Nitrogen and Carbon Mineralization from Organic Amendments and Fertilizers Using Incubations with Sandy Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil N Analyses
2.3. CO2 Emissions
2.4. Data Analyses
3. Results
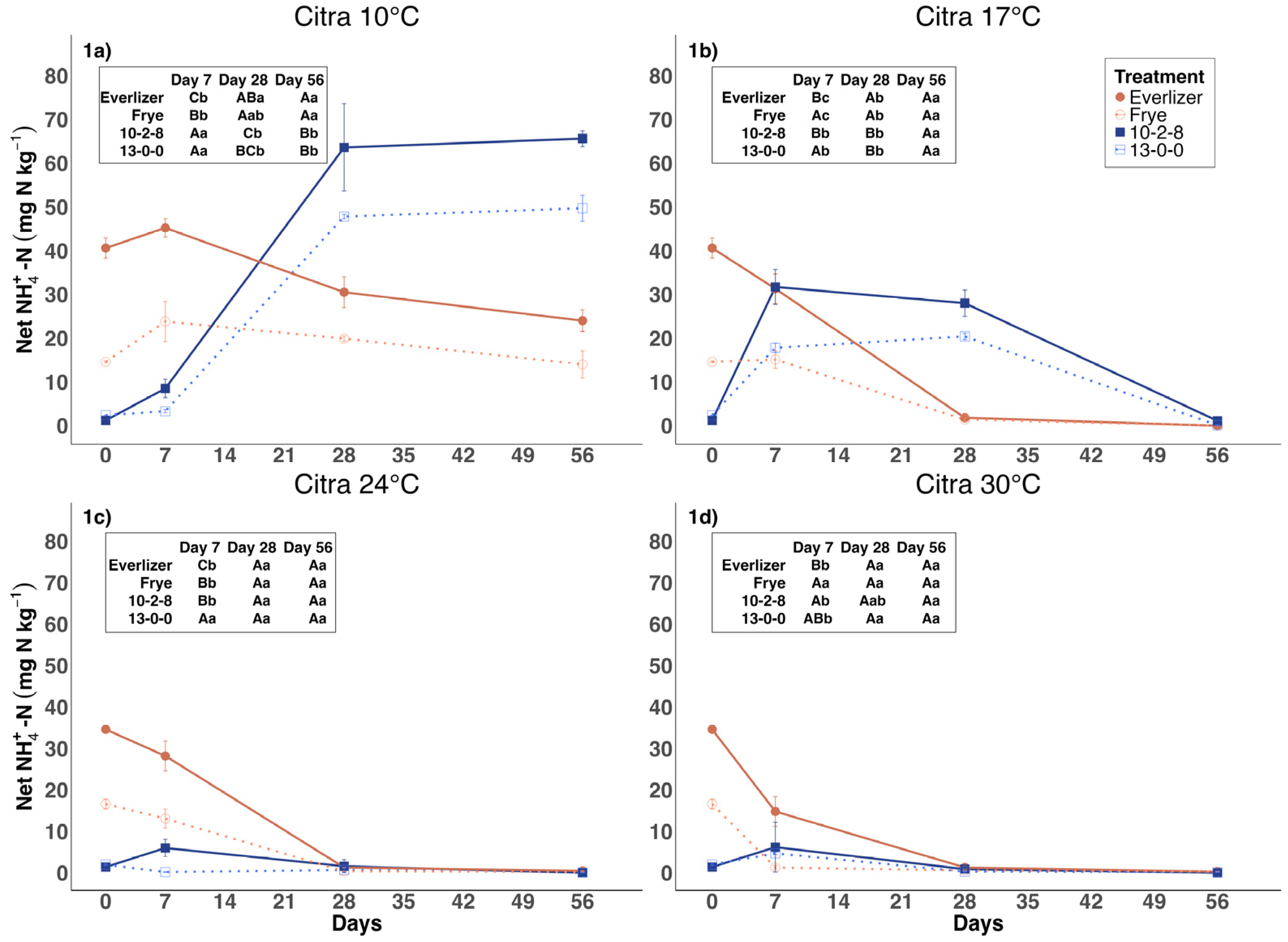
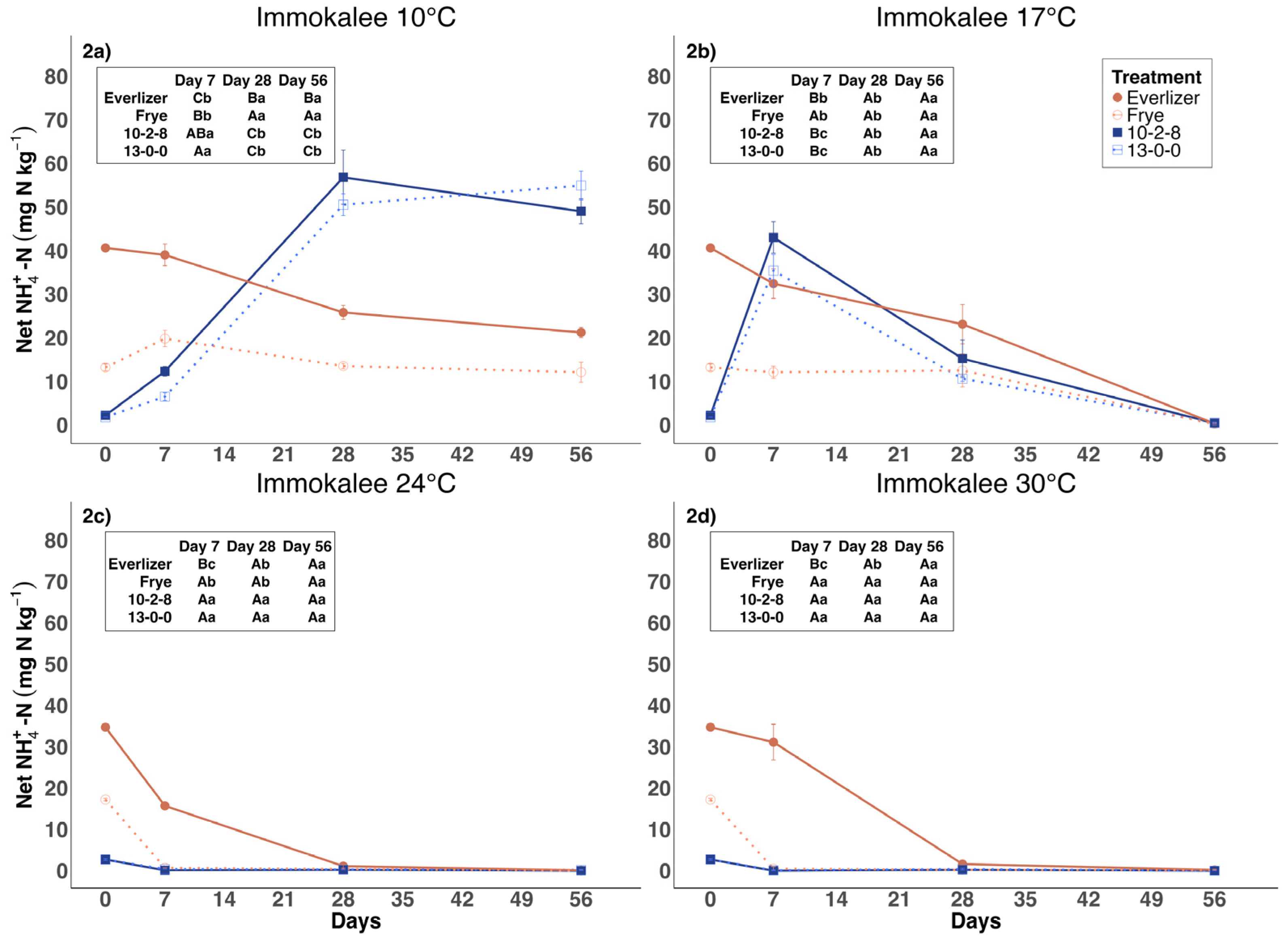
3.1. Ammonium Nitrogen
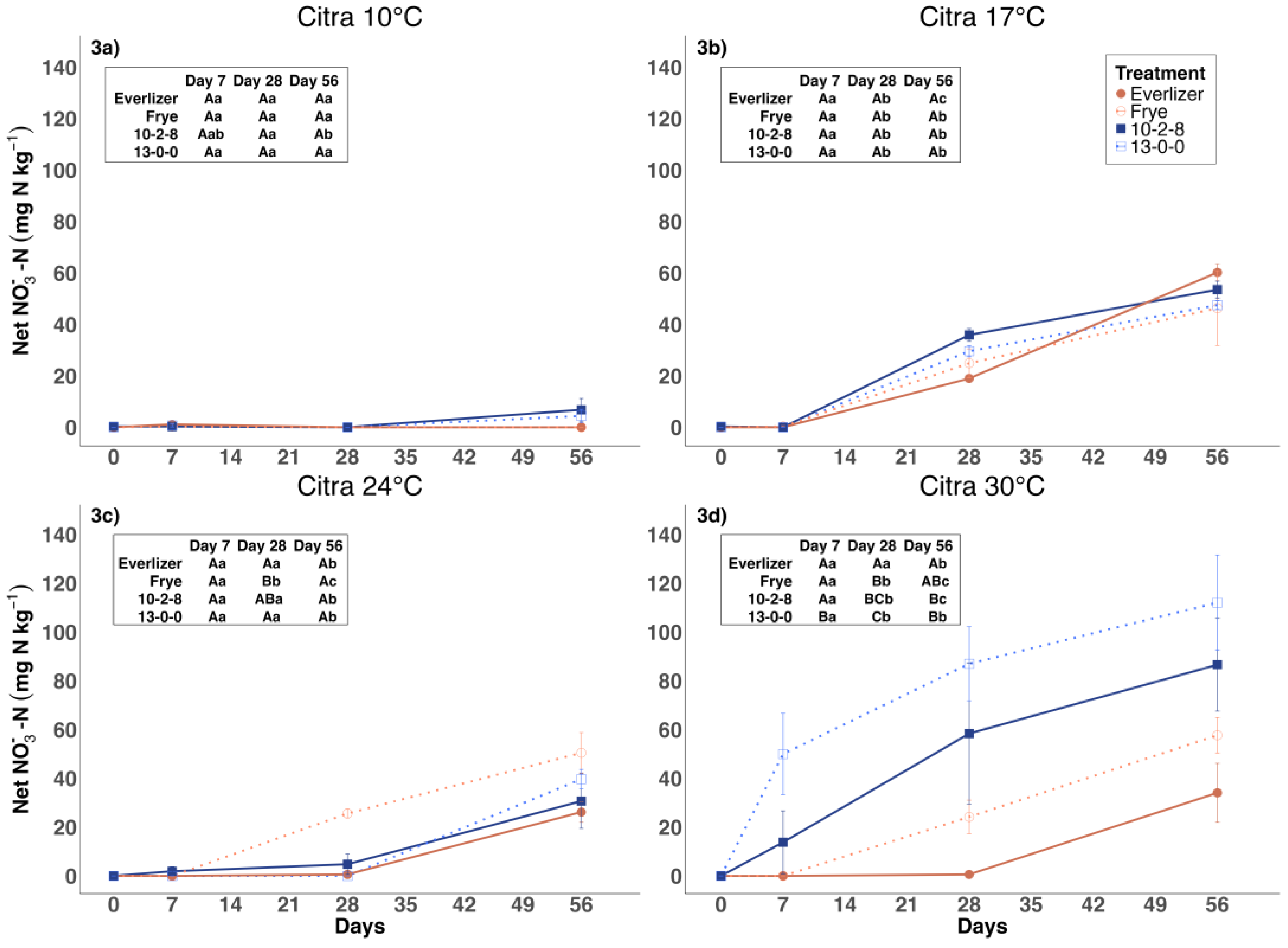
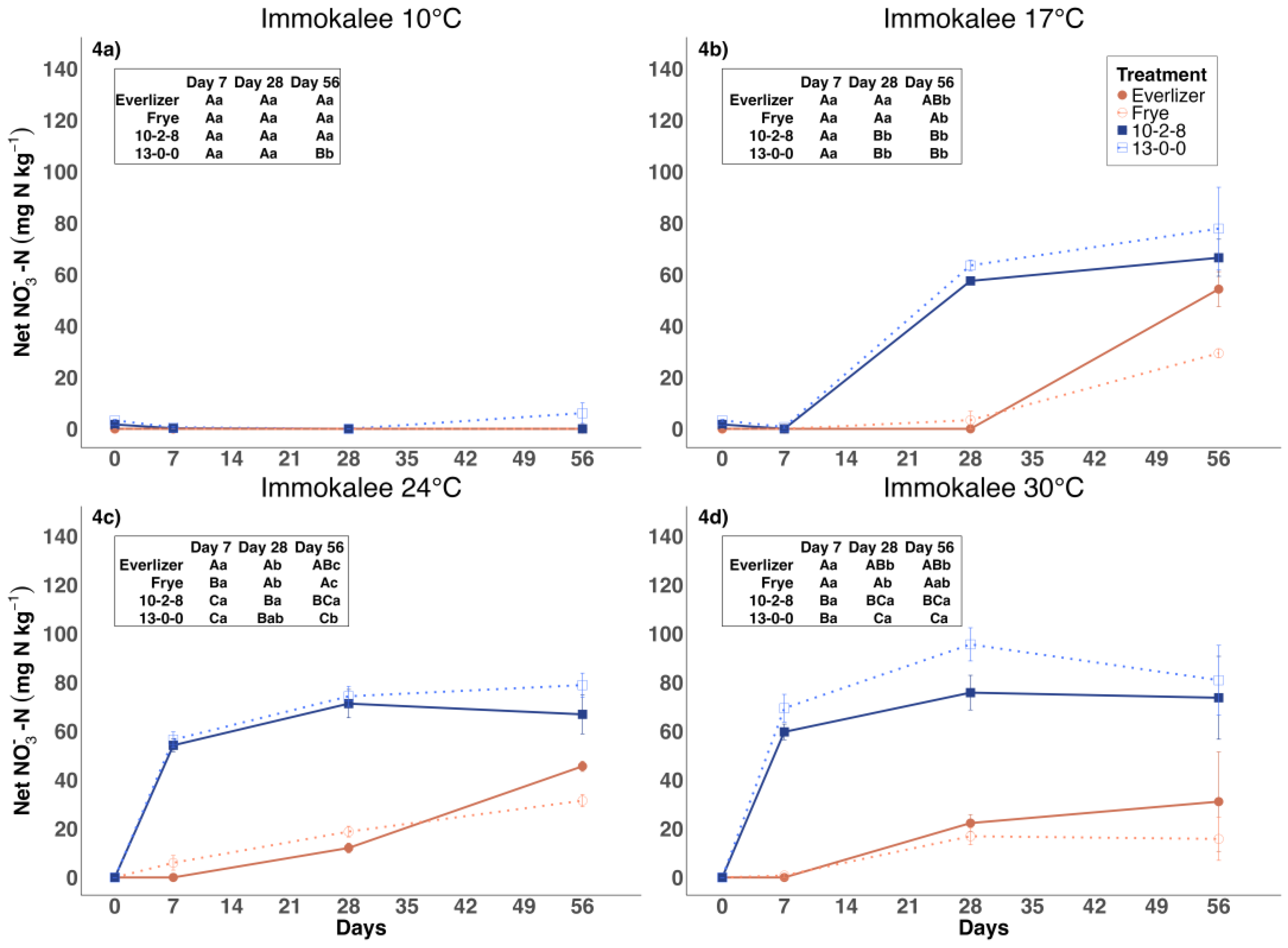
3.2. Nitrate Nitrogen
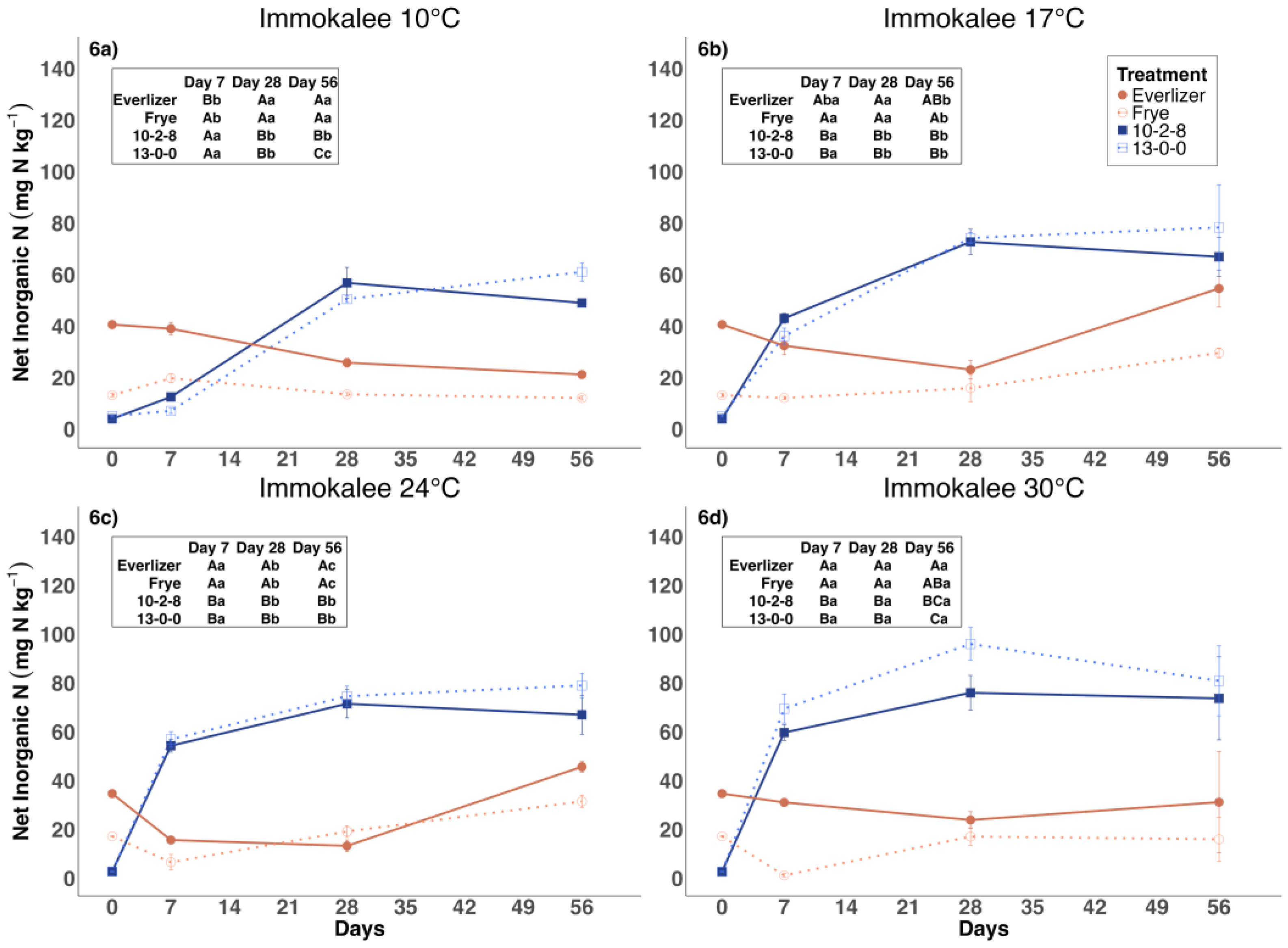
3.3. Inorganic Nitrogen
3.4. CO2-C Emissions
4. Discussion
4.1. Effects of Amendment Type and Temperature on Nitrogen Release
4.2. Effects of Amendment Type and Temperature on CO2 Emissions
4.3. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabry, K. Synthetic fertilizers; role and hazards. Fertil. Technol. 2015, 1, 110–133. [Google Scholar] [CrossRef]
