.; Younis, N.S.; Khattab, S. Bio-stimulant for improving Simmondsia chinensis secondary metabolite production, as well as antimicrobial activity and wound healing abilities. Plants 2023, 12, 3311. [Google Scholar] [CrossRef]
- Alyousif, N.A.; El Sherif, F.; Yap, Y.-K.; Khattab, S. Selection of salt-tolerant jojoba (Simmondisa chinensis L.) cultivars via in vitro culture. Horticulturae 2023, 9, 675. [Google Scholar] [CrossRef]
- Aboryia, M.S.; El-Dengawy, E.-R.F.A.; El-Banna, M.F.; El-Gobba, M.H.; Kasem, M.M.; Hegazy, A.A.; Hassan, H.M.; El-Yazied, A.A.; El-Gawad, H.G.A.; Al-Qahtani, S.M.; et al. Anatomical and physiological performance of jojoba treated with proline under salinity stress condition. Horticulturae 2022, 8, 716. [Google Scholar] [CrossRef]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sturtevant, D.; Lu, S.; Zhou, Z.-W.; Shen, Y.; Wang, S.; Song, J.-M.; Zhong, J.; Burks, D.J.; Yang, Z.-Q.; Yang, Q.-Y.; et al. The genome of jojoba (Simmondsia chinensis): A taxonomically isolated species that directs wax ester accumulation in its seeds. Sci. Adv. 2020, 6, 2375–2548. [Google Scholar] [CrossRef]
- Al-Dossary, O.; Alsubaie, B.; Kharabian-Masouleh, A.; Al-Mssallem, I.; Furtado, A.; Henry, R.J. The jojoba genome reveals wide divergence of the sex chromosomes in a dioecious plant. Plant J. 2021, 108, 1283–1294. [Google Scholar] [CrossRef]
- Zheng, L.; Li, B.; Zhou, Y.; Gao, F. A WRKY-regulated TLP gene mediates the response to cold, drought, and wound stress in jojoba. Ind. Crops Prod. 2024, 220, 119224. [Google Scholar] [CrossRef]
- Llorente, B.E.; Apóstolo, N.M. In vitro propagation of jojoba. Methods Mol. Biol. 2013, 11013, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Unique, S. Tissues culture and regeneration in jojoba. Plant Aging Basic Appl. Approaches 1990, 186, 339–343. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef] [PubMed]
- Christey, M.C. Use of ri-mediated transformation for production of transgenic plants. In Vitro Cell. Dev. Biol. Plant 2001, 37, 687–700. [Google Scholar] [CrossRef]
- Pala, Z.; Shukla, V.; Alok, A.; Kudale, S.; Desai, N. Enhanced production of an anti-malarial compound artesunate by hairy root cultures and phytochemical analysis of Artemisia pallens Wall. 3 Biotech 2016, 6, 182. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Wang, X.; Cao, L.; Ji, J.; Liu, T.; Duan, K. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean. Plant Methods 2021, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Meng, X.; Xu, K.; Li, M.; Gmitter, F.G.; Liu, N.; Gai, Y.; Huang, S.; Wang, M.; Wang, M.; et al. Highly efficient hairy root genetic transformation and applications in citrus. Front. Plant Sci. 2022, 13, 1039094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, H.; Lu, X.; Anees, M.; He, N.; Yang, D.; Chen, Z.; Hong, Z.; Zhang, J.; Liu, W. Streamlined Agrobacterium rhizogenes-Mediated hairy root transformation for efficient CRISPR/Cas9-based gene editing evaluation in diverse Citrullus varieties. Hortic. Plant J. 2024; in press. [Google Scholar] [CrossRef]
- Liu, L.; Qu, J.; Wang, C.; Liu, M.; Zhang, C.; Zhang, X.; Guo, C.; Wu, C.; Yang, G.; Huang, J.; et al. An efficient genetic transformation system mediated by Rhizobium rhizogenes in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol. J. 2024, 22, 2093–2103. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, D.; Fu, J.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. Agrobacterium rhizogenes-mediated hairy root transformation as an efficient system for gene function analysis in Litchi chinensis. Plant Methods 2021, 17, 103. [Google Scholar] [CrossRef]
- Su, Y.; Lin, C.; Zhang, J.; Hu, B.; Wang, J.; Li, J.; Wang, S.; Liu, R.; Li, X.; Song, Z.; et al. One-Step regeneration of hairy roots to induce high tanshinone plants in Salvia miltiorrhiza. Front. Plant Sci. 2022, 13, 913985. [Google Scholar] [CrossRef]
- Martins, C.P.S.; Fernandes, D.; Guimarães, V.M.; Du, D.; Silva, D.C.; Almeida, A.F.; Gmitter, F.G., Jr.; Otoni, W.C.; Costa, M.G.C. Comprehensive analysis of the GALACTINOL SYNTHASE (GolS) gene family in citrus and the function of CsGolS6 in stress tolerance. PLoS ONE 2022, 17, e0274791. [Google Scholar] [CrossRef]
