Abstract
The availability of genome-sequencing and genome-editing techniques has increased the applicability of innovative solutions, opening up revolutionary prospects for developments in horticultural plant breeding. The Cucurbitaceae family is a group of plants of great importance in horticulture due to their high nutritional and economic value. These plants serve as important models for elucidating the principles of plant development and refining yield improvement strategies. While traditional breeding approaches have made significant contributions to the production of cucurbits, they have also been limited by the reduced genetic diversity and lower rates of variation inherent in these species. This comprehensive review summarises the latest developments in genome editing in cucurbits. It covers various aspects of enhancing plant traits to resist biotic stresses such as pathogenic fungi and viruses, as well as abiotic stresses such as adverse climate change, especially stresses caused by drought and salinity. This study focused on improvements in plant quality and on the optimisation of plant architecture, sex determination of flowers and fruit features. This review provides insights that may hold great promise for the future of horticultural crop improvement and serves as an important reference for the advancement of genome-sequencing and gene-editing technologies in cucurbits.
1. Introduction
Growing human populations combined with the challenges posed by climate change highlight the need for resilient and high-yielding crops. Genome-editing technologies such as CRISPR/Cas9 systems have the potential to contribute to groundbreaking advances in plant breeding. However, in order to use these techniques effectively, an in-depth knowledge of plant genomics, physiology and ecology is essential [1,2]. Through genome sequencing and functional analyses, researchers have gained insights into the genes and proteins that play pivotal roles in key aspects of crop development, offering a multitude of possibilities for enhancing the traits of selected plants [3]. Trait association studies provide a wide range of knowledge that can be used to plan new traits in crop development, including basic information on phenotypic traits, genomic and transcriptomic data, and knowledge from epigenetic studies through comparative analysis across a wide range of plants (Figure 1).
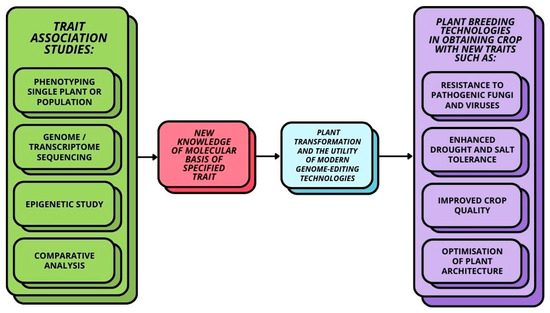
Figure 1.
Overall scheme of modern technology in plant breeding.
Next-generation sequencing technologies have significantly improved this cognitive process, offering a cost-effective and efficient analysis of DNA or RNA at high throughput rates [4]. Sequencing offers insight into the genetics of organisms, including specific gene identification, and provides an understanding of gene function and interactions [5]. This includes the ability to interpret complex genetic information, decipher regulatory elements and identify the genetic basis of traits, including complex traits [6].
Using this specific information, researchers and breeders have acquired efficient and powerful tools for precise manipulation of genetic material through precise genome-editing techniques. CRISPR/Cas9-based genome-editing techniques have triggered a revolution in genetic modification, allowing the targeted modification of specific genes [7]. Genome editing offers unprecedented opportunities to apply an understanding of genomics to improve crop traits in many plant species, including in Cucurbits.
Cucurbitaceae is a plant family belonging to the order Cucurbitales, consisting of over 965 species grouped into 95 genera [8]. Members of the Cucurbitaceae family are vulnerable to frost and are primarily found in warmer regions of the world. Those that extend into temperate zones either survive winters underground below the frost line as tubers or exist as annuals, enduring winter in the form of seeds. The Cucurbitaceae family is most prevalent in tropical regions, particularly in tropical Africa and the neotropics [9]. Cucurbits have been integral to human nutrition and culture for over 12,000 years in both the Old and New Worlds. Consequently, alongside the Brassicaceae and Asteraceae families, the Cucurbitaceae family has exceptional significance for humans, trailing only cereals and legumes in economic importance, which makes cucurbits significant candidates for genetic enhancement [10]. Notably, the genera Cucumis, Cucurbita and Citrullus are deemed highly economically important [11]. Apart from these globally cultivated genera, there are other noteworthy cucurbit genera of local or regional economic importance, including Benincasa, Lagenaria, Luffa, Momordica and Sechium [10].
As many cucurbit genomes are known, it has been possible to use the CRISPR/Cas9 technique to target the enhancement of crucial agricultural traits [12], such as improvements in crop features, plant quality and architecture, influence on plant sex determination, resistance to biotic or abiotic stresses, including the very important aspects of greater tolerance to adverse climate change to cope with drought and salinity. Climate change constitutes a barrier to the cultivation of cucurbit crops. The increasing pressure from environmental stresses, along with extreme natural events, presents a challenge to the development of new varieties that are resilient to environmental conditions [1]. Climate change affects plants in two main ways: abiotic stress (non-living factors) and biotic stress (living factors). Rising carbon dioxide (CO2) levels help some plants grow, but other factors such as higher temperatures and radiation can harm them, leading to desertification and more severe droughts [13]. Some regions experience droughts followed by heavy rains, causing soil salinisation [14,15]. Drought is a major problem for crops, particularly water-demanding ones such as cucumbers. It not only reduces crop quantity, but quality too. In the last decade, droughts have cost about USD 30 billion in crop losses worldwide [16]. Plants are also sensitive to soil salinity, which comes in two forms: osmotic stress (from sodium ions) and ionic stress (from toxic ions). Climate change also affects plants by exposing them to new pests and diseases due to changing temperatures. This can lead to the emergence of new strains of pathogens [17,18]. Understanding the molecular basis of a plant’s reaction to changing environmental conditions and discerning the determinants contributing to multi-stress tolerance are fundamental goals in crop breeding and research on stresses in plants [19].
New and rapid technologies are therefore needed to produce varieties with the desired characteristics. One such technology is the ability to edit the genome precisely. CRISPR/Cas9 genome editing has ushered in a new era of significance in breeding and agriculture thanks to its precision and versatility. This revolutionary technology allows scientists to modify the DNA of plants precisely, offering several crucial advantages. Traditional breeding methods can be time-consuming and imprecise, whereas CRISPR/Cas9 enables specific gene edits with unprecedented accuracy. Speed is essential in addressing food security challenges caused by a growing global population.
This paper presents the accomplishments of genome editing within the Cucurbitaceae family, discussing its potential to revolutionise crop improvement, as well as the challenges and applications of this technique. By analysing new research and technological advancements, this paper aims to examine the potential opportunities and intricate methodologies to enhance the genetic capacity of cucurbit crops. One of its primary objectives is to elucidate how modern genomic techniques, with CRISPR as a central player, enable researchers to unlock the genetic potential of cucurbit plants. By sequencing their genomes and utilising CRISPR technology, scientists can precisely edit specific genes responsible for desirable traits such as disease resistance, improved yield and enhanced nutritional content. The article aims to show how CRISPR technology has become an invaluable tool for enhancing crucial plant traits and enables the development of cucurbit varieties that are more resilient to climate change, environmental stressors, pests and diseases. Furthermore, CRISPR allows for the improvement of traits relevant to human nutrition, thus contributing to food security and health. By harnessing the power of CRISPR, the article emphasises how the process of crop breeding in Cucurbitaceae can be significantly accelerated. This acceleration is vital in order to address the ever-growing global demand for food production and ensure the sustainability of agriculture.
2. Genome Sequencing
2.1. Tracing Advances in Sequencing Technologies
The overall history of genome sequencing began with the publication of an article by Frederick Sanger and his colleagues [20] in 1977 featuring the first-ever genome sequence. In detail, it was the DNA sequence of bacteriophage φX174 with only 5386 bp. Sanger was awarded his second Nobel Prize in Chemistry in 1980, shared with Walter Gilbert and Paul Berg, for developing and improving sequencing methods. It was the era of first-generation sequencing methods. Mankind had to wait 23 years, until 2000, to learn the DNA sequence of the first plant: Arabidopsis thaliana, a popular model organism in plant biology and genetics [21]. The human desire to understand the basic building blocks of life has driven the creation and development of sequencing technologies. Since the completion of the Human Genome Discovery Project [22], genomics has advanced rapidly around the world. It launched the development of new sequencing technologies based on the shotgun technique to learn about the human genome. These technologies are now known as second-generation sequencing technologies. The evolution of next-generation sequencing (NGS) techniques represents a significant advancement in the field of genomics and molecular biology and has been widely described [23]. NGS technologies have revolutionised the way genetic information is studied and understood.
First-generation sequencing involved the use of chemicals to cleave bases within a DNA molecule or of chain-terminating nucleotides, followed by manual separation of fragments generated via electrophoresis [23]. Second-generation sequencing technology emerged in 2005 and marked the arrival of a new generation of sequencers to address the limitations of the first generation. The basic characteristics of second-generation sequencing technology are the generation of many millions of short reads in parallel and an acceleration of the sequencing of the process compared with the first generation. Third-generation sequencing, also known as long-read sequencing, is a class of DNA sequencing that has the capability to produce substantially longer reads than second-generation sequencing. Third-generation sequencing technologies allow direct sequencing of single DNA molecules, but they have much higher error rates than previous technologies, which can complicate downstream genome assembly and analysis of the resulting data. Third-generation sequencing technologies are undergoing active development, and it is expected that there will be further improvements in future [24,25]
Second- and third-generation sequencing technologies have greatly expanded knowledge not only of the code stored in DNA, but also of its function, interactions, modifications and evolutionary context [26]. Second-generation technologies have emerged as a faster, more accurate and more cost-effective alternative to Sanger sequencing pioneered by platforms such as Illumina’s Solexa, Roche’s 454 and ABI’s SOLiD, and allow the sequencing process to be carried out on multiple samples at the same time, enabling millions of fragments to be sequenced simultaneously [4]. This enormous throughput has been achieved by bridging amplification or emulsion PCR, followed by cyclic synthesis or pyrosequencing. The development of NGS technology has made whole-genome sequencing possible, revealing previously undetected variants, mutations and structural changes. Whole-genome sequencing of RNA transcriptomes (RNA-Seq) has also become achievable, providing a snapshot of gene expression at any point in time, enabling transcriptome mapping and the detection of alternative splicing events [27]. An important step in understanding the complexity of genomes has been the development of third-generation sequencing technologies, often referred to as long-read sequencing, which have moved away from amplification-based NGS methods. Pacific Biosciences (PacBio) and Oxford Nanopore Technologies (ONT) are pioneers in this field [4,26]. While PacBio’s single-molecule real-time sequencing (SMRT) uses zero-mode waveguides to monitor the incorporation of individual nucleotides, ONT’s technology uses nanopores to detect changes in electrical currents as DNA strands move through them [28]. By obtaining longer and longer reads of sequence fragments, scientists have been able to detect large structural changes, inversions and translocations in genomes with greater accuracy. It has also allowed for a more complete analysis of repetitive regions, which had been a major challenge for second-generation sequencing read assembly algorithms. In addition, SMRT sequencing has made it possible to directly detect DNA modifications such as methylation without the need for bisulfite, revealing complex layers of epigenetic regulation [29].
NGS methods have become more prevalent, allowing for the rapid and cost-effective sequencing of large portions of genomes or even entire genomes [30]. This has led to the possibility of reference genomes being created. However, the resequencing of genomes is also worthy of mention. The primary purpose of resequencing is not to determine the sequence itself but to identify variations or mutations in the DNA of a specific individual or species compared with the reference [30]. While sequencing provides fundamental insights into the genetic makeup of an organism, resequencing offers insights into genetic diversity and evolution, and can help identify mutations associated with diseases or specific traits [31].
Also noteworthy is epigenetics research, the study of heritable changes in gene function that are not accompanied by changes in the underlying DNA sequence. They play a key role in plant development, stress response and adaptation. One of the most powerful tools in modern epigenetic research is NGS, which provides a high-resolution, genome-wide view of epigenetic changes. In plants, NGS has revolutionised the understanding of epigenomics and its impact on phenotype. DNA methylation, the addition of a methyl group to cytosine or adenine DNA nucleotides, is the major epigenetic change in plants that leads to inhibition of gene expression. Whole-genome bisulfite sequencing (WGBS), an NGS-based method, is widely used to generate comprehensive methylation maps in various plant genomes [32]. Chromatin immunoprecipitation sequencing (ChIP-Seq) is a technique for studying protein–DNA interactions, such as transcription factor binding sites or the locations of histone modifications throughout the genome. In plants, ChIP-Seq has played a key role in identifying the distribution of various histone modifications and elucidating their role in processes such as flowering time regulation, seed development and response to biotic and abiotic stresses [33]. Small RNAs (sRNAs), typically 20–24 nucleotides in length, play a key role in gene silencing and endogenous gene regulation in plants. NGS-based sRNA sequencing enables the identification and quantification of these small RNA molecules, leading to insights into their biogenesis, modification and function [34]. Another level of epigenetic regulation is chromatin accessibility, which often correlates with active gene expression. The assay for transposase-accessible chromatin with sequencing (ATAC-Seq) is an NGS-based method that examines open regions of chromatin. In plants, ATAC-Seq has been used to study dynamic changes in chromatin accessibility during processes such as fruit ripening and seed germination [35]. Next-generation sequencing has had a groundbreaking impact on unravelling complex epigenetic networks in plants. As the technology continues to evolve and computational tools improve, knowledge of plant epigenetics will only increase, paving the way for innovative applications in agriculture and plant biology.
In addition, genome-wide association studies (GWAS) use sequenced genomes to correlate specific genetic changes with observable traits [36]. These methods have allowed the detection of genomic variants associated with either traditional agronomic phenotypes or biochemical and molecular phenotypes. These associations, in turn, enable applications in gene cloning and accelerated crop breeding through marker-assisted selection or genetic engineering. Furthermore, the integration of genome sequencing with transcriptomics has enabled the identification of regulatory elements such as enhancers and promoters, providing insight into the diverse regulatory networks that control gene expression [37].
When comparing second- and third-generation sequencing technologies, it is worth noting that while second-generation sequencing offers high sequencing throughput at a reduced cost per base, it is unable to read longer DNA fragments, making de novo assembly and detection of structural variants difficult. However, it offers much longer reads, often exceeding 10 kb or even 100 kb, at the cost of slightly lower accuracy. Combining the strengths of both technologies has become a common strategy in modern genomics to ensure comprehensive genome analyses.
However, the development of NGS technologies has revolutionised the view of the genetic code and its functions. With NGS democratising access to genomic data and providing unprecedented transparency of complex genomic structures, the understanding of how genes interact in complex organisms has improved immeasurably, paving the way for innovations in medicine, agriculture and environmental sciences.