- Nair, V.D.; Nair, P.K.R.; Dari, B.; Freitas, A.M.; Chatterjee, N.; Pinheiro, F.M. Biochar in the agroecosystem-climate-change-sustainability nexus. Front. Plant Sci. 2017, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.; ten Berge, H.F.M.; de Ruiter, P.C.; Jørgensen, H.B.; Kowalchuk, G.A.; Bloem, J. Plant biomass, soil microbial community structure and nitrogen cycling under different organic amendment regimes; a 15N tracer-based approach. Appl. Soil Ecol. 2016, 107, 251–260. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Gale, E.S.; Sullivan, D.M.; Cogger, C.G.; Bary, A.I.; Hemphill, D.D.; Myhre, E.A. Estimating plant-available nitrogen release from manures, composts, and specialty products. J. Environ. Qual. 2006, 35, 2321–2332. [Google Scholar] [CrossRef]
- Geisseler, D.; Smith, R.; Cahn, M.; Muramoto, J. Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. J. Environ. Qual. 2021, 50, 1325–1338. [Google Scholar] [CrossRef]
- Kelley, A.; Wilkie, A.C.; Maltais-Landry, G. Food-based composts provide more soil fertility benefits than cow manure-based composts in sandy soils. Agriculture 2020, 10, 69. [Google Scholar] [CrossRef]
- Agehara, S.; Warncke, D.D. Soil moisture and temperature effects on nitrogen release from organic nitrogen sources. Soil Sci. Soc. Am. J. 2005, 69, 1844–1855. [Google Scholar] [CrossRef]
- de Jesus, H.I.; Cassity-Duffey, K.; Dutta, B.; da Silva, A.L.B.R.; Coolong, T. Influence of soil type and temperature on nitrogen mineralization from organic fertilizers. Nitrogen 2024, 5, 47–61. [Google Scholar] [CrossRef]