- Ma, S.; Lv, J.; Li, X.; Ji, T.; Zhang, Z.; Gao, L. Galactinol synthase gene 4 (CsGolS4) increases cold and drought tolerance in Cucumis sativus L. by inducing RFO accumulation and ROS scavenging. Environ. Exp. Bot. 2021, 185, 104406. [Google Scholar] [CrossRef]
- He, F.; Xu, J.; Jian, Y.; Duan, S.; Hu, J.; Jin, L.; Li, G. Overexpression of galactinol synthase 1 from Solanum commersonii (ScGolS1) confers freezing tolerance in transgenic potato. Hortic. Plant J. 2023, 9, 541–552. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef]
- Ajdanian, L.; Niazian, M.; Torkamaneh, D. Optimizing ex vitro one-step RUBY-equipped hairy root transformation in drug- and hemp-type Cannabis. Plant Biotechnol. J. 2024, 22, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Belzile, F.; Curtin, S.J.; de Ronne, M.; Torkamaneh, D. Optimization of in vitro and ex vitro Agrobacterium rhizogenes-mediated hairy root transformation of soybean for visual screening of transformants using RUBY. Front. Plant Sci. 2023, 14, 1207762. [Google Scholar] [CrossRef]
- Yu, J.; Deng, S.; Huang, H.; Mo, J.; Xu, Z.-F.; Wang, Y. Exploring the potential applications of the noninvasive reporter gene RUBY in plant genetic transformation. Forests 2023, 14, 637. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Liu, X.; Yao, W.; Wu, S.; Hou, W. The RUBY reporter for visual selection in soybean genome editing. aBIOTECH 2024, 5, 209–213. [Google Scholar] [CrossRef]
- Geng, H.; Shi, L.; Li, W.; Zhang, B.; Chu, C.; Li, H.; Zhang, G. Gene expression of jojoba (Simmondsia chinensis) leaves exposed to drying. Environ. Exp. Bot. 2008, 63, 137–146. [Google Scholar] [CrossRef]
- Mohammed, I.A.T.S.; Sakai, H.; Wada, N.; Fukuia, K. Agrobacterium-mediated transformation of jojoba [Simmondsia chinensis (Link.) Schneider]. Trop. Agric. Dev. 2017, 61, 23–31. [Google Scholar] [CrossRef]
- Giri, A.; Ravindra, S.T.; Dhingra, V.; Narasu, M.L. Influence of different strains of Agrobacterium rhizogenes on induction of hairy roots and artemisinin production in Artemisia annua. Curr. Sci. 2001, 81, 378–382. [Google Scholar]
- Sathasivam, R.; Choi, M.; Radhakrishnan, R.; Kwon, H.; Yoon, J.; Yang, S.H.; Kim, J.K.; Chung, Y.S.; Park, S.U. Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front. Plant Sci. 2022, 13, 983776. [Google Scholar] [CrossRef]
- Petit, A.; David, C.; Dahl, G.A.; Ellis, J.G.; Guyon, P.; Casse-Delbart, F.; Tempé, J. Further extension of the opine concept: Plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol. Gen. Genet. MGG 1983, 190, 204–214. [Google Scholar] [CrossRef]
- Yan, H.; Ma, D.; Yi, P.; Sun, G.; Chen, X.; Yi, Y.; Huang, X. Highly efficient Agrobacterium rhizogenes-mediated transformation for functional analysis in woodland strawberry. Plant Methods 2023, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lu, M.; Li, X.; Sun, H.; Cheng, Z.; Miao, Y.; Fu, Y.; Zhang, X. An Efficient Agrobacterium rhizogenes-Mediated hairy root transformation method in a Soybean root biology study. Int. J. Mol. Sci. 2022, 23, 12261. [Google Scholar] [CrossRef]
- Park, S.U.; Facchini, P.J. Agrobacterium rhizogenes-mediated transformation of opium poppy, Papaver somniferum L., and California poppy, Eschscholzia californica Cham., root cultures. J. Exp. Bot. 2000, 51, 1005–1016. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Hoang, T.T.; Le, N.T.; Tran, H.T.; Nguyen, C.X.; Moon, Y.H.; Chu, H.H.; Do, P.T. An efficient hairy root system for validation of plant transformation vector and CRISPR/Cas construct activities in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2021, 12, 770062. [Google Scholar] [CrossRef]
- Sujatha, G.; Zdravković-Korać, S.; Calic, D.; Flamini, G.; Ranjitha Kumari, B.D. High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Artemisia vulgaris: Hairy root production and essential oil analysis. Ind. Crops Prod. 2013, 44, 643–652. [Google Scholar] [CrossRef]
- Sugiyama, N.; Izawa, T.; Oikawa, T.; Shimamoto, K. Light regulation of circadian clock-controlled gene expression in rice. Plant J. 2001, 26, 607–615. [Google Scholar] [CrossRef]
- Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The role of light-regulated auxin signaling in root development. Int. J. Mol. Sci. 2023, 24, 5253. [Google Scholar] [CrossRef]