2.2. Cucurbits Genomes, Complexity and Characterisation
Plant genomes are inherently complex, often characterised by polyploidy, extensive repetitive sequences and epigenetic modifications [38,39,40]. Comprehensive knowledge of these complexities is essential for the design of effective genome-editing strategies. A well-described genome sequence is the foundation of genome editing. However, an understanding of gene function, regulatory elements and synteny is equally important [41]. Genome complexity is influenced by factors such as repeated DNA sequences, polyploid events and transposable elements (TEs). TEs can change their position in the genome and contribute significantly to the size and complexity of the plant genome, especially retrotransposons with long terminal repeats [42]. TEs not only enlarge the genome but also induce genome reorganisation and affect gene regulation, providing an evolutionary playground for adaptation and diversification [43]. In addition, complex regulatory networks modulate gene expression in response to environmental cues. The presence of cis-regulatory elements, transcription factor binding sites and epigenetic markers adds another layer of complexity to the genome, facilitating adaptation and phenotypic plasticity [44]. Understanding the complexity of the plant genome is of great importance in the era of precision agriculture. Complex genome annotation, facilitated by advanced sequencing technologies, is paving the way for targeted crop improvement. By unravelling the complex network of genes, regulatory elements and structural changes, breeders can improve yields, resistance and nutritional profiles [45]. Focusing on the genomes of plants in the Cucurbitaceae family, the year 2009 represented a significant milestone as it provided the first genome within this family and, additionally, the genome of the first vegetable crop, i.e., cucumber (Cucumis sativus) [46]. Since then, scientists around the world have described the genomes of 87 different plant varieties and cultivars within the Cucurbitaceae family. Summarised data on sequenced genomes and the annual distribution of papers on this topic are presented in Figure 2. Moreover, detailed information on the cultivars, sequencing technologies, chromosome number, genome size and coverage, as well as the number of scaffolds, contigs and protein-coding genes, is presented in Table 1. Information concerning whether a given genome is a reference genome comes from the National Center for Biotechnology Information (NCBI) [47] and the updated database CuGenDBv2 (Cucurbit Genomics Database) [48].

Figure 2.
Overview of sequenced genomes (n = 88) and the annual distribution of publications (n = 46, black solid line) that refer to genome sequencing in the Cucurbitaceae family.
As mentioned above, the cucumber species was the first in this family to be sequenced. Cucumis sativus, commonly known as cucumber, is characterised by well-established reference lines, such as 9930 (a Chinese line) and Gy14 (a North American line) and the longest B10v3 (North European) [49,50,51]. Recently, a system for integrating experimental data with high-throughput genomic data was used by Turek et al. [52], where a model of genome comparison and a merging of experimental data were proposed [52]. As more and more genomes are sequenced and assembled, comparative genomics methods and adaptive genome drafts are increasingly needed to standardise gene location and annotation information. Research is also being carried out on so-called pangenomes, an example of which is the research on Cucumis sativus, in which a pangenome was created using 12 sequences of cucumber lines [53]. This analysis revealed structural, functional and sequence differences related to agronomic and domestication traits. The resulting pangenome contains information from cucumber genomes assembled at the chromosome level, highlighting the need for a comprehensive reference that captures structural details at the gene level as well as larger chromosomal structures [53].

Table 1.
Summary of the sequenced genomes in the Cucurbitaceae family.
Table 1.
Summary of the sequenced genomes in the Cucurbitaceae family.
| Species | Cultivar | Year | Sequencing Technologies | Chromosome Number | Genome Size (Mb) | Percentage Assembly | Genome Coverage | Number of Scaffolds | Number of Contigs | Protein-Coding Genes | Reference Genome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cucumber (C. sativus var. sativus) | “Chinese long” inbred line 9930 | 2019 | PacBio RSII, PacBio Sequel, 10x Genomics, and Hi-C technologies | 7 | 226.2 | 93.3 | 50.0x | 85 | 174 | 24,317 | YES | [49] |
| “Chinese long” inbred line 9930 | 2009 | Sanger and Illumina GA | 7 | 243.5 | 72.8 | 72.2x | 47,837 | 62,412 | 26,682 | No | [46] | |
| Borszczagowski | 2011 | 454 Sequencing and Sanger—Celera/Arachne | 7 | 323.0 | N/A * | 12.0x | 13,129 | 16,547 | 26,587 | No | [54] | |
| Gy14 | 2012 | No data | 7 | 173.1 | 86.0 | 4.3x | 244 | N/A | N/A | No | [50] | |
| B10v3 (Borszczagowski) | 2020 | PacBio RS II and Illumina HiSeq 2000 | 7 | 342.3 | 93.9 | 69.8x | N/A | 8035 | 27,271 | No | [51] | |
| MSC19 | 2020 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 32.5x | N/A | 8035 | 27,271 | No | [55] | |
| 320 | 2020 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 36.8x | N/A | 8035 | 27,271 | No | ||
| y-gc | 2020 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 34.8x | N/A | 8035 | 27,271 | No | ||
| 212 | 2021 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 34.6x | N/A | 8035 | 27,271 | No | [56] | |
| 224 | 2021 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 34.6x | N/A | 8035 | 27,271 | No | ||
| 225 | 2021 | Illumina HiSeq 2000 | 7 | 342.3 ** | N/A | 34.5x | N/A | 8035 | 27,271 | No | ||
| XTMC (East Asian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 240.1 | N/A | 53.0x | N/A | 926 | 25,167 | No | [53] | |
| Cu2 (East Asian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 247.1 | N/A | 64.0x | N/A | 851 | 25,382 | No | ||
| Cuc37 (Eurasian line) | 2022 | PacBio RSII and PacBio Sequel, 10x Genomics, and Hi-C | 7 | 238.4 | N/A | 54.0x | 865 | 967 | 24,490 | No | ||
| Gy14 (Eurasian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 239.4 | N/A | 47.0x | N/A | 926 | 25,042 | No | ||
| 9110gt (Eurasian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 242.9 | N/A | 58.0x | N/A | 830 | 24,992 | No | ||
| Cuc80 (Xishuangbanna line) | 2022 | PacBio RSII and PacBio Sequel, 10x Genomics, and Hi-C | 7 | 237.4 | N/A | 47.0x | 887 | 923 | 24,578 | No | ||
| Hx14 (Indian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 234.6 | N/A | 52.0x | N/A | 865 | 24,914 | No | ||
| Hx117 (Indian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 243.7 | N/A | 49.0x | N/A | 1015 | 26,033 | No | ||
| Cucumber (C. sativus var. hardwickii) | PI183967 (CG0002) | 2013 | Illumina GA IIx and Illumina HiSeq 2000 | 7 | 204.8 | 95.3 | 20.9x | 187 | 6113 | 23,836 | No | [56] |
| Cuc64 (Indian line) | 2022 | PacBio RSII and PacBio Sequel, 10x Genomics, and Hi-C | 7 | 232.5 | N/A | 46.0x | 796 | 842 | 24,583 | No | [53] | |
| W4 (Indian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 251.1 | N/A | 58.0x | N/A | 894 | 25,703 | No | ||
| W8 (Indian line) | 2022 | PacBio RSII and PacBio Sequel | 7 | 241.9 | N/A | 56.0x | N/A | 907 | 25,531 | No | ||
| Cucumis hystrix | - | 2021 | PacBio, Illumina HiSeq X-Ten, Illumina HiSeq 2500 and 10x Genomics | 12 | 289.9 | 90.4 | 360.0x | 2284 | 6072 | 23,864 | YES | [57] |
| Cucumis × hytivus | - | 2021 | Illumina HiSeq 2000, Illumina X-Ten, PacBio SMRT, BioNano, and Hi-C | 19 | 540.7 | 97.2 | 104.0x | 562 | 771 | 45,687 | No | [58] |
| Bitter gourd (Momordica charantia) | OHB3-1 | 2016 | Illumina MiSeq and Illumina HiSeq 2500 | 11 | 285.6 | 84.0 | 110.0x | 1052 | 20,427 | 45,859 | YES | [59] |
| OHB3-1 | 2020 | PacBio Sequel and Illumina HiSeq 2500 | 11 | 303.0 | 96.3 | 84.0x | 193 | 221 | N/A | No | [60] | |
| Dali-11 | 2020 | Illumina HiSeq 2000 | 11 | 296.3 | 97.9 | 251.0x | 297 | 8600 | 264,27 | No | [61] | |
| TR | 2020 | Illumina HiSeq 2000 | 11 | 296.3 | 98.7 | 185.0x | 1643 | 23,789 | 28,827 | No | ||
| Bottle gourd (Lagenaria siceraria) | Hangzhou gourd | 2018 | PacBio SMRT and Illumina HiSeq 4000 | 11 | 297.9 | N/A | 77.0x | 27 | 71 | 23,541 | YES | [62] |
| USVL1VR-Ls | 2017 | Illumina HiSeq 2500 | 11 | 313.4 | 93.8 | 395.0x | 444 | 18,083 | 22,472 | No | [63] | |
| Chayote (Sechium edule) | - | 2021 | Nanopore and Hi-C | 14 | 608.2 | 99.7 | 151.0x | 103 | 356 | 28,237 | No | [64] |
| Crookneck pumpkin (Cucurbita moschata) | Rifu | 2017 | Illumina HiSeq 2500 | 20 | 269.9 | 72.6 | 215.5x | 3500 | 17,340 | 32,205 | YES | [65] |
| Herpetospermum pedunculosum | - | 2023 | PacBio Sequel IIe and HiC | 10 | 804.1 | 90.45 | 27.3x | 189 | 250 | 23,924 | YES | [66] |
| Horned cucumber (Cucumis metuliferus) | PI 482,460 (CM27) | 2021 | PacBio SMART and Hi-C | 12 | 329.1 | 98.0 | 93.0x | N/A | 432 | 29,214 | No | [67] |
| Jiaogulan (Gynostemma pentaphyllum) | - | 2023 | DNBSEQ™ and PromethION | 11 | 609.0 | 99.99 | 275.0x | 18 | 158 | 26,588 | YES | [68] |
| - | 2021 | Illumina, PacBio Sequel II, and Hi-C | 11 | 582.9 | 91.65 | 403.0x | 578 | 1232 | 25,285 | No | [69] | |
| Melon (Cucumis melo subsp. melo) | AY | 2022 | PacBio Sequel II | 12 | 438.3 | N/A | 44.0x | 1309 | 1548 | 28,628 | YES | [70] |
| Double-haploid line DHL92 | 2020 | PacBio SMRT and Illumina | 12 | 357.6 | 96.2 | 50.0x | 13 | 1178 | 29,980 | No | [71] | |
| MR1 | 2022 | PacBio Sequel II | 12 | 438.3 | N/A | 53.0x | 1030 | 1374 | N/A | No | [70] | |
| Melon (C. melo) | Double-haploid line DHL92 | 2012 | 454 Sequencing and Sanger | 12 | 374.8 | 83.3 | 13.5x | 1594 | 60,752 | 27,427 | No | [72] |
| Melon (C. melo subsp. agrestis var. chinensis) | BAHC | 2022 | Oxford Nanopore MinION | 12 | 361.9 | N/A | 60.0x | 170 | 247 | 36,981 | No | [73] |
| PI161375 | 2022 | Oxford Nanopore MinION | 12 | 360.1 | N/A | 44.0x | 242 | 458 | 36,593 | No | ||
| Melon (C. melo subsp. agrestis var. conomon) | TOG | 2022 | Oxford Nanopore MinION | 12 | 361.2 | N/A | 46.0x | 161 | 273 | 36,802 | No | [73] |
| Melon (C. melo subsp. agrestis var. makuwa) | SW3 | 2019 | Illumina HiSeq 2500 | 12 | 354.0 | 94.9 | 258.0x | 7202 | 29,154 | 38,173 | No | [74] |
| Chang Bougi | 2019 | Illumina HiSeq 2500 | 12 | 344.0 | 96.9 | 258.0x | 11,309 | 43,251 | 36,235 | No | ||
| ESL | 2022 | Oxford Nanopore MinION | 12 | 358.6 | N/A | 39.0x | 163 | 560 | 36,345 | No | [73] | |
| OHG | 2022 | Oxford Nanopore MinION | 12 | 360.7 | N/A | 54.0x | 113 | 174 | 36,883 | No | ||
| SAS | 2022 | Oxford Nanopore MinION | 12 | 361.0 | N/A | 41.0x | 309 | 432 | 36,725 | No | ||
| Melon (C. melo subsp. agrestis var. momordica) | PI414723 | 2022 | Oxford Nanopore MinION | 12 | 363.4 | N/A | 101.0x | 157 | 230 | 36,458 | No | |
| Melon (C. melo subsp. agrestis) | - | 2020 | Illumina HiSeq, PacBio SMRT, PacBio Sequel, and Hi-C | 12 | 366.2 | 98.2 | 100.0x | 101 | 298 | 28,898 | No | [75] |
| IVF77 | 2021 | PacBio SMART and Hi-C | 12 | 364.3 | 96.3 | 84.0x | N/A | 1698 | 27,073 | No | [67] | |
| Melon (C. melo subsp. melo var. adzhur) | PI164323 | 2022 | Oxford Nanopore MinION | 12 | 367.9 | N/A | 53.0x | 750 | 1029 | 36,394 | No | [73] |
| Melon (C. melo subsp. melo var. ameri) | AY | 2022 | Oxford Nanopore MinION | 12 | 367.5 | N/A | 53.0x | 176 | 280 | 37,183 | No | |
| Melon (C. melo subsp. melo var. cantalupensis) | BEL | 2022 | Oxford Nanopore MinION | 12 | 373.8 | N/A | 54.0x | 142 | 226 | 37,193 | No | [73] |
| NDD1 | 2022 | Oxford Nanopore MinION | 12 | 365.1 | N/A | 59.0x | 208 | 282 | 37,122 | No | ||
| NY | 2022 | Oxford Nanopore MinION | 12 | 365.7 | N/A | 39.0x | 148 | 221 | 36,919 | No | ||
| VEP | 2022 | Oxford Nanopore MinION | 12 | 365.4 | N/A | 48.0x | 392 | 528 | 36,984 | No | ||
| Charmono | 2022 | PacBio RSII, 10x Genomics, and Hi-C | 12 | 366.8 | 99.5 | 100.0x | 43 | 236 | 31,348 | No | [76] | |
| Melon (C. melo subsp. melo var. duda’im) | DUD | 2022 | Oxford Nanopore MinION | 12 | 362.9 | N/A | 49.0x | 214 | 357 | 36,602 | No | [73] |
| Melon (C. melo subsp. melo var. flexuosus) | DOYA | 2022 | Oxford Nanopore MinION | 12 | 366.7 | N/A | 49.0x | 277 | 473 | 36,513 | No | [73] |
| Melon (C. melo subsp. melo var. inodorus) | Payzawat | 2019 | PacBio RSII and Illumina X-Ten | 12 | 386.5 | 94.1 | 81.0x | 623 | 882 | 22,924 | No | [77] |
| BDR | 2022 | Oxford Nanopore MinION | 12 | 366.0 | N/A | 57.0x | 346 | 462 | 37,136 | No | [73] | |
| NA | 2022 | Oxford Nanopore MinION | 12 | 367.3 | N/A | 80.0x | 155 | 239 | 37,259 | No | ||
| PSR | 2022 | Oxford Nanopore MinION | 12 | 368.7 | N/A | 44.0x | 165 | 258 | 37,232 | No | ||
| TAD | 2022 | Oxford Nanopore MinION | 12 | 364.9 | N/A | 72.0x | 98 | 173 | 37,120 | No | ||
| TVT | 2022 | Oxford Nanopore MinION | 12 | 364.8 | N/A | 55.0x | 142 | 245 | 36,970 | No | ||
