Response of Nicandra physalodes (Linn.) Gaertn. and Its Rhizospheric Organisms to the Selective Pressures of High-Concentration Oxytetracycline, Ciprofloxacin, and Tobramycin
Abstract
:1. Introduction
2. Material and Methods
2.1. Plants, Earthworms, Antibiotics, and Reagents
2.2. Soil Treatment, Earthworm Stocking, and Seed Sowing
2.3. Effects of Antibiotics on the Growth Behavior and Biomass of Nicandra physalodes (Linn.) Gaertn
2.4. Blood Coagulation Test
2.5. Effects of Antibiotics on Soil Enzyme Activities
2.6. Effects of Antibiotics on Nicandra Physalodes (Linn.) Gaertn. Rhizosphere Microorganism Diversity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Growth Behavior and Biomass
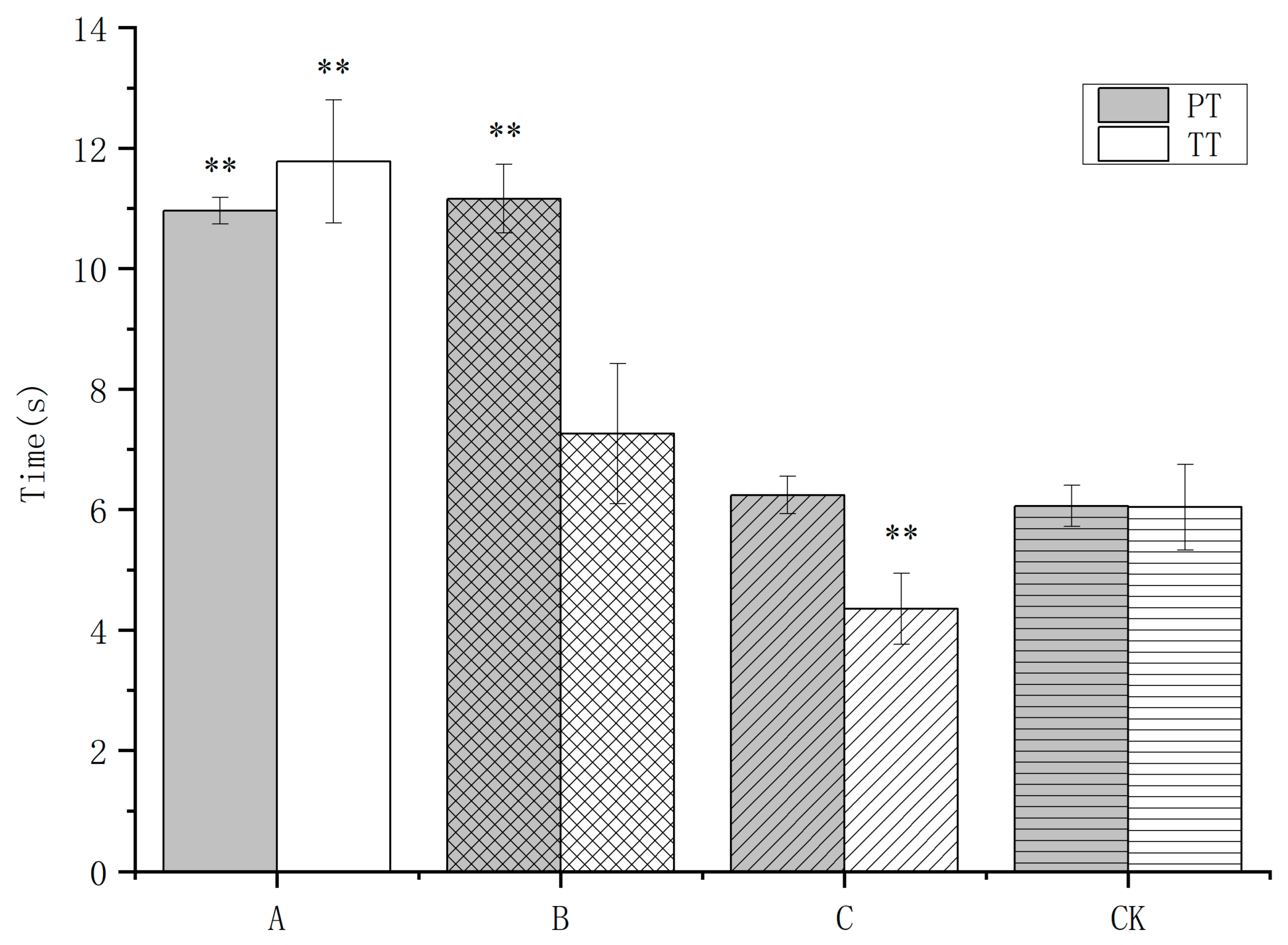
3.2. Earthworm Extract on the Coagulation Parameters PT and TT
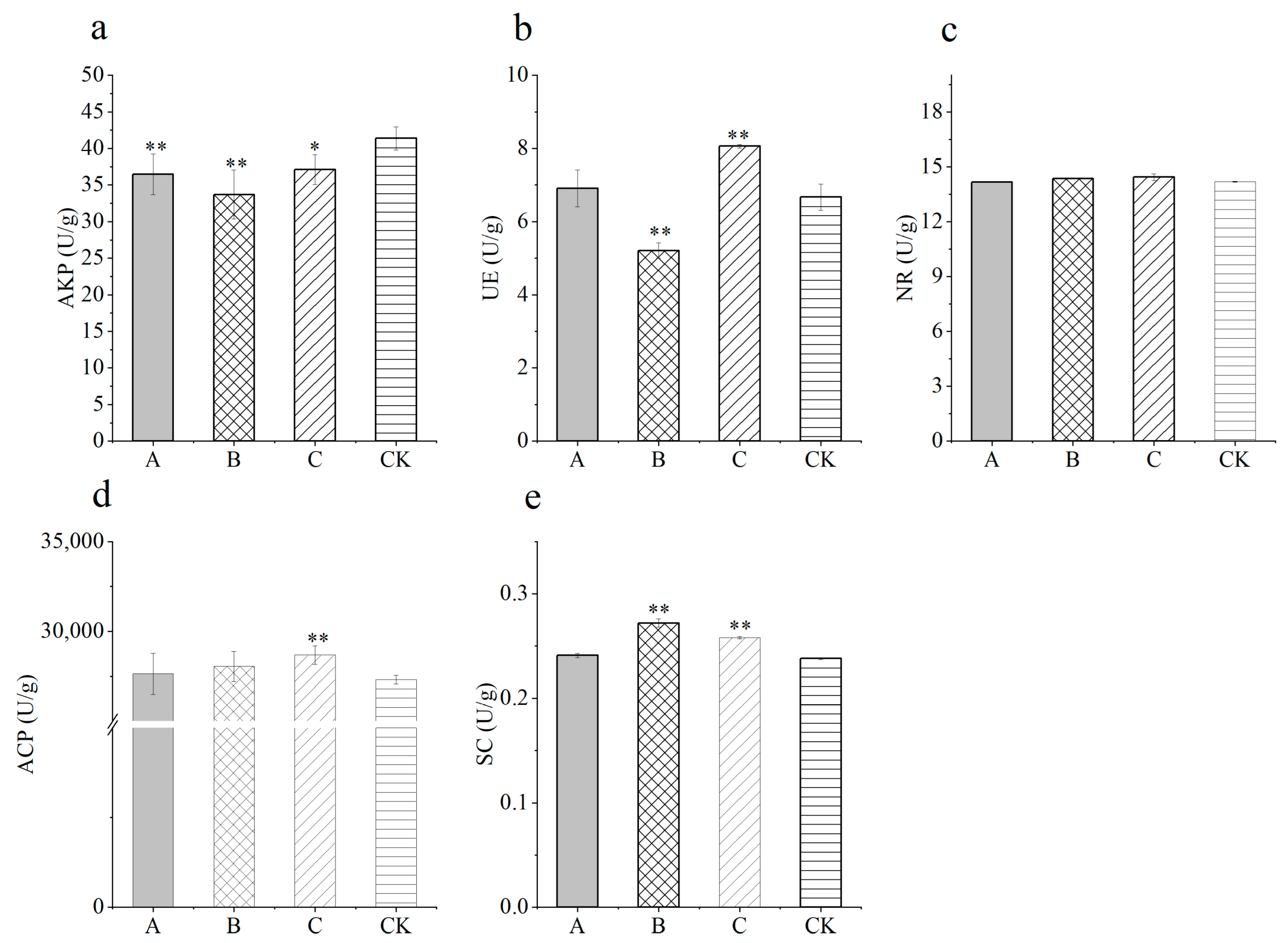
3.3. Soil Enzyme Activities
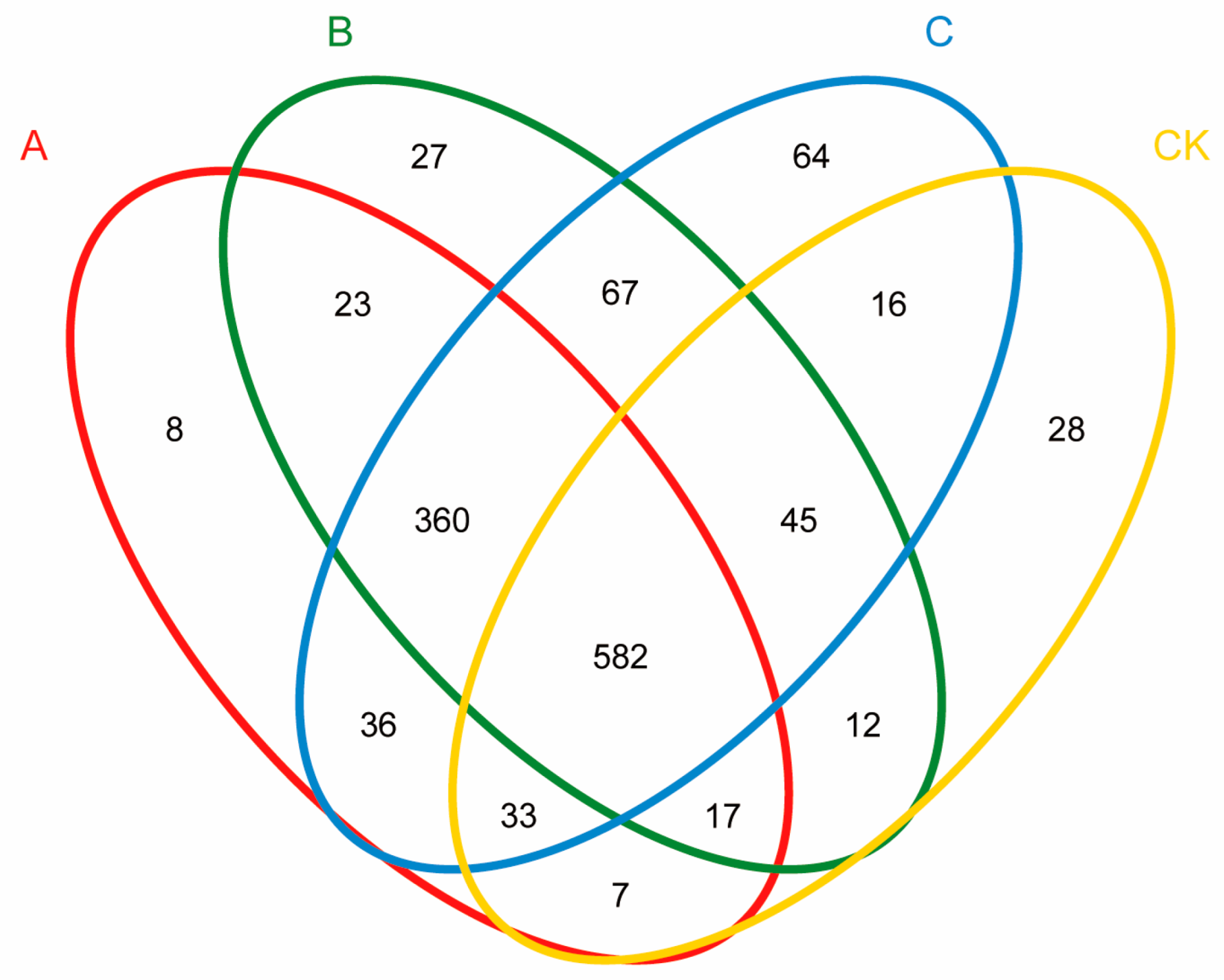
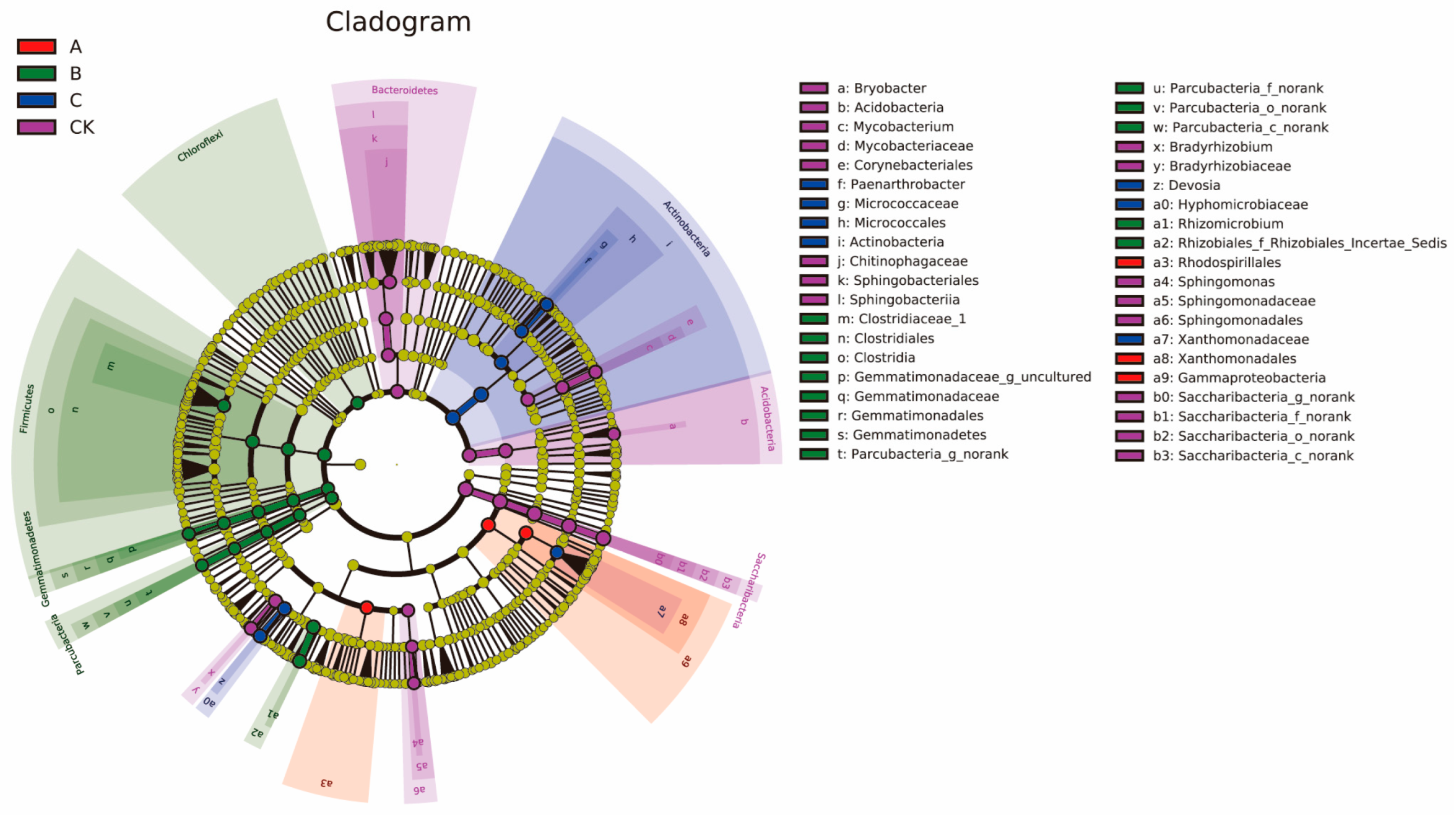
3.4. Influence of the Different Antibiotic Groups on Rhizosphere Bacterial Community Diversity
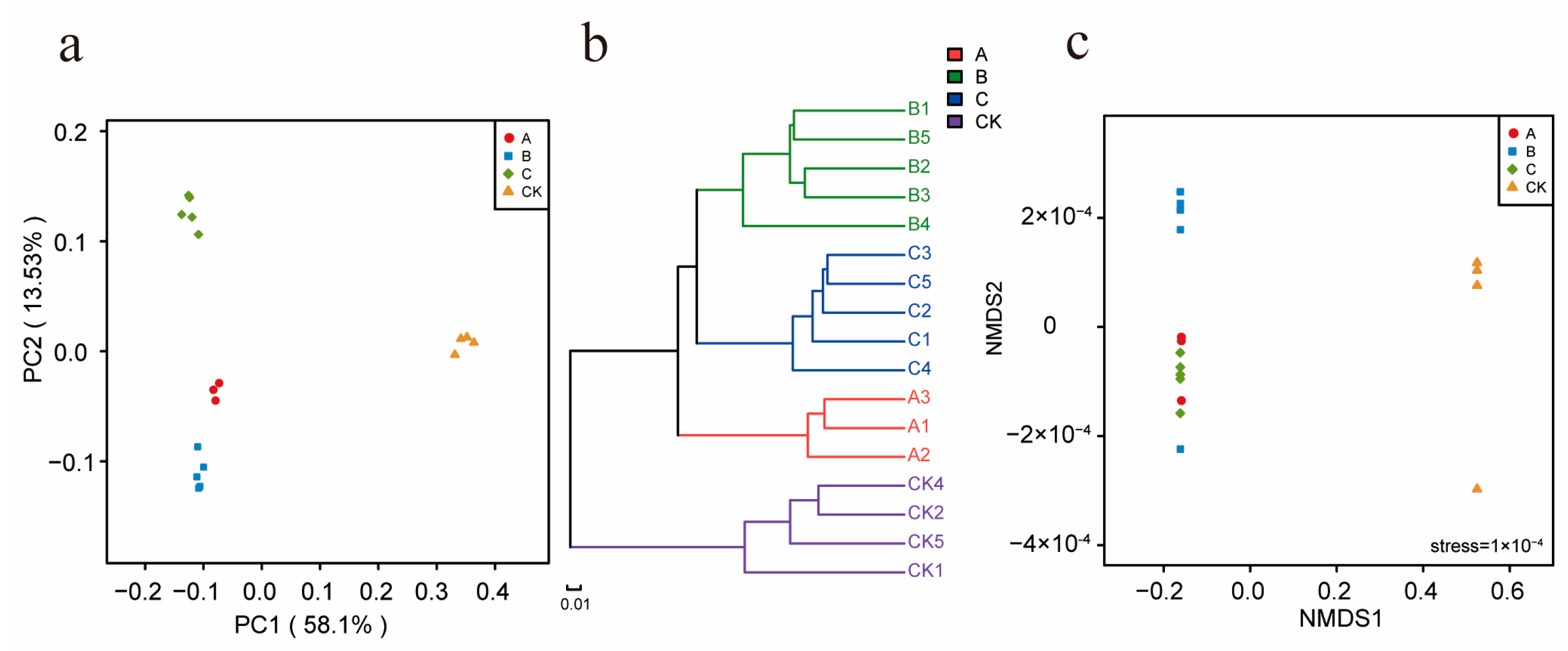
3.5. Impact of the Different Antibiotic Groups on the Rhizosphere Bacterial Community Structure