- Haynes, R.J. The decomposition process: Mineralization, immobilization, humus formation, and degradation. In Mineral Nitrogen in the Plant-Soil System; Haynes, R.J., Ed.; Academic Press: Cambridge, MA, USA, 1986; pp. 52–126. [Google Scholar]
- van der Sloot, M.; Kleijn, D.; de Deyn, G.B.; Limpens, J. Carbon to nitrogen ratio and quantity of organic amendment interactively affect crop growth and soil mineral N retention. Crop Environ. 2022, 1, 161–167. [Google Scholar] [CrossRef]
- Janssen, B.H. Nitrogen mineralization in relation to C ratio and decomposability of organic materials. Plant Soil 1996, 181, 39–45. [Google Scholar] [CrossRef]
- Delin, S.; Stenberg, B.; Nyberg, A.; Brohede, L. Potential methods for estimating nitrogen fertilizer value of organic residues. Soil Use Manag. 2012, 28, 283–291. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2019, 49, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Sikora, L.; Szmidt, R.; Stofella, P.; Kahn, B. Nitrogen sources, mineralization rates and nitrogen nutrition benefits to plants of compost. In Compost Utilization in Horticultural Cropping Systems; Stofella, P.J., Kahn, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 287–305. [Google Scholar]
- Żołnowski, A.C.; Bakuła, T.; Rolka, E.; Klasa, A. Effect of Mineral–Microbial Deodorizing Preparation on the Value of Poultry Manure as Soil Amendment. Int. J. Environ. Res. Public Health 2022, 19, 16639. [Google Scholar] [CrossRef]
- Olfs, H.-W.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil- and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.R. Nitrogen availability from high-nitrogen-containing organic fertilizers. HortTechnology 2006, 16, 39–42. [Google Scholar] [CrossRef]
- Cassity-Duffey, K.; Cabrera, M.; Franklin, D.; Gaskin, J.; Kissel, D. Effect of soil texture on nitrogen mineralization from organic fertilizers in four common southeastern soils. Soil Sci. Soc. Am. J. 2020, 84, 534–542. [Google Scholar] [CrossRef]
- Cassity-Duffey, K.; Cabrera, M.; Gaskin, J.; Franklin, D.; Kissel, D.; Saha, U. Nitrogen mineralization from organic materials and fertilizers: Predicting N release. Soil Sci. Soc. Am. J. 2020, 84, 522–533. [Google Scholar] [CrossRef]
- Nyabami, P.; Maltais-Landry, G.; Lin, Y. Nitrogen release dynamics of cover crop mixtures in a subtropical agroecosystem were rapid and species-specific. Plant Soil 2023, 492, 399–412. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Harris, W.; Hochmuth, G. Agricultural Soils of Florida. 2019. Available online: https://edis.ifas.ufl.edu/publication/SS655 (accessed on 1 July 2024).