| Melon (C. melo subsp. melo var. khandalak) | ARJ | 2022 | Oxford Nanopore MinION | 12 | 365.0 | N/A | 72.0x | 327 | 525 | 36,773 | No | |
| INB | 2022 | Oxford Nanopore MinION | 12 | 363.6 | N/A | 45.0x | 117 | 231 | 36,626 | No | ||
| Melon (C. melo subsp. melo var. reticulatus) | Harukei-3 | 2020 | PacBio RSII, Illumina HiSeq 2000, and Oxford Nanopore MinION | 12 | 368.5 | N/A | 73.0x | 80 | 112 | 33,829 | No | [78] |
| DUL | 2022 | Oxford Nanopore MinION | 12 | 365.5 | N/A | 57.0x | 63 | 124 | 36,175 | No | [73] | |
| KRY | 2022 | Oxford Nanopore MinION | 12 | 369.4 | N/A | 54.0x | 295 | 441 | 37,158 | No | ||
| Melon (C. collosus var. feral) | QME | 2022 | Oxford Nanopore MinION | 12 | 363.6 | N/A | 70.0x | 184 | 269 | 36,578 | No | [73] |
| Monk fruit (Siraitia grosvenorii) | - | 2016 | Illumina TSLR | 14 | 420.1 | N/A | 36.9x | 12,772 | 25,166 | N/A | No | [79] |
| “Qingpiguo” variety | 2018 | Illumina HiSeq X-Ten and PacBio SMRT | 14 | 469.5 | N/A | 73.8x | N/A | 4128 | 30,565 | No | [80] | |
| Ridge gourd (Luffa acutangula) | AG-4 | 2020 | PacBio SMRT, Chicago, and HiC | 13 | 735.6 | 92.2 | 47.5x | 7871 | 17,812 | 42,211 | YES | [81] |
| Silver-seed gourd (Cucurbita argyrosperma subsp. sororia) | - | 2021 | Illumina HiSeq 4000 and PacBio Sequel | 20 | 255.2 | 92.8 | 288.4x | 72 | 959 | 30,592 | YES | [82] |
| Silver-seed gourd (C. argyrosperma subsp. argyrosperma) | - | 2019 | Illumina HiSeq 2000, Illumina MiSeq, and PacBio RS II | 20 | 228.8 | 95.6 | 151.0x | 920 | 1481 | 28,298 | No | [83] |
| Snake gourd (Trichosanthes anguina) | - | 2020 | PromethION and Hi-C | 11 | 919.8 | 99.9 | 108.5x | 69 | 202 | 22,874 | No | [84] |
| Sponge gourd/smooth loofah (Luffa cylindrica, syn. L. aegyptiaca) | P93075 | 2020 | Illumina HiSeq X-Ten, 10x Genomics, PacBio Sequel, and Hi-C | 13 | 656.2 | 96.9 | 100.0x | 332 | 480 | 25,508 | YES | [85] |
| - | 2020 | Illumina Hiseq X-Ten, PacBio Sequel, and Hi-C | 13 | 669.7 | 99.5 | 101.0x | 798 | 1156 | 31,661 | No | [86] | |
| Telfairia occidentalis | - | 2022 | Illumina HiSeq | N/A | 745.3 | N/A | 105.0x | 852,383 | 874,487 | N/A | YES | [87] |
| Watermelon (Citrullus lanatus) | 242-1 | 2023 | Oxford Nanopore MinION and Illumina HiSeq 2500 | 11 | 361.7 | 95.9 | 22.0x | N/A | 43 | 23,921 | YES | [88] |
| Charleston Gray | 2019 | Illumina GAIIx, Illumina HiSeq 2500, and Illumina MiSeq | 11 | 396.4 | 94.6 | 228.0x | 2034 | 21,498 | 22,546 | No | [89] | |
| 159-1 | 2023 | Oxford Nanopore MinION and Illumina | 11 | 362.1 | 95.8 | 25.0x | N/A | 103 | 24,451 | No | [88] | |
| Watermelon (C. lanatus subsp. vulgaris) | 97103 | 2013 | Illumina GAII and Illumina HiSeq 2000 | 11 | 353.5 | 83.2 | 108.6x | 1793 | 41,945 | 23,440 | No | [90] |
| Watermelon (C. lanatus subsp. cordophanus) | - | 2021 | PacBio Sequel SMRT, Illumina, and Hi-C technologies | 11 | 367.9 | 84.1 | 388.8x | 33 | 86 | 23,043 | No | [91] |
| Citron melon (C. amarus) | USVL246-FR2 | 2023 | PacBio and Illumina | 11 | 356.8 | 93.6 | 284.2x | 1422 | 38,258 | 22,028 | YES | [92] |
| Colocynth (C. colocynthis) | PI 537277 | 2023 | PacBio and Illumina | 11 | 360.2 | 99.7 | 370.1x | 1536 | 15,928 | 22,723 | YES | |
| Watermelon (C. mucosospermus) | USVL531-MDR | 2023 | PacBio and Illumina | 11 | 365.3 | 99.4 | 84.8x | N/A | 77 | 22,377 | YES | |
| Wax gourd (Benincasa hispida) | B227 | 2019 | PacBio RSII and Illumina HiSeq 4000 | 12 | 913.0 | 94.1 | 50.0x | 2197 | 26,315 | 27,467 | YES | [93] |
| pf3 | 2023 | PacBio Sequel II, Illumina NovaSeq 6000, and Hi-C | 12 | 975.6 | 94.9 | 86.0x | 1862 | 1897 | 31,562 | No | [94] | |
| Winter squash (Cucurbita maxima) | Rimu | 2017 | Illumina HiSeq 2500 | 20 | 271.4 | 70.2 | 282.7x | 8299 | 25,524 | 32,076 | YES | [65] |
| Zucchini (C. pepo subsp. pepo) | MU-CU-16 | 2017 | Illumina HiSeq 2000 | 20 | 263.5 | 93.0 | 198.0x | 26,025 | 32,754 | 27,870 | YES | [95] |
* Not applicable. ** Resequencing performed.
Between 2009 and today, scientists have sequenced a total of 13 cucumber (C. sativus var. sativus) genomes, with their results described in six papers. Two varieties of cucumber have been sequenced: “sativus” and “hardwickii”. The latter is a wild form of cucumber considered to be the progenitor of C. sativus var. sativus. Qi et al. [56] performed de novo sequencing and assembly of this wild cucumber of PI183967 accession. Through the application of Illumina GA IIx and Illumina HiSeq 2000 with 20.9x coverage, a total length of the assembly of 204.8 Mb was obtained, and the authors predicted a total of 23,836 genes [56]. In the study of Li et al. [53], three “hardwickii” Indian line cultivars, W4, W8 and Cuc64, were also sequenced, and the assembled genome size ranged from 232.5 to 251.5 Mb, with the number of protein-coding genes ranging from 24,583 to 25,703. Qin et al. [57] reported the genome assembly of another Cucumis representative, i.e., C. hystrix, a monoecious climbing vine. A genome size of 289.9 Mb was revealed, where 90.4% of the sequences were anchored onto 12 chromosomes and 23,846 genes were annotated. Moreover, a comparative genomic analysis conducted by the authors stated that C. hystrix is phylogenetically closer to cucumber than to melon [57].
Yu et al. [58] undertook sequencing of the Cucumis × hytivus genome, which is a synthetic allotetraploid species consisting of 2n = 4x = 38 chromosomes containing both genomes of two different species described above (C. sativus and C. hystrix). The use of different sequencing approaches (Illumina, PacBio, BioNano, and Hi-C) allowed a total assembly size of 540.7 Mb and 45,687 predicted genes to be obtained, and to date it is the first fully sequenced synthetic allopolyploid [58].
Subsequently, four genomes of bitter gourd (Momordica charantia) have been revealed. The first from the OHB3-1 cultivar, officially recognised by the NCBI as the reference genome, was sequenced in 2017 by Urasaki et al. [59]. M. charantia is characterised by 11 chromosomes, and the authors described a genome size of 285.6 Mb and 45,859 predicted genes. Matsumura et al. [60] again sequenced the genome of this cultivar and using PacBio Sequel and Illumina HiSeq 2500 obtained a size of 303.0 Mb. Cui et al. [61] also contributed to the research on bitter gourds, and two other cultivars, i.e., Dali-11 and TR, were tested. The authors achieved similar results on the genome size, in detail about 296.3 Mb for both samples. In contrast to the reference genome, the number of protein-coding genes was significantly lower (26,427 and 28,827 vs. 45,859) [61].
Among other gourds, Lagenaria siceraria (bottle gourd) was another species under consideration in the study of the genomes of Cucurbitaceae. Both Hangzhou gourd [62] and USVL1VR-Ls [63] varieties were analysed. In the case of the former, a genome size of 297.9 Mb was provided, while for the latter it was 313.4 Mb. Bottle gourds have 11 chromosomes, and in both studies about 23,000 genes were predicted.
Another example of a gourd is chayote (Sechium edule). In 2021, Fu et al. [64] applied Nanopore and Hi-C technologies to obtain a dataset of 151.0x coverage, and then based on the 103 scaffolds and 356 contigs, the authors described the 14 chromosomes of gourd with a relatively large genome size of 608.2 Mb [64].
Rifu and Rimu are the names of two cultivars, Cucurbita moschata (crookneck pumpkin) and C. maxima (winter squash), respectively, for whom Sun et al. [65] reported genome sequences. In both cases, Illumina HiSeq 2500 was used and high-quality reads with the coverage of 215.5x and 282.7x for Rifu and Rimu, respectively, were obtained. Moreover, de novo assemblies resulted in draft genomes of 269.9 and 271.4 Mb, and in comparison with cucumber, a higher number of protein-coding genes were predicted: 32,205 for C. moschata and 32,076 for C. maxima [65].
Moreover, Herpetospermum pedunculosum [66], horned cucumber (Cucumis metuliferus) [67] and jiaogulan (Gynostemma pentaphyllum, n = 2) [68,69] are subsequent species within the Cucurbitaceae family whose genomes have been reported in the last three years.
Interestingly, melon (Cucumis melo) was the most frequently studied species within this plant family in terms of genome sequencing. Over the years, ten papers [67,70,71,72,73,74,75,76,77,78] have described the sequences of different melon subspecies, varieties and cultivars. The first genome sequence of C. melo was revealed in 2012, as the second species after the cucumber genome in the Cucurbitaceae family. The genome of double-haploid line DHL92 was obtained via 454 and Sanger sequencing methods. Low genome coverage by current standards of 13.5x allowed a genome size to be obtained of 374.8 Mb with 83.3% percentage assembly and prediction of 27,427 protein-coding genes [72]. In comparison, sequencing and assembly of C. melo subsp. melo AY, the NCBI reference genome, was accomplished in 2022 [70]. The authors applied PacBio Sequel II with a coverage of 44.0x, and ultimately a genome size of 438.3 Mb was obtained.
An important work contributing greatly to the discovery of melon genomes was published in 2022 by the team of Oren et al. [73]. The authors sequenced the genomes of 25 different melons, i.e., they provided 17 accessions of “melo” subspecies (in detail: 5x inodorus, 4x cantalupensis, 2x reticulatus, 2x khandalak, 1x duda’im, 1x flexuosus, 1x ameri and 1x adzhur cultivars), 7 for “agrestis” subspecies (2x chinensis, 1x conomon, 3x makuwa and 1x momordica), and 1 for C. collosus var. feral, an under-exploited wild melon fruit traditionally used in folk remedies [73]. Among all the sequenced melon genomes, their size ranged from 357.6 to 438.3 Mb, and predicted genes from 22,924 to 38,173 (Table 1).
The development of genome cognition techniques and the interest of different scientific groups in this issue allowed the discovery of the genomes of subsequent cucurbits: monk fruit (Siraitia grosvenorii) [79,80], ridge gourd (Luffa acutangula) [81] and silver-seed gourds, i.e., Cucurbita argyrosperma subsp. sororia [82] or C. argyrosperma subsp. argyrosperma [83]. Moreover, the study by Ma et al. [84] revealed that the genome of the snake gourd (Trichosanthes anguina) is one the largest genomes ever within the Cucurbitaceae family. The use of PromethION and Hi-C technologies with 108.5x coverage led to the genome size of 919.8 Mb being obtained and compared with the large genome the authors described 22,874 protein-coding genes.
More recently, i.e., between 2019 and 2023, the genomes have been identified of well-known tropical vines grown in Asia, i.e., sponge gourd known also as smooth loofah (Luffa cylindrica, syn. L. aegyptiaca) and Telfairia occidentalis. The referenced genome for loofah is organised in 13 chromosomes with a size of 656.2 Mb [85], and the second paper for this species showed a size of 669.7 Mb [86]. In the case of the African fluted pumpkin (T. occidentalis), Pirro et al. [87] applied the Illumina HiSeq sequencing system to reveal its genome (745.3 Mb), where the genome assembly includes 852,383 scaffolds but no assembled chromosomes.
Another important species in relation to a high cultivation rate worldwide is watermelon (Citrullus lanatus). The referenced genome of 242-1 isolate was published in 2023 [88], where the authors utilised Oxford Nanopore MinION and Illumina HiSeq 2500 systems and described the genome size of 361.7 Mb with 22.0x data coverage and 23,921 protein-coding genes. Due to the existence of a huge number of watermelon varieties, the genome sequencing of other subspecies and species has also been attempted several times by other researchers. DNA sequences for the Korean cultivar 159-1 [88], as well as for Charleston Gray [89], a dessert watermelon cultivar, have also been obtained. Moreover, in 2013 the genome of C. lanatus subsp. vulgaris 97,103 was accomplished [90], and in 2021 the sequencing for C. lanatus subsp. cordophanus was performed [91]. In addition, Wu et al. [92] sequenced the genomes of C. amarus, C. colocynthis, and C. mucosospermus, i.e., other wild and cultivated watermelons, for a better understanding of the evolution and domestication of watermelons. All watermelons tested so far have been characterised by a similar genome size, ranging from 353.5 to 396.4 Mb (Table 1).
Finally, the genomes of the two edible cucurbits, i.e., wax gourd (Benincasa hispida) and zucchini (C. pepo subsp. pepo) MU-CU-16, have been sequenced. The genome sequence of the former and its two cultivars, i.e., B227 and pf3, were discovered in 2019 [93] and 2023 [94]. The first had a size of 913.0 Mb, making it one of the largest genomes in the Cucurbitaceae family, and pf3 had the largest described sequence amounting to 975.6 Mb. Furthermore, a genome sequence of the zucchini was accomplished using the Illumina HiSeq 2000 technique, which had a size of 263.5 Mb [95].