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brandt, K.K.; Amezquita, A.; Backhaus, T.; Boxall, A.; Coors, A.; Heberer, T.; Lawrence, J.R.; Lazorchak, J.; Schonfeld, J.; Snape, J.R.; et al. Ecotoxicological assessment of antibiotics: A call for improved consideration of microorganisms. Environ. Int. 2015, 85, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Picó, Y.; Andreu, V. Fluoroquinolones in soil—Risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Vazquez-Roig, P.; Blasco, C.; Pico, Y. Determination of tetracycline residues in soil by pressurized liquid extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1329–1339. [Google Scholar] [CrossRef]
- Bian, K.; Liu, Y.H.; Wang, Z.N.; Zhou, T.; Song, X.Q.; Zhang, F.Y.; He, L.M. Determination of multi-class antimicrobial residues in soil by liquid chromatography-tandem mass spectrometry. RSC Adv. 2015, 5, 27584–27593. [Google Scholar] [CrossRef]
- Yang, X.; Guo, C.; Yang, Y.; Yuan, K.; Yang, X.; Guo, Y. Rheological and gelling properties of Nicandra physalodes (Linn.) Gaertn. pectin in acidic media. Food. Chem. 2022, 373 Pt B, 131711. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, L.; Li, S.; Xie, J.; Xue, X.; Jiang, Y. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef]