- UF IFAS Extension. FAWN Soil Temperature. University of Florida. 2024. Available online: https://fawn.ifas.ufl.edu (accessed on 1 July 2024).
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online: https://soilseries.sc.egov.usda.gov (accessed on 1 July 2024).
- Food and Agriculture Organization of the United Nations. World Reference Base. Available online: https://www.fao.org/soils-portal/data-hub/soil-classification/world-reference-base/en/ (accessed on 1 November 2024).
- Allar, J.; Maltais-Landry, G. Limited benefits of summer cover crops on nitrogen cycling in organic vegetable production. Nutr. Cycl. Agroecosyst. 2022, 122, 119–138. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Should soil testing services measure soil biological activity? Agric. Environ. Lett. 2016, 1, 150009. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Flavel, T.C.; Murphy, D.V. Carbon and nitrogen mineralization rates after application of organic amendments to soil. J. Environ. Qual. 2006, 35, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Buragienė, S.; Šarauskis, E.; Romaneckas, K.; Adamavičienė, A.; Kriaučiūnienė, Z.; Avižienytė, D.; Marozas, V.; Naujokienė, V. Relationship between CO₂ emissions and soil properties of differently tilled soils. Sci. Total Environ. 2019, 662, 786–795. [Google Scholar] [CrossRef]
- Juhász, C.; Huzsvai, L.; Kovács, E.; Kovács, G.; Tuba, G.; Sinka, L.; Zsembeli, J. Carbon dioxide efflux of bare soil as a function of soil temperature and moisture content under weather conditions of warm, temperate, dry climate zone. Agronomy 2022, 12, 3050. [Google Scholar] [CrossRef]
- Raich, J.W.; Kaiser, M.S.; Dornbush, M.E.; Martin, J.G.; Valverde-Barrantes, O.J. Multiple factors co-limit short-term in situ soil carbon dioxide emissions. PLoS ONE 2023, 18, e0279839. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Pinotti, C.R.; Hirai, W.Y.; de Moraes, M.L.T.; Montanari, R.; Filho, M.C.M.T.; Milori, D.M.B.P.; Júnior, N.L.S.; Panosso, A.R. CO₂ emission and its relation to soil temperature, moisture, and O₂ absorption in the reforested areas of Cerrado biome, Central Brazil. Plant Soil 2019, 444, 193–211. [Google Scholar] [CrossRef]
- Nyberg, M.; Hovenden, M.J. Warming increases soil respiration in a carbon-rich soil without changing microbial respiratory potential. Biogeosciences 2020, 17, 4405–4420. [Google Scholar] [CrossRef]
- Bezyk, Y.; Dorodnikov, M.; Górka, M.; Sówka, I.; Sawiński, T. Temperature and soil moisture control CO2 flux and CH2 oxidation in urban ecosystems. Geochemistry 2023, 83, 125989. [Google Scholar] [CrossRef]
- Schipper, L.A.; Hobbs, J.K.; Rutledge, S.; Arcus, V.L. Thermodynamic theory explains the temperature optima of soil microbial processes and high Q10 values at low temperatures. Glob. Chang. Biol. 2014, 20, 3578–3586. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sánchez-Monedero, M.A.; Paredes, C.; Roig, A. Carbon mineralization from organic wastes at different composting stages during their incubation with soil. Bioresour. Technol. 1998, 64, 179–187. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K.; Schimel, J.P. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]