3. Genome Editing
3.1. The Evolution of Genome Editing Technologies
Deciphering the vast expanse of genomic sequences is just the tip of the iceberg; the profound value lies in interpreting the role of individual genes and their organised functions in organisms. Genome sequencing is a fundamental tool of functional genomics, which aims to understand the relationship between a genome sequence and the resulting physiological traits. A well-described genome sequence is the basis for genome editing. However, understanding gene function, regulatory elements and synteny is equally important [41]. High-resolution genomic maps obtained by sequencing are the foundation of genomic editing as they provide the basis for designing precise genome-editing systems to manipulate target genes, thus influencing their phenotypic consequences [96]. Gene-editing techniques have significantly evolved, directly impacting phenotypic outcomes in targeted genes. The progression of these methods can be charted from the 1970s, with the use of restriction enzymes to manipulate DNA; in the 1980s, zinc finger nucleases (ZFNs) were introduced, which are artificially engineered restriction enzymes for custom site-specific genome editing; in 2011, transcription activator-like effector nucleases (TALENs) were developed, which are similar to ZFNs, but use a different DNA-binding domain; in 2013, the CRISPR/Cas9 system was discovered, which is a simple and efficient genome-editing tool; in 2017, base editing was introduced, which allows for precise single-base changes; and in 2019, prime editing was developed, which enables all types of base conversion, small deletions and insertions [97,98,99]. ZFNs, TALENs and CRISPR/Cas9 are three widely used gene-editing techniques that have revolutionised genome engineering. ZFNs are composed of a zinc finger domain and a Fok1 endonuclease domain [97,99,100]. Each zinc finger can recognise three to six nucleotide bases and requires the endonuclease domain to dimerise before creating a double-strand break in the DNA. The ZFNs technique is less flexible and more expensive than TALENs. The TALENs technique is cheaper and produces faster results than ZFNs [100]. It is more flexible and easier to design due to well-defined target specificities. CRISPR/Cas9 is an RNA-based bacterial defence mechanism composed of two types of RNA (trans-activating crRNA and a single guide RNA) and Cas9 endonuclease. It is simpler, cheaper and more efficient than ZFNs and TALENs [97,98,99]. In summary, each technique has its advantages and disadvantages, and the choice of which one to use depends on the specific requirements of the research. CRISPR/Cas9 is currently the most popular choice due to its simplicity, cost-effectiveness and efficiency.
The widespread use of gene-editing techniques in many plant species has been made possible by major advances in transformation methodology. In gene editing, an endonuclease recognises specific DNA or guides RNA sequences through its DNA binding domain (DBD), facilitating precise and efficient incisions in the target DNA sequence, resulting in changes in specific regions of DNA [101]. Modern research has led to a number of gene-editing techniques using nucleases that recognise their loci through protein–DNA interactions, examples of which include meganucleases (MNs), zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) [102,103]. A separate category of nucleases includes CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated) and CRISPR/Cpf1 (class 2/type V CRISPR RNA-guided endonuclease), which identify their loci by RNA–DNA base complementarity [104].
It is important to consider performance, specificity, versatility and cost when comparing these genome-editing tools. CRISPR/Cas9 generally shows a superior performance compared with ZFNs and TALENs. This is primarily due to the ease of designing targeting RNAs for CRISPR compared with custom proteins for ZFNs and TALENs [105]. All systems may have off-target effects when considering the specificity of the techniques. However, modifications in the design of the guide RNA and Cas9 protein may improve the specificity [105]. In terms of versatility, although ZFNs and TALENs are limited by the protein engineering requirements for each target, CRISPR/Cas9’s reliance on guide RNAs makes it easily programmable and versatile for many targets [105]. CRISPR technology is considered the optimal method for generating modified genomes due to its low cost, high flexibility and reliability [106,107,108].
In recent times, numerous plant viral vectors have been effectively designed to transport CRISPR/Cas reagents for genome editing in both model and non-model plants. Virus-induced gene editing (VIGE) has emerged as a powerful technique, providing substantial advantages over alternative methods, including enhanced efficiency, precision and ease of use [109]. Notably, VIGE facilitates genome editing without the need for tissue culture, as it directly delivers transgenes to the meristem. In the past decade, VIGE systems have been developed and applied across various host plants, demonstrating successful outcomes in genome editing [110]. In cucurbits, there is a potential application of cucumber green mottle mosaic virus (CGMMV)) as a VIGE vector, which has been successfully employed in virus-induced gene silencing [111].
3.2. Genome Editing in Cucurbitaceae
Recently, scientists have been using gene-editing technologies, particularly CRISPR, on cucurbits (Figure 3) such as cucumber, melon, watermelon and squash. Analysing the data presented in Figure 3, there have been a total of 63 scientific papers on CRISPR-based genome editing in the Cucurbitaceae family registered in the Scopus database between 2016 and 2023. Of these, 73% were research articles, 21% were review papers and the remainder (6%) were conference papers, book chapters and letters. In recent years a dynamic increase in the number of publications in this subject has been observed. In particular, in 2022 the number of papers reached 19 and the same in 2023, but it should be noted that at the time of publication of this review article, more articles may still appear by the end of 2023. The growing interest in the application of CRISPR-based technologies may also be linked to the number of genomes sequenced so far within this plant family, as well as to the possibility of different gene functions by deleting or editing them, leading to the improvement of plant traits in terms of their resistance to many biotic and abiotic stresses, climate change and global warming.
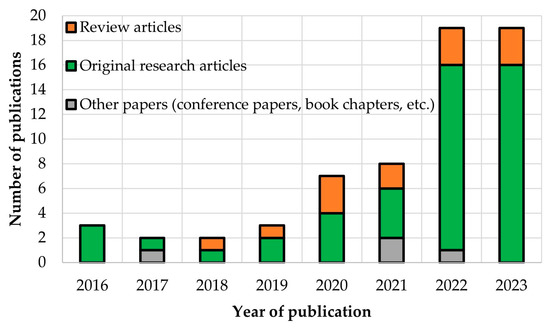
Figure 3.
Number of publications connected with genome editing in Cucurbitaceae (Source: Scopus; n = 63, accessed on 16 October 2023).
Figure 4 presents a network visualisation of keywords based on their co-occurrence, where the size of each frame is proportional to the number of occurrences. The given keywords are divided into five clusters, but all of them are strictly related to “CRISPR/Cas9”. The grouping into these clusters results from the common appearance of individual words in articles from the Scopus database, and the detailed connections will be described below. One of the clusters refers to virus resistance and genome editing within this subject, and the eIF4E (eukaryotic translation initiation factor 4E) gene appears here, whose disruption in cucumber led to the development of virus-resistant plants [112]. Furthermore, the green and blue boxes show an interest in CRISPR-based genome editing in the case of physiological aspects and climate correlation, respectively, with the following keywords: “auxin”, “ethylene”, “salinity”, etc.

Figure 4.
Network visualisation of keywords based on their co-occurrence, obtained by VOSviewer.
Plants have developed a range of physiological, biochemical and anatomical mechanisms to withstand abiotic stresses such as drought, flooding and salinity, which are becoming more prevalent due to climate change. Knowledge of the genomic sequences of cucurbit plants combined with novel genome-editing techniques have enabled the elucidation of the functions of numerous key genes in the physiology and stress response of Cucurbitaceae. These insights, in conjunction with the ongoing development of genome-editing methodologies, constitute an impressive tool for breeders, facilitating the rapid generation of new plant cultivars. New cultivars should be characterised not only by improved yield quantity and quality but also by their enhanced adaptability to the challenges posed by a changing environment [1]. In Table 2, examples of genome editing in Cucurbitaceae plants are presented, which enhances understanding of their physiology and the application of this knowledge in breeding. The sections below present the potential utilisation of genome editing in obtaining cucurbit plants that will confront the challenges of a dynamic environment (drought, salinity, limited arable land and new biotic stressors). It is noteworthy that induced modifications aimed at various objectives (e.g., improving crop quality and understanding of sex determination) may also be beneficial in breeding plants resistant to multistress conditions posed by climate change.

Table 2.
Examples of genome editing found in the Cucurbitaceae family.
3.3. Gene Editing of Phytohormone Metabolism to Improve Fruit Quality and Enhance Multiple Stress Resistance
Phytohormones and growth regulators play a crucial role not only in regulating plant growth, development and flower sex determination but also in responding to stress throughout a plant’s life cycle. Ethylene is involved in regulating various developmental processes such as organ abscission, seed germination, transition from vegetative to generative phases, flowering, seed maturation and responses to biotic and abiotic stresses. Depending on the species and interactions with other phytohormones, ethylene can induce opposing effects in some of these processes. The biosynthesis of ethylene begins with methionine, which is converted into S-adenosylmethionine (SAM) by SAM synthetase. SAM is then transformed into 1-aminocyclopropane (ACC) by ACC synthase (ACS). Finally, ethylene is released from ACC due to the activity of ACC oxidase (ACO). A very important aspect in which ethylene plays a crucial role is the regulation of sex determination [165]. The sex determination system in the family Cucurbitaceae is conserved, which inspires a broader understanding of sex differentiation in plants. Genetic studies have revealed that sex expression in cucumber is controlled by loci connected with ethylene biosynthesis genes: F (ACS1), M (ACS2) and A (ACS11) in combination with G (WIP) (the WIP family C2H2 zinc finger transcription factor gene WIP1) and the ethylene biosynthetic genes ACC oxidase 2 (ACO2) [165]. Selected aspects of the above reports are confirmed in some works presenting gene editing (Table 2). Using CRISPR/Cas9, the downregulation of ACO genes resulted in a significant decrease in ethylene production in generative organs and extended flower longevity. However, this reduction in ethylene production in seeds also negatively affected germination. Upregulating the expression of ACS1G in cucumber induces the development of female flowers, but in combination with ACO2 activity leads to the over-production of ethylene [114]. Maize (Zea mays L.) mutants with reduced ACS expression (ZmACS6) were characterised by increased drought resistance associated with delayed leaf senescence (increased chlorophyll content, proteins, including RuBisCO) [166]. The cucumber mutant SF1 (Short-fruit1), which produces short fruits (due to arrested cell divisions), is characterised by ACS2 accumulation. Using CRISPR/Cas9 to generate null mutants of ACS2 results in androecy and the production of male flowers only [114]. A knockout of ACS2 mutants produces only male flowers [115]. Beyond sex determination control, ACS2 plays a crucial role in cucumber fruit development, potentially accompanied by changes in drought resistance [115]. Another gene related to sex expression is WIP, a transcription factor that could block the expression of ethylene biosynthesis genes and inhibit stamen development. In watermelon, a direct mutation in the ClWIP gene, introduced by CRISP/Cas, resulted in the formation of female flowers [150]. Other studies linked to sex determination were connected to, among other things, transcription factors due to the bHLH transcription factor CsALC expressed in the ovaries, which plays a role in cucumber pollen tube emergence [116]. A study of watermelon showed that the CRISPR/Cas9-edited lines (ClATM1) exhibited typical vegetative growth, but displayed male flower abnormalities, including reduced petal size and degraded anthers with nonviable pollen [151]. Gynoecious inbred lines of cucumber have been successfully generated using CRISPR/Cas9 by introducing mutations in CsVFB1 [122]. In melon, a mutation in the eIF4E gene introduced by CRISP/Cas9 is responsible for virus resistance, but it can also lead to the development of male sterile lines [138].
Cucumber sf2, carrying a recessive allelic variation in the hdc1 Arabidopsis homolog, produces shorter fruits due to inhibited cell proliferation by 70%. Through the CRISPR/Cas9 technique, the function of the cucumber sf2 gene, encoding histone deacetylase homologous to HDC1 in Arabidopsis, was elucidated (Table 2). Genomic analysis indicated that SF2 promotes histone deacetylation of genes involved in multiple pathways related to phytohormone biosynthesis and transduction. SF2 promotes the actions of auxins, gibberellins and cytokinins by repressing negative regulators of these phytohormones, while repression of positive regulators of abscisic acid, jasmonic acid and ethylene reduces the impact of these hormones. Furthermore, SF2 targets and inhibits several SAM decarboxylase genes, which code enzymes involved in polyamine biosynthesis [113]. Undoubtedly, polyamines present a prominent metabolic feature in plants when exposed to a range of abiotic stressors, including drought, salinity, cold, heat, waterlogging, ultraviolet radiation, heavy metals and herbicides [167]. Polyamines assume a crucial role in upholding the protein balance, countering the effects of reactive oxygen species (ROS), instigating protective antioxidative mechanisms, and serving as molecular chaperones in stressful circumstances. Consequently, due to polyamines, plants acquire a versatile capacity to withstand a multitude of stress factors [167].
Certain varieties of melons produce climacteric fruits, which, as they mature, exhibit a respiratory burst and increase in ethylene emission [168]. Delaying melon post-harvesting ripening improves fruit shelf life. Understanding the mechanisms behind climacteric melon fruit physiology is of interest to researchers, but changes in ethylene metabolism may bring about alterations in plant sensitivity to stresses [136]. Mutation of the CmNAC-NOR transcription factor gene led to inhibited ethylene production, the absence of an abscission layer, and also any change in fruit colour (Table 2) [132]. CmROS1 (MELO3C024516) encodes a homolog of the main DNA demethylase AtROS1 in Arabidopsis, primarily acting on sequences of transposable elements and regulating certain genes involved in pathogen responses and epidermal cell organisation [169]. Methylome analysis of ROS1 knockout mutants revealed changes in DNA methylation in promoter regions of key ripening genes, such as ACS1 and ACO1, suggesting the importance of DNA demethylation through ROS1 in initiating fruit ripening in melon [134]. Potential utilisation of these findings in stress-resistant plant breeding should encompass functional analyses of these genes not only in fruits but also in whole plants. Knockout of CmACO1 (which is predominantly expressed in ripe fruits) using CRISPR/Cas9 significantly reduced ethylene emission, extending the shelf life of fruits (Table 2) [136]. Understanding the precise functions of the remaining four CmACO genes, which are expressed in different organs, could be of great relevance in the context of breeding more stress-resistant varieties.
Auxins are among the most thoroughly characterised phytohormones, which play a significant role at all stages of plant growth and development. Currently, the attention of an expanding group of researchers is drawn to the involvement of auxins not only in growth and development processes but also in plant responses to stress [170]. Cytokinins are commonly characterised as growth-promoting phytohormones, although various compounds with cytokinin-like properties have been identified as regulators of a wide spectrum of developmental and physiological processes in plants. Numerous studies have demonstrated that cytokinins can exert both beneficial and adverse effects on stress tolerance [171]. Many reports suggest that the response to drought is accompanied by a reduction in cytokinin biosynthesis in the roots and their transport to the shoots. Increased cytokinin levels enhance stomatal apertures and stimulate transpiration. However, elevated cytokinin concentrations result in the mitigation of stress-related symptoms, such as the delay in leaf senescence [172] (Table 2). Auxins, along with other phytohormones and growth regulators, are responsible for the architecture of the whole plant [173]. Modifying plant architecture not only enhances yield by optimising resource utilisation but also potentially increases resistance to biotic and abiotic stresses [174].