- Jalilian, F.; Valeo, C.; Chu, A.; Bhiladvala, R. Sensors for biomass monitoring in vegetated green infrastructure: A review. Sensors 2023, 23, 6404. [Google Scholar] [CrossRef]
- Fu, Y.T.; Chen, K.Y.; Chen, Y.S.; Yao, C.H. Earthworm (Pheretima aspergillum) extract stimulates osteoblast activity and inhibits osteoclast differentiation. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef]
- Albert, V.; Arulselvi, S.; Agrawal, D.; Pati, H.P.; Pandey, R.M. Early posttraumatic changes in coagulation and fibrinolysis systems in isolated severe traumatic brain injury patients and its influence on immediate outcome. Hematol. Oncol. Stem Cell Ther. 2019, 12, 32–43. [Google Scholar] [CrossRef]
- Telesinski, A.; Platkowski, M.; Cybulska, K.; Telesinska, N.; Wrobel, J.; Pawlowska, B. Response of soil enzymes to two antibiotics: Polymyxin B and penicillin G. Fresenius Environ. Bull. 2018, 27, 3837–3845. [Google Scholar] [CrossRef]
- Vasileiadis, S.; Puglisi, E.; Arena, M.; Cappa, F.; Cocconcelli, P.S.; Trevisan, M. Soil bacterial diversity screening using single 16s rRNA gene V regions coupled with multi-million read generating sequencing technologies. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef]