| Soil | Clay | Silt | Sand | pH | Total C | Total N | P | K | Ca | Mg | S |
| ________ % _______ | _______ % _______ | __ mg kg−1 __ | |||||||||
| Citra | 0.7 | 2.6 | 96.7 | 6.6 | 0.62 | 0.04 | 87 | 11 | 559 | 11 | 6 |
| Immokalee | 0.7 | 3.5 | 95.9 | 6.9 | 1.60 | 0.14 | 23 | 37 | 882 | 13 | 10 |
| Amendment | Type | Total C | Total N | C:N | C Inputs | N Inputs | P2O5 | K2O |
| ________ % ________ | ______ mg kg−1 ______ | ____ % ____ | ||||||
| Everlizer | Heat-treated poultry manure | 30.3 | 3.1 | 9.8 | 3710 | 369 | 4.0 | 5.2 |
| Frye | Charred poultry manure | 28.0 | 3.4 | 8.3 | 3875 | 373 | 5.7 | 6.6 |
| 10-2-8 | Protein-based fertilizer | 40.2 | 10.8 | 3.7 | 499 | 125 | 2.4 | 7.7 |
| 13-0-0 | Protein-based fertilizer | 45.3 | 12.6 | 3.6 | 428 | 113 | 1.1 | 0.7 |
| Soil | Amendment | 10 °C | 17 °C | 24 °C | 30 °C |
|---|---|---|---|---|---|
| Citra | Everlizer | 7% ab | 16% a | 7% a | 9% a |
| Frye | 4% a | 12% a | 14% a | 16% ab | |
| 10-2-8 | 58% c | 44% a | 25% a | 69% bc | |
| 13-0-0 | 48% bc | 42% a | 35% a | 99% c | |
| Immokalee | Everlizer | 6% a | 15% ab | 12% ab | 8% a |
| Frye | 3% a | 8% a | 8% a | 4% a | |
| 10-2-8 | 39% b | 54% b | 54% ab | 59% b | |
| 13-0-0 | 54% b | 69% b | 70% b | 72% b |
| Soil | Amendment | 10 °C | 17 °C | 24 °C | 30 °C |
|---|---|---|---|---|---|
| Citra | Everlizer | 10.4% d | 14.5% d | 14.1% d | 14.8% d |
| Frye | 6.4% c | 8.4% c | 9.0% c | 8.8% c | |
| 10-2-8 | 5.1% c | 4.6% b | 3.8% b | 5.3% b | |
| 13-0-0 | 2.8% b | 4.0% b | 3.4% b | 5.9% b | |
| Control | 0.7% a | 0.6% a | 1.4% a | 1.9% a | |
| Immokalee | Everlizer | 6.7% d | 9.3% d | 9.8% c | 11.1% c |
| Frye | 3.8% c | 5.7% c | 5.3% b | 6.8% b | |
| 10-2-8 | 2.7% b | 3.9% b | 4.7% b | 5.6% ab | |
| 13-0-0 | 2.3% b | 3.3% b | 4.8% b | 6.3% ab | |
| Control | 0.7% a | 1.5% a | 3.4% a | 4.9% a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, C.; Tucker, K.; Victores, S.; Lin, Y.; Obreza, T.; Maltais-Landry, G. Nitrogen and Carbon Mineralization from Organic Amendments and Fertilizers Using Incubations with Sandy Soils. Agriculture 2024, 14, 2009. https://doi.org/10.3390/agriculture14112009
Gil C, Tucker K, Victores S, Lin Y, Obreza T, Maltais-Landry G. Nitrogen and Carbon Mineralization from Organic Amendments and Fertilizers Using Incubations with Sandy Soils. Agriculture. 2024; 14(11):2009. https://doi.org/10.3390/agriculture14112009
Chicago/Turabian StyleGil, Cristina, Kaitlyn Tucker, Samantha Victores, Yang Lin, Thomas Obreza, and Gabriel Maltais-Landry. 2024. "Nitrogen and Carbon Mineralization from Organic Amendments and Fertilizers Using Incubations with Sandy Soils" Agriculture 14, no. 11: 2009. https://doi.org/10.3390/agriculture14112009
APA StyleGil, C., Tucker, K., Victores, S., Lin, Y., Obreza, T., & Maltais-Landry, G. (2024). Nitrogen and Carbon Mineralization from Organic Amendments and Fertilizers Using Incubations with Sandy Soils. Agriculture, 14(11), 2009. https://doi.org/10.3390/agriculture14112009