The CsHEC1 gene is responsible for fruit neck development in cucumber. Genetic editing has allowed a detailed understanding of its function. CsHeC1 knockout results in the formation of fruits with shorter necks, attributed to reduced auxin accumulation (Table 2) [119]. CsHEC1 directly activates the CsYuc4 gene, a member of the YUCCA family that catalyses the conversion of indole-3-pyruvic acid to IAA, the final step in the most common natural auxin biosynthesis pathway. The function of the numerous spines (ns) gene Csa2G264590 in cucumber was studied by Liu et al. [120]. The ns gene encodes an auxin transporter of the AUX1/LAX type. Mutants with ns-cr had inhibited auxin transport to the epidermis, resulting in numerous spines. However, the study was limited to fruit peel and spines. The authors indicated the expression of auxin transporter genes in other plant organs such as stems (Csa4G308640 and Csa5G201310), roots (Csa5G20131 and Csa5G374730I), which exhibited expression in most tissues (Table 2). Regulating auxin biosynthesis and transport within the plant and various organs could be crucial in breeding plants more adapted to changing environmental conditions [175].
CsHEC2 in cucumber is responsible for spine formation on the fruit skin and its function is related to cytokinin content. Knockout of CsHEC2 reduced cytokinin levels, while overexpression increased them. CsHEC2 regulates cytokinin levels by controlling the expression of CTK hydroxylase-like1, an enzyme involved in cytokinin biosynthesis (Table 2). Utilising CRISPR/Cas9, the CsHEC2 function can be precisely studied to develop cucumber varieties with enhanced resistance to abiotic stresses [121].
3.4. Increasing Salt Stress Tolerance through Gene Editing
A plant’s response to salinity depends on the species and its adaptation. Initial symptoms of salinity stress include inhibited root and shoot growth. Under conditions of salinity stress, leaves age prematurely, chlorophyll and protein concentrations decrease, and membrane permeability increases. Plants also experience an imbalance in ion content, with higher levels of chloride and sodium ions and lower concentrations of calcium and potassium ions. However, plants have the capacity to adapt to salinity stress by utilising biochemical pathways that maintain or promote growth through improved water or ion management [176].
Homeostasis of K+/Na+ is a key factor in plant resistance to salt stress. In cucumber, the K+ transporters CsAKT control K+ influx. Understanding the function of CsAKT can be useful for breeding cultivars more resistant to sodium toxicity and improving the efficacy of salt-mitigating treatments, such as cerium oxide nanoparticle spraying [124] (Table 2).
Salt stress induces CsBPC2 expression, and mutated CsBPC2 leads to reduced osmolyte content, antioxidant enzyme activity and activity of ATP-dependent ion pumps. Mutated CsBPC2 impedes osmotic adjustments and eliminates ROS, simultaneously causing unfavourable anion balance disturbances. These changes negatively affect plant tolerance to salinity stress. CsBPC2 is also involved in the abscisic acid signalling pathway and is essential for ABA-induced biosynthesis and transcription of ABA-related signalling genes [128].
The basic pentacysteine (BPC) transcription factor, encoded by the BPC gene, plays a role in plant responses to abiotic stress [177]. In Arabidopsis, the homologous transcription factor BPC1/BPC2 enhances plant salt resistance by suppressing the expression of galactan synthase 1 [178], but other studies suggest that BPC2 may increase sensitivity to osmotic stress by repressing the expression of the LEA protective protein [179].
Knocking out the gene encoding RBOHD (Respiratory Burst Oxidase Homolog D, NADPH-dependent enzyme catalysing •OH formation) in salt-tolerant pumpkin (Cucurbita moschata) cv. Chaojiquanwang reduced the H2O2 levels in the root, resulting in decreased K+ concentration and increased susceptibility to drought stress [139]. These studies have contributed to a better understanding of the signalling function of reactive oxygen species in Cucurbitaceae in response to salt stress.
An essential determinant of fruit quality pertains to the abundance of simple sugars and disaccharides in fruit. Modern genome-editing techniques are employed to investigate fundamental mechanisms underlying the transport of oligosaccharides, their unloading from the phloem, and hydrolysis within watermelon plants (Table 2) [143,144,146]. Simple sugars and disaccharides, classified as osmolytes, play a crucial role in osmoregulation, consequently mitigating the adverse effects of drought and salinity. The study by Xuan et al. [180] revealed that the majority of ClSWEET genes (encoding plasma membrane-localised hexose transporters) exhibited higher expression levels in response to stress.
3.5. Enhancing Resistance to Biotic Stresses through Genome Editing
Climate change exacerbates the likelihood of disease outbreaks through its influence on pathogen evolution, interactions between hosts and pathogens, and the promotion of novel pathogenic strains. It can also lead to shifts in the geographic range of pathogens, thereby expanding the prevalence of plant diseases into previously unaffected regions [17]. The most environmentally friendly approach to addressing new pathogen issues is through resistance breeding, which is greatly enhanced by genome sequencing and emerging genome-editing techniques.
Powdery mildew poses a significant threat to cucumber cultivation [181]. Resistance to the pathogens that cause this disease is polygenic. Modern gene-editing techniques, however, can elucidate the role of individual genes, which can be crucial for effective resistance breeding. Tek et al. [126] used CRISPR/Cas9 to investigate the function of genes responsible for mlo resistance to powdery mildew. A mutation in CsaMLO8 corresponded to pre-invasion response resistance, while genes CsaMLO1 and CsaMLO11 were identified as negative regulators in a post-invasive response to Podosphaera xanthii (Table 2). A double mutation of CsaMLO1 and CsaMLO11 was suggested as a potential approach to powdery mildew resistance relying on over-sensitivity. Therefore, CRISPR/Cas9 can be used to create cucumber varieties resistant to powdery mildew, exhibiting strong pre-invasion resistance with CsaMLO8 mutations or post-invasion resistance with CsaMLO1/CsaMLO11 mutations. Zhang et al. [162], applying CRISPR/Cas9 technology, generated a knockout line of watermelon clpsk1, which exhibited resistance to Fusarium oxysporum f.sp. niveum, thus confirming that phytosulfokine-associated signalling mitigates the plant’s response to pathogens.
Plant-parasitic nematodes represent a serious category of plant pathogens. Their effective control is hampered by the restricted availability of nematocides. Furthermore, these nematodes themselves may serve as vectors for viruses. By knockout of the CsMS gene (using CRISPR/Cas9), cucumber plants resistant to root-knot nematodes were obtained (Table 2) [153].
Another example of CRISPR/Cas9 application in cucumber genome editing, which may be useful in breeding for increased stress resistance, is introducing virus resistance. Chandrasekaran et al. [112] used CAS9/subgenomic RNA to disrupt the function of the eukaryotic translation initiation factor 4E (eIF4E) transcription factor (Table 2). The resulting mutants were resistant to Ipomovirus (cucumber vein yellowing virus) and potyviruses (zucchini yellow mosaic virus and papaya ring spot mosaic virus-W). A mutation in the gene encoding the cap-binding protein eIF4E induced resistance to the Moroccan watermelon mosaic virus in melon [133,138]. CRISPR/Cas9 mutations in eIF4E were successfully applied for breeding cucumber plants resistant to zucchini yellow mosaic virus, watermelon mosaic virus and papaya ringspot virus (Table 2) [117]. Through the induction of mutations in the gene CmRDR1c1/c2 encoding RNA-dependent RNA polymerase 1b, it has been possible to gain a deeper understanding of the mechanisms of diversified resistance in melon against viruses from various families [161].
3.6. Modifications to Plant Architecture Resulting from Genome Editing
The world’s increasing population and decreasing arable land necessitate a higher crop yield per unit area. Plant architecture, a complex agronomic trait determining crop yield under high planting density, comprises many factors, including plant height and branching. Leaf angle is a significant factor determining plant canopy architecture, which can increase photosynthetic efficiency and facilitate dense plant spacing. Indole-3-acetic acid is an essential hormone that appears to play a crucial role in leaf angle regulation. A decrease in IAA content leads to a greater leaf angle in maize, while an increase in IAA concentration results in a smaller leaf angle [182]. The petiole angle (LPA) in cucumbers can impact not only yield and planting density but also fruit quality and disease occurrence due to light capture and air circulation. The gene encoding indole-3-octenoic acid glucosyltransferase (CsIAGLU) negatively regulates the leaf petiole angle by modifying the active IAA pool (glycosylation of free IAA) at the petiole base and the size of adaxial cells in cucumber (Table 2) [127]. CsIAGLU acts as a negative regulator of leaf petiole angle development by expanding cells, mediated by auxin. This provides a valuable strategy for breeding cucumbers with smaller leaf petiole angles by increasing CsIAGLU expression, likely through gene editing using CRISPR/Cas9 in the promoter region [127]. Being the outermost layer on terrestrial plants, the lipophilic cuticle primarily coats aerial plant structures, including non-woody stems, leaves, flowers and fruits. It serves as a protective barrier against various abiotic and biotic stresses [183]. Mutation using CRISPR/Cas9 has allowed the understanding of the function of the CsSEC23 gene, which modifies the deposition of cutin wax on fruit surfaces and has a significant impact on resistance to abiotic stresses [130]. To sum this up, an overview of crop improvement through CRISPR/Cas9-mediated genome editing in plants of the Cucurbitaceae family is presented in Figure 5.
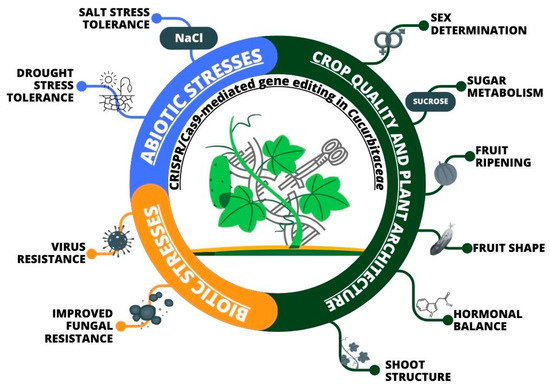
Figure 5.
An overview of crop improvement through genome editing in Cucurbitaceae.
4. Legal Framework for Plant Genome Editing in Agriculture
The agricultural sector has experienced significant technological advancements leading to groundbreaking innovations. Among these innovations is plant genome editing, a technique that has attracted significant attention. As described above, this cutting-edge technique holds the promise of revolutionising agriculture by improving crop traits, increasing yields and ensuring food security. However, implementing plant genome editing in agriculture poses legal complexities and challenges. This analysis covers the crucial legal aspects and established conventions related to the use of plant genome editing in agriculture. There is a need for strict international regulatory frameworks to govern the application of this technique. The legal framework regulating plant genome editing is intricate and encompasses global agreements, regional regulations and domestic laws. The Cartagena Protocol on Biosafety [184], established under the Convention on Biological Diversity, is a significant factor at the international level. The protocol was adopted on 29 January 2000 in Cartagena, Colombia, and came into force on 11 September 2003. It has been ratified by numerous countries and plays a crucial role in the regulation of genetically modified organisms (GMOs) and biotechnology on a global scale. This protocol has the objective of guaranteeing the safe management, conveyance and utilisation of altered living organisms, comprising genetically modified plants. Additionally, the International Union for the Protection of New Varieties of Plants (UPOV) [185] offers a framework to secure the intellectual property rights of breeders who generate new plant species through genome editing. UPOV establishes guidelines to protect plant breeders’ rights and distribute benefits resulting from the utilisation of new plant varieties equitably. Additionally, compliance with national legislation and regulations is necessary. At the national level, different countries have differing approaches to regulating plant genome editing. The United States, for instance, has adopted a relatively lenient position, with the United States Department of Agriculture (USDA) asserting that certain genome-edited crops might not be subject to the same regulations as conventional genetically modified organisms (GMOs) [186]. This view is based on the belief that genome-edited crops may be identical to those created through conventional breeding methods. In contrast, the European Union (EU) has adopted a cautious approach to GMOs by imposing stringent regulations. In 2018 the European Court of Justice (ECJ) issued a significant ruling stating that organisms obtained through genome-editing techniques, such as CRISPR-Cas9, must be treated as genetically modified organisms (GMOs) under European Union (EU) regulations [187,188]. This ruling clarified that these genetically edited crops and organisms would be subject to the same regulatory framework and labelling requirements as traditional GMOs. The decision emphasised that the exemption previously applied to mutagenesis techniques did not extend to genome editing.
This ruling had a substantial impact on the regulation of genetically edited crops and organisms in the EU, aligning them with the precautionary approach to GMOs. It meant that developers and producers of genetically edited crops had to undergo rigorous safety assessments and comply with labelling and traceability requirements, similar to conventional GMOs. The ECJ’s decision aimed to ensure transparency and consumer choice regarding genetically edited products in the European market and reflected the EU’s commitment to maintaining strict controls over genetically modified organisms. Intellectual property and access to genetic resources are critical legal considerations in plant genome editing. Therefore, it is important to address the issue of intellectual property rights and access to genetic resources. As scientists create new plant varieties through this method, questions arise regarding ownership of the rights to these innovations and their accessibility to others. The Nagoya Protocol, under the Convention on Biological Diversity [189], provides a framework for accessing and distributing benefits linked to genetic resources. It aims to ensure equitable distribution of such benefits, thereby promoting agricultural equity and sustainability.
Beyond the legal framework, ethics plays a significant role in the use of plant genome editing in agriculture. Questions concerning the ethical implications of creating genetically modified crops, potential environmental impacts and equitable benefit sharing are central to the discourse surrounding genome editing. Various organisations and institutions have developed ethical guidelines and principles for scientists and stakeholders involved in genome editing in response to ethical concerns. These guidelines prioritise transparency, risk assessment and the responsible utilisation of this influential technology. Even when using sophisticated tools such as CRISPR, there is a need to monitor unintended mutations. Understanding the genome landscape, potential locations of atypical mutations and techniques for their detection are of key importance [190]. Edited plants may crossbreed with wild relatives, which may have unintended consequences, thus knowledge of reproductive biology, pollen dispersal and hybridisation potential is essential [191]. Recognising the interactions of edited crops can aid in the safe placement of edited crops [192]. Edited crops can influence the agricultural economy, trade and farmers’ choices. Anticipating these impacts and providing equitable benefits can promote sustainable progress [193]. Although genome-edited plants may not have introduced foreign DNA, distinguishing them from traditional genetically modified organisms (GMOs) is the subject of regulatory and public debate. Understanding international regulations, ethical issues and public perceptions can guide research directions and applications [194].
In summary, the legal aspects of utilising plant genome editing in agriculture involve various international agreements, national regulations, intellectual property rights and ethical considerations. Successfully operating in this complex field requires a comprehensive understanding of relevant legal frameworks and a strong commitment to ethical and responsible conduct. As plant genome editing evolves and shapes the future of agriculture, it is crucial that scientists, policymakers and stakeholders collaborate to establish a cohesive regulatory framework that balances innovation and security.
5. Conclusions and Prospects
Cucurbits are of particular interest owing to their high nutritional and economic value. They are also models for the study of plant development and the refinement of yield improvement strategies. However, while traditional breeding approaches are valuable, they are limited due to the reduced genetic diversity and lower rates of variation in cucurbit species. A broad scope of genome editing has been presented in this review, which covers different aspects of genome editing, including resistance to biotic stresses (pathogenic fungi and viruses) and abiotic stresses (climate change, drought and salinity). It also focused on improving plant quality, optimising plant architecture and influencing cucurbit sex determination. In conclusion, this comprehensive review provides valuable insights into the latest developments in genome editing in cucurbits. It serves as an important reference for the advancement of genome-sequencing and gene-editing technologies in the Cucurbitaceae family and has the potential to have a significant impact on the improvement of horticultural crops.