- Oliveira, C.; Shakiba, E.; North, D.; McGraw, M.; Ballard, E.; Barrett-D’Amico, M.; Glazko, G.; Rahmatallah, Y. 16S rRNA Gene-based metagenomic analysis of rhizosphere soil bacteria in Arkansas rice Crop Fields. Agronomy 2022, 12, 13. [Google Scholar] [CrossRef]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.J.; Lavoie, M.; Jin, Y.J.; Fan, X.J.; Zhang, Z.Y.; Fu, Z.W.; Sun, L.W.; Gillings, M.; Penuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 12. [Google Scholar] [CrossRef]
- Zhu, T.; Meng, X.B.; Dong, D.X.; Zhao, L.Y.; Qu, M.W.; Sun, G.B.; Sun, X.B. Xuesaitong injection (lyophilized) combined with aspirin and clopidogrel protect against focal cerebral ischemic/reperfusion injury in rats by suppressing oxidative stress and inflammation and regulating the NOX2/IL-6/STAT3 pathway. Ann. Palliat. Med. 2021, 10, 1650–1667. [Google Scholar] [CrossRef]
- Ren, Y.; Ai, J.; Liu, X.; Liang, S.; Zheng, Y.; Deng, X.; Li, Y.; Wang, J.; Deng, X.; Chen, L.L. Anticoagulant active ingredients identification of total saponin extraction of different Panax medicinal plants based on grey relational analysis combined with UPLC-MS and molecular docking. J. Ethnopharmacol. 2020, 260, 112955. [Google Scholar] [CrossRef]