- Lv, Q.; Chen, C.; Xu, Y.; Hu, S.; Wang, L.; Sun, K.; Chen, X.; Li, X. Optimization of Agrobacterium tumefaciens-mediated transformation systems in tea plant (Camellia sinensis). Hortic. Plant J. 2017, 3, 105–109. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Liang, J.; Hu, X.; He, Y.; Miao, T.; Ouyang, Z.; Yang, Z.; Amin, A.K.; Ling, C.; et al. Agrobacterium rhizogenes-mediated marker-free transformation and gene editing system revealed that AeCBL3 mediates the formation of calcium oxalate crystal in kiwifruit. Mol. Hortic. 2024, 4, 1. [Google Scholar] [CrossRef]
- Boobalan, S.; Kamalanathan, D. Tailoring enhanced production of aervine in Aerva lanata (L.) Juss. Ex Schult by Agrobacterium rhizogenes- mediated hairy root cultures. Ind. Crops Prod. 2020, 155, 112814. [Google Scholar] [CrossRef]
- Wang, M.; Qin, Y.-Y.; Wei, N.-N.; Xue, H.-Y.; Dai, W.-S. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation in citrus seeds and its application in gene functional analysis. Front. Plant Sci. 2023, 14, 1293374. [Google Scholar] [CrossRef] [PubMed]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schoffl, F. Galactinol synthase1. A Novel Heat Shock Factor Target Gene Responsible for Heat-Induced Synthesis of Raffinose Family Oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mo, F.; Li, D.; Zheng, J.; Liang, S.; Liu, S.; Wang, P.; Cheng, M.; Chen, X.; Wang, A. Genome-wide identification and expression analysis of the GT8 gene family in tomato (Solanum lycopersicum) and the functional of SlGolS1 under cold stress. Sci. Hortic. 2024, 338, 113686. [Google Scholar] [CrossRef]
- Levengood, H.; Zhou, Y.; Zhang, C. Advancements in plant transformation: From traditional methods to cutting-edge techniques and emerging model species. Plant Cell Rep. 2024, 43, 273. [Google Scholar] [CrossRef]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Establishment of optimized in vitro disinfection protocol of Pistacia vera L. explants mediated a computational approach: Multilayer perceptron–multi−objective genetic algorithm. BMC Plant Biol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Cui, M.L.; Liu, C.; Piao, C.L.; Liu, C.L. A stable Agrobacterium rhizogenes-mediated transformation of Cotton (Gossypium hirsutum L.) and plant regeneration from transformed hairy root via embryogenesis. Front. Plant Sci. 2020, 11, 604255. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Wang, P.; Wang, C.; Wang, J.; Wang, X.; Cheng, H. Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene editing analysis in cotton. Front. Plant Sci. 2022, 13, 1059404. [Google Scholar] [CrossRef]
- Yan, Z.; Hossain, M.S.; Arikit, S.; Valdés-López, O.; Zhai, J.; Wang, J.; Libault, M.; Ji, T.; Qiu, L.; Meyers, B.C.; et al. Identification of microRNAs and their mRNA targets during soybean nodule development: Functional analysis of the role of miR393j-3p in soybean nodulation. New Phytol. 2015, 207, 748–759. [Google Scholar] [CrossRef]
- Dong, B.; Meng, D.; Song, Z.; Cao, H.; Du, T.; Qi, M.; Wang, S.; Xue, J.; Yang, Q.; Fu, Y. CcNFYB3-CcMATE35 and LncRNA CcLTCS-CcCS modules jointly regulate the efflux and synthesis of citrate to enhance aluminium tolerance in pigeon pea. Plant Biotechnol. J. 2024, 22, 181–199. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, Y.; Ma, W.; Bing, J.; Zhou, Y.; Gen, Y.; Gao, F. Establishment of Hairy Root Transformation System for Evaluating Stress-Tolerant Gene in Jojoba. Agriculture 2024, 14, 2132. https://doi.org/10.3390/agriculture14122132
Li B, Wang Y, Ma W, Bing J, Zhou Y, Gen Y, Gao F. Establishment of Hairy Root Transformation System for Evaluating Stress-Tolerant Gene in Jojoba. Agriculture. 2024; 14(12):2132. https://doi.org/10.3390/agriculture14122132
Chicago/Turabian StyleLi, Bojing, Yan Wang, Wenguo Ma, Jie Bing, Yijun Zhou, Yuke Gen, and Fei Gao. 2024. "Establishment of Hairy Root Transformation System for Evaluating Stress-Tolerant Gene in Jojoba" Agriculture 14, no. 12: 2132. https://doi.org/10.3390/agriculture14122132
APA StyleLi, B., Wang, Y., Ma, W., Bing, J., Zhou, Y., Gen, Y., & Gao, F. (2024). Establishment of Hairy Root Transformation System for Evaluating Stress-Tolerant Gene in Jojoba. Agriculture, 14(12), 2132. https://doi.org/10.3390/agriculture14122132