The prospects for the recent innovations in genome sequencing and gene editing in the context of plant science are promising and offer significant potential for various applications. In particular, they have the capacity to improve crop traits to address pressing global issues such as food security. By manipulating genes responsible for yield, disease resistance and nutritional content, these technologies could revolutionise crop production and ensure an abundant and healthy food supply for a growing population. These innovations also go beyond traditional crop improvement. They can help protect biodiversity, particularly in demanding crops such as cucurbits. By creating new mutants and precisely modifying plant genomes, genetic diversity can be preserved, helping to protect important plant species and ecosystems. Genome editing is at the forefront of sustainable agriculture. Its precision and reduced side effects lessen reliance on chemical interventions. This not only minimises the environmental impact but also increases the resilience of crops to the ever-increasing challenges of abiotic and biotic stresses, making agriculture more sustainable and environmentally friendly. In addition, these technologies are taking research on plants such as cucurbits to a new level. They enable a deeper understanding of plant developmental processes, shedding light on the intricacies of growth, adaptation and response to environmental factors. Finally, the application of gene-editing tools extends to many horticultural crops. By using these technologies, breeders and farmers can improve quality, yield and adaptability, ensuring that these plants meet consumer demands and adapt to changing conditions. In summary, genetic transformation and gene editing offer many benefits, from revolutionising agriculture to deepening the understanding of plant biology.
Author Contributions
Conceptualisation, M.P.; resources, B.Z., P.S. and M.P.; writing—original draft preparation, B.Z., P.S. and M.P.; writing—review and editing, A.P.; visualisation, B.Z.; supervision, M.P.; project administration, M.P.; funding acquisition, A.P. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a project from the National Science Center UMO-2020/37/B/NZ9/00586.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Massel, K.; Godwin, I.D.; Gao, C. Applications and potential of genome editing in crop improvement. Genome. Biol. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, K.; Miladinović, D.; Sweet, J.; Akin, M.; Galović, V.; Kavas, M.; Zlatkovic, M.; de Andrade, E. Genome editing for healthy crops: Traits, tools and impacts. Front. Plant Sci. 2023, 14, 1231013. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Chu, H.D.; Le, N.Q. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genomics. 2016, 17, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef]
- Coradini, A.L.; Hull, C.B.; Ehrenreich, I.M. Building genomes to understand biology. Nat. Commun. 2020, 11, 6177. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Imbeaud, S.; Roux-Rouquié, M.; Hood, L. From functional genomics to systems biology: Concepts and practices. C. R. Biol. 2003, 326, 879–892. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Jeffrey, C. A review of the Cucurbitaceae. Bot. J. Linn. Soc. 1980, 81, 233–247. [Google Scholar] [CrossRef]
- Lebeda, A.; Widrlechner, M.P.; Staub, J.; Ezura, H.; Zalapa, J.; Kristkova, E. Cucurbits (Cucurbitaceae; Cucumis spp., Cucurbita spp., Citrullus spp.). In Genetic Resources, Chromosome Engineering, and Crop Improvement, Vegetable Crops, 1st ed.; Singh, R.J., Ed.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2007; Volume 3, pp. 271–376. [Google Scholar]
- Chikh-Rouhou, H.; Abdedayem, W.; Solmaz, I.; Sari, N.; Garcés-Claver, A. Melon (Cucumis melo L.): Genomics and breeding. In Smart Plant Breeding for Vegetable Crops in Post-Genomics, 1st ed.; Singh, E.S., Sharma, D., Sharma, S.K., Singh, R., Eds.; Springer: Singapore, 2023; pp. 25–52. [Google Scholar] [CrossRef]
- Feng, J.; Wang, N.; Li, Y.; Wang, H.; Zhang, W.; Wang, H.; Chai, S. Recent progress in genetic transformation and gene editing technology in cucurbit crops. Agronomy 2023, 13, 755. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Ainsworth, E.A.; Bahuguna, R.N.; Broadley, M.R.; Busch, W.; Carpita, N.C.; Castrillo, G.; Chory, J.; DeHaan, L.R.; Duarte, C.M.; et al. Climate change challenges, plant science solutions. Plant Cell 2023, 35, 24–66. [Google Scholar] [CrossRef] [PubMed]
- Sidău, M.R.; Croitoru, A.-E.; Alexandru, D.-E. Comparative analysis between daily extreme temperature and precipitation values derived from observations and gridded datasets in north-western Romania. Atmosphere 2021, 12, 361. [Google Scholar] [CrossRef]
- Trușcă, M.; Gâdea, Ș.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the research challenges and perspectives in ecophysiology of plants affected by salinity stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Juenger, T.E.; Verslues, P.E. Time for a drought experiment: Do you know your plants’ water status? Plant Cell 2023, 35, 10–23. [Google Scholar] [CrossRef]
- Sanger, F.; Air, G.M.; Barrell, B.G.; Brown, N.L.; Coulson, A.R.; Fiddes, J.C.; Hutchison, C.A., III; Slocombe, P.M.; Smith, M. Nucleotide sequence of bacteriophage φX174 DNA. Nature 1977, 265, 687–695. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Nerlich, B.; Dingwall, R.; Clarke, D.D. The book of life: How the completion of the Human Genome Project was revealed to the public. Health 2002, 6, 445–469. [Google Scholar] [CrossRef]
- Akintunde, O.; Tucker, T.; Carabetta, V.J. The evolution of next-generation sequencing technologies. arXiv 2023, arXiv:2305.08724v1. [Google Scholar]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Quinquis, B.; Da Silva, K.; Oñate, F.P.; Pons, N.; Roume, H.; Podar, M.; Almeida, M. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Sci. Data 2022, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Kchouk, M.; Gibrat, J.-F.; Elloumi, M. Generations of sequencing technologies: From first to next generation. Biol. Med. 2017, 9, 395. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cuber, P.; Chooneea, D.; Geeves, C.; Salatino, S.; Creedy, T.J.; Griffin, C.; Sivess, L.; Barnes, I.; Price, B.; Misra, R. Comparing the accuracy and efficiency of third generation sequencing technologies, oxford nanopore technologies, and Pacific Biosciences, for DNA barcode sequencing applications. Ecol. Genet. Genom. 2023, 28, 100181. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—The next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef]
- Schuster, S.C. Next-generation sequencing transforms today’s biology. Nature 2008, 200, 16–18. [Google Scholar] [CrossRef]
- Lister, R.; Ecker, J.R. Finding the fifth base: Genome-wide sequencing of cytosine methylation. Genome Res. 2009, 19, 959–966. [Google Scholar] [CrossRef]
- He, G.M.; Chen, B.B.; Wang, X.C.; Li, X.Y.; Li, J.; He, H.; Yang, M.; Lu, L.; Qi, Y.; Wang, X.P.; et al. Conservation and divergence of transcriptomic and epigenomic variation in maize hybrids. Genome Biol. 2013, 14, R57. [Google Scholar] [CrossRef] [PubMed]
- Szwacka, M.; Pawełkowicz, M.; Skarzyńska, A.; Osipowski, P.; Wojcieszek, M.; Przybecki, Z.; Pląder, W. Biological significance, computational analysis, and applications of plant microRNAs. Acta Physiol. Plant. 2018, 40, 146. [Google Scholar] [CrossRef]
- Lu, Z.; Marand, A.P.; Ricci, W.A.; Ethridge, C.L.; Zhang, X.; Schmitz, R.J. The prevalence, evolution and chromatin signatures of plant regulatory elements. Nat. Plants 2019, 5, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Cortes, L.T.; Zhang, Z.; Yu, J. Status and prospects of genome-wide association studies in plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef]
- Muhammad, I.; Li, W.-Q.; Jing, X.-Q.; Zhou, M.-R.; Shalmani, A.; Ali, M.; Wei, X.-Y.; Sharif, R.; Liu, W.-T.; Chen, K.-M. A systematic in silico prediction of gibberellic acid stimulated GASA family members: A novel small peptide contributes to floral architecture and transcriptomic changes induced by external stimuli in rice. J. Plant Physiol. 2019, 234–235, 117–132. [Google Scholar] [CrossRef]
- Michael, T.P.; VanBuren, R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020, 54, 26–33. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genomic plasticity and the diversity of polyploid plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef]
- Michael, T.P.; Jackson, S. The first 50 plant genomes. Plant Genome 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.S.; Pasternak, S.; Liang, C.Z.; Zhang, J.W.; Fulton, L.; Graves, T.A.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef]
- Lisch, D. How important are transposons for plant evolution? Nat. Rev. Genet. 2013, 14, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Bäurle, I.; Dean, C. The timing of developmental transitions in plants. Cell 2006, 125, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Pandey, M.K.; Bohra, A.; Singh, V.K.; Thudi, M.; Saxena, R.K. Toward the sequence-based breeding in legumes in the post-genome sequencing era. Theor. Appl. Genet. 2019, 132, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The Genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Cucurbitaceae Genomes Data Set. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/?taxon=3650 (accessed on 13 October 2023).
- Yu, J.; Wu, S.; Sun, H.; Wang, X.; Tang, X.; Guo, S.; Zhang, Z.; Huang, S.; Xu, Y.; Weng, Y.; et al. CuGenDBv2: An updated database for Cucurbit genomics. Nucleic Acids Res. 2023, 51, D1457–D1464. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Huang, W.; Xu, Y.; Zhou, Q.; Wang, S.; Ruan, J.; Huang, S.; Zhang, Z. A chromosome-scale genome assembly of cucumber (Cucumis sativus L.). GigaScience 2019, 8, giz072. [Google Scholar] [CrossRef]
- Yang, L.; Koo, D.-H.; Li, Y.; Zhang, X.; Luan, F.; Havey, M.J.; Jiang, J.; Weng, Y. Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J. 2012, 71, 895–906. [Google Scholar] [CrossRef]
- Osipowski, P.; Pawełkowicz, M.; Wojcieszek, M.; Skarzyńska, A.; Przybecki, Z.; Pląder, W. A high-quality cucumber genome assembly enhances computational comparative genomics. Mol. Genet. Genom. 2019, 295, 177–193. [Google Scholar] [CrossRef]
- Turek, S.; Pląder, W.; Hoshi, Y.; Skarzyńska, A.; Pawełkowicz, M. Insight into the organization of the b10v3 cucumber genome by integration of biological and bioinformatic data. Int. J. Mol. Sci. 2023, 24, 4011. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Q.; Xin, H.; Xu, Y.; Lin, S.; Chen, X.; Yao, Z.; et al. Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 2022, 13, 682. [Google Scholar] [CrossRef]
- Wóycicki, R.; Witkowicz, J.; Gawroński, P.; Dąbrowska, J.; Lomsadze, A.; Pawełkowicz, M.; Siedlecka, E.; Yagi, K.; Pląder, W.; Seroczyńska, A.; et al. The genome sequence of the north-european cucumber (Cucumis sativus L.) unravels evolutionary adaptation mechanisms in plants. PLoS ONE 2011, 6, e22728. [Google Scholar] [CrossRef] [PubMed]
- Skarzyńska, A.; Pawełkowicz, M.; Pląder, W. Genome-wide discovery of DNA variants in cucumber somaclonal lines. Gene 2020, 736, 144412. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, X.; Shen, D.; Miao, H.; Xie, B.; Li, X.; Zeng, P.; Wang, S.; Shang, Y.; Gu, X.; et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 2013, 45, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, Z.; Lou, Q.; Xia, L.; Li, J.; Li, M.; Zhou, J.; Zhao, X.; Xu, Y.; Li, Q.; et al. Chromosome-scale genome assembly of Cucumis hystrix—A wild species interspecifically cross-compatible with cultivated cucumber. Hortic. Res. 2021, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, P.; Li, J.; Zhao, Q.; Ji, C.; Zhu, Z.; Zhai, Y.; Qin, X.; Zhou, J.; Yu, H.; et al. Whole-genome sequence of synthesized allopolyploids in Cucumis reveals insights into the genome evolution of allopolyploidization. Adv. Sci. 2021, 8, 2004222. [Google Scholar] [CrossRef]
- Urasaki, N.; Takagi, H.; Natsume, S.; Uemura, A.; Taniai, N.; Miyagi, N.; Fukushima, M.; Suzuki, S.; Tarora, K.; Tamaki, M.; et al. Draft genome sequence of bitter gourd (Momordica charantia), a vegetable and medicinal plant in tropical and subtropical regions. DNA Res. 2017, 24, 51–58. [Google Scholar] [CrossRef]
- Matsumura, H.; Hsiao, M.-C.; Lin, Y.-P.; Toyoda, A.; Taniai, N.; Tarora, K.; Urasaki, N.; Anand, S.S.; Dhillon, N.P.S.; Schafleitner, R.; et al. Long-read bitter gourd (Momordica charantia) genome and the genomic architecture of nonclassic domestication. Proc. Natl. Acad. Sci. USA 2020, 117, 14543–14551. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Y.; Luo, S.; Wang, L.; Huang, R.; Wen, Q.; Han, X.; Miao, N.; Cheng, J.; Liu, Z.; et al. Whole-genome sequencing provides insights into the genetic diversity and domestication of bitter gourd (Momordica spp.). Hortic. Res. 2020, 7, 85. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Sun, F.; Wu, R.; Du, H.; Wang, Y.; Jiang, L.; Wu, X.; Wu, X.; Yang, L.; et al. Long-read genome assembly and genetic architecture of fruit shape in the bottle gourd. Plant J. 2021, 107, 956–968. [Google Scholar] [CrossRef]
- Wu, S.; Shamimuzzaman, M.; Sun, H.; Salse, J.; Sui, X.; Wilder, A.; Wu, Z.; Levi, A.; Xu, Y.; Ling, K.S.; et al. The bottle gourd genome provides insights into Cucurbitaceae evolution and facilitates mapping of a papaya ring-spot virus resistance locus. Plant J. 2017, 92, 963–975. [Google Scholar] [CrossRef]