- Jastrzbska, E.; Kucharski, J. Dehydrogenases, urease and phosphatases activities of soil contaminated with fungicides. Plant Soil Environ. 2007, 53, 51–57. [Google Scholar] [CrossRef]
- Berg, G.; Zachow, C.; Lottmann, J.; Gotz, M.; Costa, R.; Smalla, K. Impact of plant species and site on rhizosphere-associated fungi antagonistic to Verticillium dahliae Kleb. Appl. Environ. Microbiol. 2005, 71, 4203–4213. [Google Scholar] [CrossRef]
- Song, L.Y.; Yang, S.; Liu, H.J.; Xu, J. Geographic and environmental sources of variation in bacterial community composition in a large-scale municipal landfill site in China. Appl. Microbiol. Biotechnol. 2017, 101, 761–769. [Google Scholar] [CrossRef]
- Panda, S.; El khader, I.; Casellas, F.; López Vivancos, J.; García Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef]
- Knecht, H.; Neulinger, S.C.; Heinsen, F.A.; Knecht, C.; Schilhabel, A.; Schmitz, R.A.; Zimmermann, A.; Dos Santos, V.M.; Ferrer, M.; Rosenstiel, P.C.; et al. Effects of β-lactam antibiotics and fluoroquinolones on human gut microbiota in relation to Clostridium difficile associated diarrhea. PLoS ONE 2014, 9, e89417. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chellemi, D.O.; Graham, J.H.; Martin, K.J.; Rosskopf, E.N. Comparison of soil bacterial communities under diverse agricultural land management and crop production practices. Microb. Ecol. 2008, 55, 293–310. [Google Scholar] [CrossRef] [PubMed]





| Groups | OTUs (Total in the All Replicates/Shared by the All Replicates) | OTUs Richness (X ± SD) | Ace | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|---|
| CK | 740/572 | 572.25 ± 12.26 | 655 ± 16.83 | 655.75 ± 13.28 | 4.49 ± 0.25 | 0.033 ± 0.013 |
| A | 1047/890 | 890.33 ± 46.36 ** | 1031 ± 44.03 **, ## | 1046.33 ± 38 **, ## | 5.1 ± 0.12 ** | 0.014 ± 0.002 ** |
| B | 1133/920 | 920.6 ± 21.23 ** | 1014.2 ± 29.24 **, ## | 1016.8 ± 28.4 **, ## | 5.17 ± 0.1 ** | 0.015 ± 0.003 ** |
| C | 1203/999 | 999.2 ± 16.71 ** | 1098.6 ± 23.43 ** | 1109 ± 18.53 ** | 5.25 ± 0.07 ** | 0.014 ± 0.002 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Lai, X.; Zhao, X.; Wang, L.; A, G.; Yin, X.; Ren, Z.; Chen, C. Response of Nicandra physalodes (Linn.) Gaertn. and Its Rhizospheric Organisms to the Selective Pressures of High-Concentration Oxytetracycline, Ciprofloxacin, and Tobramycin. Agriculture 2023, 13, 1793. https://doi.org/10.3390/agriculture13091793