- Fu, A.; Wang, Q.; Mu, J.; Ma, L.; Wen, C.; Zhao, X.; Gao, L.; Li, J.; Shi, K.; Wang, Y.; et al. Combined genomic, transcriptomic, and metabolomic analyses provide insights into chayote (Sechium edule) evolution and fruit development. Hortic. Res. 2021, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype stability and unbiased fractionation in the paleo-allotetraploid Cucurbita genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, B.; Bao, Y.; Huang, P.; Li, J.; Li, R.; Zhao, Q. Chromosome-level genome assembly of Herpetospermum pedunculosum (Cucurbitaceae). Genome Biol. Evol. 2023, 15, evad005. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Xie, X.; Gu, X.; Zhao, J.; Ping, X.; Li, Y.; Yang, Y.; Mao, Z.; Xie, B. High-quality chromosome-level genomes of Cucumis metuliferus and Cucumis melo provide insight into Cucumis genome evolution. Plant J. 2021, 107, 136–148. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Kou, Y.; Chen, X.; Yang, J.; Zhang, H.; Zhao, Z.; Zhao, Y.; Zhao, G.; Li, Z. Diploid chromosome-level reference genome and population genomic analyses provide insights into gypenoside biosynthesis and demographic evolution of Gynostemma pentaphyllum (Cucurbitaceae). Hortic. Res. 2023, 10, uhac231. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ming, R.; Xu, S.; Wang, J.; Yao, S.; Li, L.; Huang, R.; Tan, Y. Chromosome-level genome assembly of Gynostemma pentaphyllum provides insights into gypenoside biosynthesis. DNA Res. 2021, 28, dsab018. [Google Scholar] [CrossRef]
- Vaughn, J.N.; Branham, S.E.; Abernathy, B.; Hulse-Kemp, A.M.; Rivers, A.R.; Levi, A.; Wechter, W.P. Graph-based pangenomics maximizes genotyping density and reveals structural impacts on fungal resistance in melon. Nat. Commun. 2022, 13, 7897. [Google Scholar] [CrossRef]
- Castanera, R.; Ruggieri, V.; Pujol, M.; Garcia-Mas, J.; Casacuberta, J.M. An improved melon reference genome with single-molecule sequencing uncovers a recent burst of transposable elements with potential impact on genes. Front. Plant Sci. 2020, 10, 1815. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef]
- Oren, E.; Dafna, A.; Tzuri, G.; Halperin, I.; Isaacson, T.; Elkabetz, M.; Meir, A.; Saar, U.; Ohali, S.; La, T.; et al. Pan-genome and multi-parental framework for high-resolution trait dissection in melon (Cucumis melo). Plant J. 2022, 112, 1525–1542. [Google Scholar] [CrossRef]
- Shin, A.-Y.; Koo, N.; Kim, S.; Sim, Y.M.; Choi, D.; Kim, Y.-M.; Kwon, S.-Y. Draft genome sequences of two oriental melons, Cucumis melo L. var. makuwa. Sci. Data 2019, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, G.; Lian, J.; Garraway, J.; Niu, Y.; Hu, Z.; Yu, J.; Zhang, M. The chromosome-scale genome of melon dissects genetic architecture of important agronomic traits. iScience 2020, 23, 101422. [Google Scholar] [CrossRef] [PubMed]
- Pichot, C.; Djari, A.; Tran, J.; Verdenaud, M.; Marande, W.; Huneau, C.; Gautier, V.; Latrasse, D.; Arribat, S.; Sommard, V.; et al. Cantaloupe melon genome reveals 3d chromatin features and structural relationship with the ancestral Cucurbitaceae karyotype. iScience 2022, 25, 103696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Yu, H.; Zhang, Y.; Li, M.; Wang, H.; Wang, D.; Wang, H.; Fu, Q.; Liu, M.; et al. A high-quality melon genome assembly provides insights into genetic basis of fruit trait improvement. iScience 2019, 22, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Ariizumi, T.; Nonaka, S.; Kawazu, Y.; Zhong, S.; Mueller, L.; Giovannoni, J.J.; Rose, J.K.C.; Ezura, H. Comparative genomics of muskmelon reveals a potential role for retrotransposons in the modification of gene expression. Commun. Biol. 2020, 3, 432. [Google Scholar] [CrossRef]
- Itkin, M.; Davidovich-Rikanati, R.; Cohen, S.; Portnoy, V.; Doron-Faigenboim, A.; Oren, E.; Freilich, S.; Tzuri, G.; Baranes, N.; Shen, S.; et al. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. USA 2016, 113, E7619–E7628. [Google Scholar] [CrossRef]
- Xia, M.; Han, X.; He, H.; Yu, R.; Zhen, G.; Jia, X.; Cheng, B.; Deng, X.W. Improved de novo genome assembly and analysis of the Chinese cucurbit Siraitia grosvenorii, also known as monk fruit or luo-han-guo. GigaScience 2018, 7, giy067. [Google Scholar] [CrossRef]
- Pootakham, W.; Sonthirod, C.; Naktang, C.; Nawae, W.; Yoocha, T.; Kongkachana, W.; Sangsrakru, D.; Jomchai, N.; U-thoomporn, S.; Sheedy, J.R.; et al. De novo assemblies of Luffa acutangula and Luffa cylindrica genomes reveal an expansion associated with substantial accumulation of transposable elements. Mol. Ecol. Resour. 2021, 21, 212–225. [Google Scholar] [CrossRef]
- Barrera-Redondo, J.; Sánchez-de la Vega, G.; Aguirre-Liguori, J.A.; Castellanos-Morales, G.; Gutiérrez-Guerrero, Y.T.; Aguirre-Dugua, X.; Aguirre-Planter, E.; Tenaillon, M.I.; Lira-Saade, R.; Eguiarte, L.E. The domestication of Cucurbita argyrosperma as revealed by the genome of its wild relative. Hortic. Res. 2021, 8, 109. [Google Scholar] [CrossRef]
- Barrera-Redondo, J.; Ibarra-Laclette, E.; Vázquez-Lobo, A.; Gutiérrez-Guerrero, Y.T.; Sánchez de la Vega, G.; Piñero, D.; Montes-Hernández, S.; Lira-Saade, R.; Eguiarte, L.E. The genome of Cucurbita argyrosperma (silver-seed gourd) reveals faster rates of protein-coding gene and long noncoding rna turnover and neofunctionalization within Cucurbita. Mol. Plant 2019, 12, 506–520. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Mu, J.; Fu, A.; Wen, C.; Zhao, X.; Gao, L.; Li, J.; Shi, K.; Wang, Y.; et al. The genome and transcriptome analysis of snake gourd provide insights into its evolution and fruit development and ripening. Hortic. Res. 2020, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, G.; Gong, H.; Li, J.; Luo, C.; He, X.; Luo, S.; Zheng, X.; Liu, X.; Guo, J.; et al. A high-quality sponge gourd (Luffa cylindrica) genome. Hortic. Res. 2020, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ren, X.; Zhang, Z.; Ming, Y.; Yang, Z.; Hu, J.; Li, S.; Wang, Y.; Sun, S.; Sun, K.; et al. Long-read sequencing and de novo assembly of the Luffa cylindrica (L.) Roem. Genome. Mol. Ecol. Resour. 2020, 20, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Pirro, S.; Obembe, O. The complete genome sequence of Telfairia occidentalis, the African fluted pumpkin. Biodivers. Genomes 2022. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Kim, M.; Lee, S.; Lim, S.; Lee, M.S.; Kim, J.H.; Noh, S.J.; Park, S.W.; Kim, S.-T.; Shin, A.-Y.; et al. High-quality genome assembly and genetic mapping reveal a gene regulating flesh color in watermelon (Citrullus lanatus). Front. Plant Sci. 2023, 14, 1142856. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, X.; Reddy, U.; Sun, H.; Bao, K.; Gao, L.; Mao, L.; Patel, T.; Ortiz, C.; Abburi, V.L.; et al. Genome of ‘Charleston Gray’, the principal American watermelon cultivar, and genetic characterization of 1,365 accessions in the U.S. National Plant Germplasm System watermelon collection. Plant Biotechnol. J. 2019, 17, 2246–2258. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef]
- Renner, S.S.; Wu, S.; Pérez-Escobar, O.A.; Silber, M.V.; Fei, Z.; Chomicki, G. A chromosome-Level genome of a Kordofan melon illuminates the origin of domesticated watermelons. Proc. Natl. Acad. Sci. USA 2021, 118, e2101486118. [Google Scholar] [CrossRef]
- Wu, S.; Sun, H.; Gao, L.; Branham, S.; McGregor, C.; Renner, S.S.; Xu, Y.; Kousik, C.; Wechter, W.P.; Levi, A.; et al. A Citrullus genus super-pangenome reveals extensive variations in wild and cultivated watermelons and sheds light on watermelon evolution and domestication. Plant Biotechnol. J. 2023, 21, 1926–1928. [Google Scholar] [CrossRef]
- Xie, D.; Xu, Y.; Wang, J.; Liu, W.; Zhou, Q.; Luo, S.; Huang, W.; He, X.; Li, Q.; Peng, Q.; et al. The wax gourd genomes offer insights into the genetic diversity and ancestral Cucurbit karyotype. Nat. Commun. 2019, 10, 5158. [Google Scholar] [CrossRef]
- Luo, W.; Yan, J.; Luo, S.; Liu, W.; Xie, D.; Jiang, B. A chromosome-level reference genome of the wax gourd (Benincasa hispida). Sci. Data 2023, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Martí-Gómez, C.; Ferriol, M.; Gómez, P.; Jamilena, M.; Mueller, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M. The genome editing revolution: Review. J. Genet. Eng. Biotechnol. 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Dhakate, P.; Sehgal, D.; Vaishnavi, S.; Chandra, A.; Singh, A.; Raina, S.N.; Rajpal, V.R. Comprehending the evolution of gene editing platforms for crop trait improvement. Front. Genet. 2022, 13, 876987. [Google Scholar] [CrossRef] [PubMed]
- Van der Oost, J.; Patinios, C. The genome editing revolution. Trends Biotechnol. 2023, 41, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Wyman, C.; Kanaar, R. DNA double-strand break repair: All’s well that ends well. Annu. Rev. Genet. 2006, 40, 363–383. [Google Scholar] [CrossRef]
- Puchta, H.; Fauser, F. Synthetic nucleases for genome engineering in plants: Prospects for a bright future. Plant J. 2014, 78, 727–741. [Google Scholar] [CrossRef]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- El-Mounadi, K.; Morales-Floriano, M.L.; Garcia-Ruiz, H. Principles, applications, and biosafety of plant genome editing using CRISPR-Cas9. Front. Plant Sci. 2020, 11, 56. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Wang, X.; Li, X.; You, C. CRISPR/Cas9 technology and its application in horticultural crops. Hortic. Plant J. 2022, 8, 395–407. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.R.; Liu, Y.; Zhu, J.-K. Gene editing in plants: Progress and challenges. Natl. Sci. Rev. 2019, 6, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, X.; Zhang, R.; Li, J. Virus-induced gene editing and its applications in plants. Int. J. Mol. Sci. 2022, 23, 10202. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, S.; Xu, X.; He, Y.; Shou, W.; Munaiz, E.D.; Yu, C.; Shen, J. Application and expansion of virus-induced gene silencing for functional studies in vegetables. Horticulturae 2023, 9, 934. [Google Scholar] [CrossRef]
- Liu, M.; Liang, Z.; Aranda, M.A.; Hong, N.; Liu, L.; Kang, B.; Gu, Q. A cucumber green mottle mosaic virus vector for virus-induced gene silencing in cucurbit plants. Plant Methods 2020, 16, 9. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wang, S.; Lin, T.; Yang, L.; Zhao, Z.; Zhang, Z.; Huang, S.; Yang, X. Genome-wide target mapping shows histone deacetylase complex1 regulates cell proliferation in cucumber fruit. Plant Physiol. 2020, 182, 167–184. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Yang, L.; Cai, G.; Chen, H.; Gao, D.; Lin, T.; Cui, Q.; Wang, D.; Li, Z.; et al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene acs1g induces female flower development in cucumber gynoecy. Plant Cell 2021, 33, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.-H.; Huang, S.; Yang, X. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, X.; Yan, S.; Liu, B.; Zhong, Y.; Song, W.; Chen, J.; Wang, Z.; Che, G.; Liu, L.; et al. Pollen tube emergence is mediated by ovary-expressed ALCATRAZ in cucumber. Nat. Commun. 2023, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Calis, O.; Ari, E.; Atasayar, A.; Sarikaya, P.; Tek, M.I.; Izmirli, A.; Oz, Y.; Firat, G. Knockout of ElF4E using CRISPR/Cas9 for large-scale production of resistant cucumber cultivar against WMV, ZYMV, and PRSV. Front. Plant Sci. 2023, 14, 1143813. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Q.; Wang, H.; Lu, T.; Li, Y.; Sui, X.; Yu, H.; Jiang, W. Ethylene signalling plays an important role in UV-B-induced ascorbic acid accumulation in Cucumis sativus leaves. Authorea 2022. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Wang, L.; Yan, S.; Cheng, Z.; Liu, X.; Han, L.; Chen, G.; Wang, S.; Song, W.; et al. The CsHEC1-CsOVATE module contributes to fruit neck length variation via modulating auxin biosynthesis in cucumber. Proc. Natl. Acad. Sci. USA 2022, 119, e2209717119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, X.; Xie, Q.; Miao, H.; Bo, K.; Dong, S.; Xin, T.; Gu, X.; Sun, J.; Zhang, S. NS encodes an auxin transporter that regulates the ‘numerous spines’ trait in cucumber (Cucumis sativus) fruit. Plant J. 2022, 110, 325–336. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Han, L.; Cheng, Z.; Liu, X.; Wang, S.; Liu, L.; Chen, J.; Song, W.; Zhao, J.; et al. HECATE2 acts with GLABROUS3 and TU to boost cytokinin biosynthesis and regulate cucumber fruit wart formation. Plant Physiol. 2021, 187, 1619–1635. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, X.; Yuan, X.; Hong, Y.; Zhang, L.; Zhang, X.; Chen, S. Identification and characterization of CsSRP43, a major gene controlling leaf yellowing in cucumber. Hortic. Res. 2022, 9, uhac212. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Zhu, L.; Cui, L.; Yang, L.; Wu, H.; Bie, Z. CsAKT1 is a key gene for the CeO2 nanoparticle’s improved cucumber salt tolerance: A validation from CRISPR-Cas9 lines. Environ. Sci. Nano 2022, 9, 4367–4381. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, Y.; Yang, Z.; Cheng, L.; Sharif, R.; Raza, A.; Chen, P.; Hou, D.; Li, Y. Exploring the agrobacterium-mediated transformation with CRISPR/Cas9 in cucumber (Cucumis sativus L.). Mol. Biol. Rep. 2022, 49, 11481–11490. [Google Scholar] [CrossRef] [PubMed]
- Tek, M.I.; Calis, O.; Fidan, H.; Shah, M.D.; Celik, S.; Wani, S.H. CRISPR/Cas9 based Mlo-mediated resistance against Podosphaera xanthii in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 1081506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Liu, X.; Chen, G.; Liu, L.; Cheng, Z.; Song, W.; Han, L.; Wang, S.; Wang, L.; et al. CsIAGLU regulates the angle of leaf petiole by affecting endogenous content of auxin in cucumber (Cucumis sativus L.). Genes 2022, 13, 2216. [Google Scholar] [CrossRef]
- Li, S.; Sun, M.; Miao, L.; Di, Q.; Lv, L.; Yu, X.; Yan, Y.; He, C.; Wang, J.; Shi, A.; et al. Multifaceted regulatory functions of CsBPC2 in cucumber under salt stress conditions. Hortic. Res. 2023, 10, uhad051. [Google Scholar] [CrossRef]
- Xin, T.; Tian, H.; Ma, Y.; Wang, S.; Yang, L.; Li, X.; Zhang, M.; Chen, C.; Wang, H.; Li, H.; et al. Targeted creation of new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in Cucurbit plants. Hortic. Res. 2022, 9, uhab086. [Google Scholar] [CrossRef]
- Gao, L.; Cao, J.; Gong, S.; Hao, N.; Du, Y.; Wang, C.; Wu, T. The COPII subunit CsSEC23 mediates fruit glossiness in cucumber. Plant J. 2023, 116, 524–540. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Hoang, T.T.-H.; Le, N.T.; Tran, H.T.; Nguyen, C.X.; Moon, Y.-H.; Chu, H.H.; Do, P.T. An efficient hairy root system for validation of plant transformation vector and CRISPR/Cas construct activities in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 12, 770062. [Google Scholar] [CrossRef]
- Liu, B.; Santo Domingo, M.; Mayobre, C.; Martín-Hernández, A.M.; Pujol, M.; Garcia-Mas, J. Knock-out of CmNAC-NOR affects melon climacteric fruit ripening. Front. Plant Sci. 2022, 13, 878037. [Google Scholar] [CrossRef]
- Shirazi Parsa, H.; Sabet, M.S.; Moieni, A.; Shojaeiyan, A.; Dogimont, C.; Boualem, A.; Bendahmane, A. CRISPR/Cas9-mediated cytosine base editing using an improved transformation procedure in melon (Cucumis melo L.). Int. J. Mol. Sci. 2023, 24, 11189. [Google Scholar] [CrossRef]
- Giordano, A.; Santo Domingo, M.; Quadrana, L.; Pujol, M.; Martín-Hernández, A.M.; Garcia-Mas, J. CRISPR/Cas9 gene editing uncovers the roles of CONSTITUTIVE TRIPLE RESPONSE 1 and REPRESSOR OF SILENCING 1 in melon fruit ripening and epigenetic regulation. J. Exp. Bot. 2022, 73, 4022–4033. [Google Scholar] [CrossRef] [PubMed]
- Hooghvorst, I.; López-Cristoffanini, C.; Nogués, S. Efficient knockout of phytoene desaturase gene using CRISPR/Cas9 in melon. Sci. Rep. 2019, 9, 17077. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Ito, M.; Ezura, H. Targeted modification of CmACO1 by CRISPR/Cas9 extends the shelf-life of Cucumis melo var. reticulatus melon. Front. Genome Ed. 2023, 5, 1176125. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wang, Z.; Zhang, X.; Zeng, H.; Ren, J.; Zhang, N.; Sun, Y.; Mi, T. Optimised agrobacterium-mediated transformation and application of developmental regulators improve regeneration efficiency in melons. Genes 2023, 14, 1432. [Google Scholar] [CrossRef] [PubMed]
- Pechar, G.S.; Donaire, L.; Gosalvez, B.; García-Almodovar, C.; Sánchez-Pina, M.A.; Truniger, V.; Aranda, M.A. Editing melon EIF4E associates with virus resistance and male sterility. Plant Biotechnol. J. 2022, 20, 2006–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef]
- Geng, S.; Sohail, H.; Cao, H.; Sun, J.; Chen, Z.; Zhou, L.; Wang, W.; Ye, R.; Yang, L.; Bie, Z. An efficient root transformation system for CRISPR/Cas9-based analyses of shoot–root communication in Cucurbit crops. Hortic. Res. 2022, 9, uhab082. [Google Scholar] [CrossRef]
- Chang, J.; Guo, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X.; et al. The role of watermelon caffeic acid o-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Li, H.; Wang, Z.; Jiang, Y.; Guo, Y.; Sun, P.; Chen, X.; Li, Q.; Tian, H.; et al. Disruption of REC8 in Meiosis I led to watermelon seedless. Plant Sci. 2022, 323, 111394. [Google Scholar] [CrossRef]
- Ren, Y.; Li, M.; Guo, S.; Sun, H.; Zhao, J.; Zhang, J.; Liu, G.; He, H.; Tian, S.; Yu, Y.; et al. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell 2021, 33, 1554–1573. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Zong, M.; Guo, S.; Ren, Z.; Zhao, J.; Li, M.; Zhang, J.; Tian, S.; Wang, J.; et al. Localization shift of a sugar transporter contributes to phloem unloading in sweet watermelons. New Phytol. 2020, 227, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrullus lanatus) using a chimeric ClGRF4-GIF1 gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhang, J.; Ren, Y.; Li, M.; Tian, S.; Yu, Y.; Zuo, Y.; Gong, G.; Zhang, H.; et al. The NAC transcription factor ClNAC68 positively regulates sugar content and seed development in watermelon by repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Guo, S.; Tian, S.; Zhang, J.; Ren, Y.; Li, M.; Gong, G.; Zhang, H.; Xu, Y. CRISPR/Cas9-mediated mutagenesis of ClBG1 decreased seed size and promoted seed germination in watermelon. Hortic. Res. 2021, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Gao, Q.; Zhang, J.; Zong, M.; Zhang, H.; Ren, Y.; Guo, S.; Gong, G.; Liu, F.; et al. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2017, 36, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, S.; Ji, G.; Zhao, H.; Sun, H.; Ren, Y.; Tian, S.; Li, M.; Gong, G.; Zhang, H.; et al. A Unique chromosome translocation disrupting ClWIP1 leads to gynoecy in watermelon. Plant J. 2020, 101, 265–277. [Google Scholar] [CrossRef]
- Zhang, R.; Chang, J.; Li, J.; Lan, G.; Xuan, C.; Li, H.; Ma, J.; Zhang, Y.; Yang, J.; Tian, S.; et al. Disruption of the BHLH transcription factor abnormal Tapetum 1 causes male sterility in watermelon. Hortic. Res. 2021, 8, 258. [Google Scholar] [CrossRef]
- Pan, W.; Cheng, Z.; Han, Z.; Yang, H.; Zhang, W.; Zhang, H. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing of watermelon assisted by genes encoding developmental regulators. J. Zhejiang Univ. Sci. B 2022, 23, 339–344. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, X.; Song, M.; Ma, S.; Tian, Y.; Gao, L. Peat-based hairy root transformation using Rhizobium rhizogenes as a rapid and efficient tool for easily exploring potential genes related to root-knot nematode parasitism and host response. Plant Methods 2023, 19, 22. [Google Scholar] [CrossRef]
- Nizan, S.; Amitzur, A.; Dahan-Meir, T.; Benichou, J.I.C.; Bar-Ziv, A.; Perl-Treves, R. Mutagenesis of the melon Prv gene by CRISPR/Cas9 breaks papaya ringspot virus resistance and generates an autoimmune allele with constitutive defense responses. J. Exp. Bot. 2023, 74, 4579–4596. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, S.; Meng, D.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is a key regulator of root growth and development. Physiol. Plant. 2023, 175, e13977. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, J.; Zhao, H.; Zong, M.; Li, M.; Gong, G.; Wang, J.; Zhang, J.; Ren, Y.; Zhang, H.; et al. Production of double haploid watermelon via maternal haploid induction. Plant Biotechnol. J. 2023, 21, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, L.; Chen, Z.; Huang, Y.; Yang, L.; Wang, P.; Xue, S.; Bie, Z. CmCNIH1 improves salt tolerance by influencing the trafficking of CmHKT1;1 in pumpkin. Plant J. 2023, 114, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, M.; Hu, Q.; Yan, W.; Pan, J.; Yan, Y.; Chen, X. A CsEIL3-CsARN6.1 module promotes waterlogging-triggered adventitious root formation in cucumber by activating the expression of CsPrx5. Plant J. 2023, 114, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.; Sun, C.; Song, X.; Li, X.; Cui, H.; Guo, J.; Liu, L.; Ying, A.; Zhang, Z.; et al. CsTRM5 regulates fruit shape via mediating cell division direction and cell expansion in cucumber. Hortic. Res. 2023, 10, uhad007. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Shen, J.; Niu, Y.; Zhao, H.; Guo, Y.; Sun, P.; Yang, T.; Jiang, Y.; Zhao, B.; Wang, Z.; et al. Cucumber NUCLEAR FACTOR-YC2/-YC9 target translocon component CsTIC21 in chloroplast photomorphogenesis. Plant Physiol. 2023, 192, 2822–2837. [Google Scholar] [CrossRef]
- Leibman, D.; Pashkovsky, E.; Shnaider, Y.; Shtarkman, M.; Gaba, V.; Gal-On, A. Analysis of the RNA-dependent RNA polymerase 1 (RDR1) gene family in melon. Plants 2022, 11, 1795. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Yang, X.; Xu, J.; Liu, G.; Yao, X.; Ren, R.; Xu, J.; Lou, L. CRISPR/Cas9-mediated mutagenesis of Clpsk1 in watermelon to confer resistance to Fusarium oxysporum f.sp. niveum. Plant Cell Rep. 2020, 39, 589–595. [Google Scholar] [CrossRef]
- Sohail, H.; Noor, I.; Nawaz, M.A.; Ma, M.; Shireen, F.; Huang, Y.; Yang, L.; Bie, Z. Genome-wide identification of plasma-membrane intrinsic proteins in pumpkin and functional characterization of CmoPIP1-4 under salinity stress. Environ. Exp. Bot. 2022, 202, 104995. [Google Scholar] [CrossRef]
- Duan, Y.; Li, H.; Amanullah, S.; Bao, X.; Guo, Y.; Liu, X.; Xu, H.; Liu, J.; Gao, Y.; Yuan, C.; et al. A single nucleotide mutation in ClphyB gene is associated with a short lateral branch phenotype in watermelon. Sci. Hortic. 2023, 321, 112378. [Google Scholar] [CrossRef]
- Pawełkowicz, M.E.; Skarzyńska, A.; Pląder, W.; Przybecki, Z. Genetic and molecular bases of cucumber (Cucumis sativus L.) sex determination. Mol. Breed. 2019, 39, 1–27. [Google Scholar] [CrossRef]
- Young, T.E.; Meeley, R.B.; Gallie, D.R. ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J. 2004, 40, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Li, Z.; Liu, M. Role of ethylene response factors (ERFs) in fruit ripening. Food Qual. Saf. 2020, 4, 15–20. [Google Scholar] [CrossRef]
- Yamamuro, C.; Miki, D.; Zheng, Z.; Ma, J.; Wang, J.; Yang, Z.; Dong, J.; Zhu, J.-K. Overproduction of stomatal lineage cells in Arabidopsis mutants defective in active DNA demethylation. Nat. Commun. 2014, 5, 4062. [Google Scholar] [CrossRef]
- Sharma, E.; Sharma, R.; Borah, P.; Jain, M.; Khurana, J.P. Emerging roles of auxin in abiotic stress responses. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G., Ed.; Springer: New York, NY, USA, 2015; pp. 299–329. [Google Scholar] [CrossRef]
- Mandal, S.; Ghorai, M.; Anand, U.; Samanta, D.; Kant, N.; Mishra, T.; Rahman, M.H.; Jha, N.K.; Jha, S.K.; Lal, M.K.; et al. Cytokinin and abiotic stress tolerance -What has been accomplished and the way forward? Front. Genet. 2022, 13, 943025. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Gallavotti, A. The role of auxin in shaping shoot architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-generation breeding strategies for climate-ready crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.N.; Rahman, F.U.; Hussain, Z.; Khan, I.A.; Aslam, M.M.; Aslam, M.; Waheed, H.; Khan, A.U.; Iqbal, S. Effect of salinity stress on germination, seedling growth, mineral uptake and chlorophyll contents of three Cucurbitaceae species. Braz. Arch. Biol. Techn. 2022, 65, e22210213. [Google Scholar] [CrossRef]
- Li, S.; Miao, L.; Huang, B.; Gao, L.; He, C.; Yan, Y.; Wang, J.; Yu, X.; Li, Y. Genome-wide identification and characterization of cucumber BPC transcription factors and their responses to abiotic stresses and exogenous phytohormones. Int. J. Mol. Sci. 2019, 20, 5048. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, Y.; Yang, L.; He, H.; Huang, Y.; Fang, L.; Scheller, H.V.; Jiang, M.; Zhang, A. Cell wall β-1,4-galactan regulated by the BPC1/BPC2-GALS1 module aggravates salt sensitivity in Arabidopsis thaliana. Mol. Plant 2021, 14, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Fang, L. BASIC PENTACYSTEINE2 negatively regulates osmotic stress tolerance by modulating LEA4-5 expression in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 168, 373–380. [Google Scholar] [CrossRef]
- Xuan, C.; Lan, G.; Si, F.; Zeng, Z.; Wang, C.; Yadav, V.; Wei, C.; Zhang, X. Systematic genome-wide study and expression analysis of SWEET gene family: Sugar transporter family contributes to biotic and abiotic stimuli in watermelon. Int. J. Mol. Sci. 2021, 22, 8407. [Google Scholar] [CrossRef] [PubMed]
- Zitter, T.A.; Hopkins, D.L.; Thomas, C.E. Compendium of Cucurbits Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Fellner, M.; Horton, L.A.; Cocke, A.E.; Stephens, N.R.; Ford, D.E.; Van Volkenburgh, E. Light interacts with auxin during leaf elongation and leaf angle development in young corn seedlings. Planta 2003, 216, 366–376. [Google Scholar] [CrossRef]
- Wang, X.; Chang, C. Exploring and exploiting cuticle biosynthesis for abiotic and biotic stress tolerance in wheat and barley. Front. Plant Sci. 2022, 13, 1064390. [Google Scholar] [CrossRef]
- Cartagena Protocol on Biosafety to the Convention on Biological Diversity. Available online: https://www.cbd.int/doc/legal/cartagena-protocol-en.pdf (accessed on 15 October 2023).
- The International Union for the Protection of New Varieties of Plants. Available online: https://www.upov.int/portal/index.html.en (accessed on 10 October 2023).
- Adoption of Agricultural Production Practices: Lessons Learned from the U.S. Department of Agriculture Area Studies Project. Available online: https://www.ers.usda.gov/webdocs/publications/41192/32131_aer792.pdf?v=0 (accessed on 20 October 2023).
- Judgment of the Court (Grand Chamber). Available online: https://www.iucn.org/sites/default/files/content/documents/2018/judgment_of_the_court_-_c-528-16.pdf (accessed on 20 October 2023).
- Callaway, E. CRISPR plants now subject to tough GM laws in European Union. Nature 2018, 560, 16–17. [Google Scholar] [CrossRef]
- The Nagoya Protocol—Convention on Biological Diversity. Available online: https://www.dcceew.gov.au/science-research/australias-biological-resources/nagoya-protocol-convention-biological (accessed on 19 October 2023).
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Dangerous Liaisons? When Cultivated Plants Mate with Their Wild Relatives; The Johns Hopkins University Press: Baltimore, MD, USA, 2003. [Google Scholar]
- Losey, J.; Rayor, L.; Carter, M. Transgenic pollen harms monarch larvae. Nature 1999, 399, 214. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.D. The anthropology of genetically modified crops. Annu. Rev. Anthropol. 2010, 39, 381–400. [Google Scholar] [CrossRef]
- Wolt, J.D.; Wang, K.; Sashital, D.; Lawrence-Dill, C.J. Achieving plant CRISPR targeting that limits off-target effects. Plant Genome 2016, 9, 0047. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).