Xia Z, Lai X, Zhao X, Wang L, A G, Yin X, Ren Z, Chen C. Response of Nicandra physalodes (Linn.) Gaertn. and Its Rhizospheric Organisms to the Selective Pressures of High-Concentration Oxytetracycline, Ciprofloxacin, and Tobramycin. Agriculture. 2023; 13(9):1793. https://doi.org/10.3390/agriculture13091793
Chicago/Turabian StyleXia, Zhaobin, Xinuo Lai, Xing Zhao, Lu Wang, Gayuebumo A, Xiangyu Yin, Zhihua Ren, and Chaoxi Chen. 2023. "Response of Nicandra physalodes (Linn.) Gaertn. and Its Rhizospheric Organisms to the Selective Pressures of High-Concentration Oxytetracycline, Ciprofloxacin, and Tobramycin" Agriculture 13, no. 9: 1793. https://doi.org/10.3390/agriculture13091793
APA StyleXia, Z., Lai, X., Zhao, X., Wang, L., A, G., Yin, X., Ren, Z., & Chen, C. (2023). Response of Nicandra physalodes (Linn.) Gaertn. and Its Rhizospheric Organisms to the Selective Pressures of High-Concentration Oxytetracycline, Ciprofloxacin, and Tobramycin. Agriculture, 13(9), 1793. https://doi.org/10.3390/agriculture13091793






