A Crop of High Nutritional Quality and Health Maintenance Value: The Importance of Tartary Buckwheat Breeding
Abstract
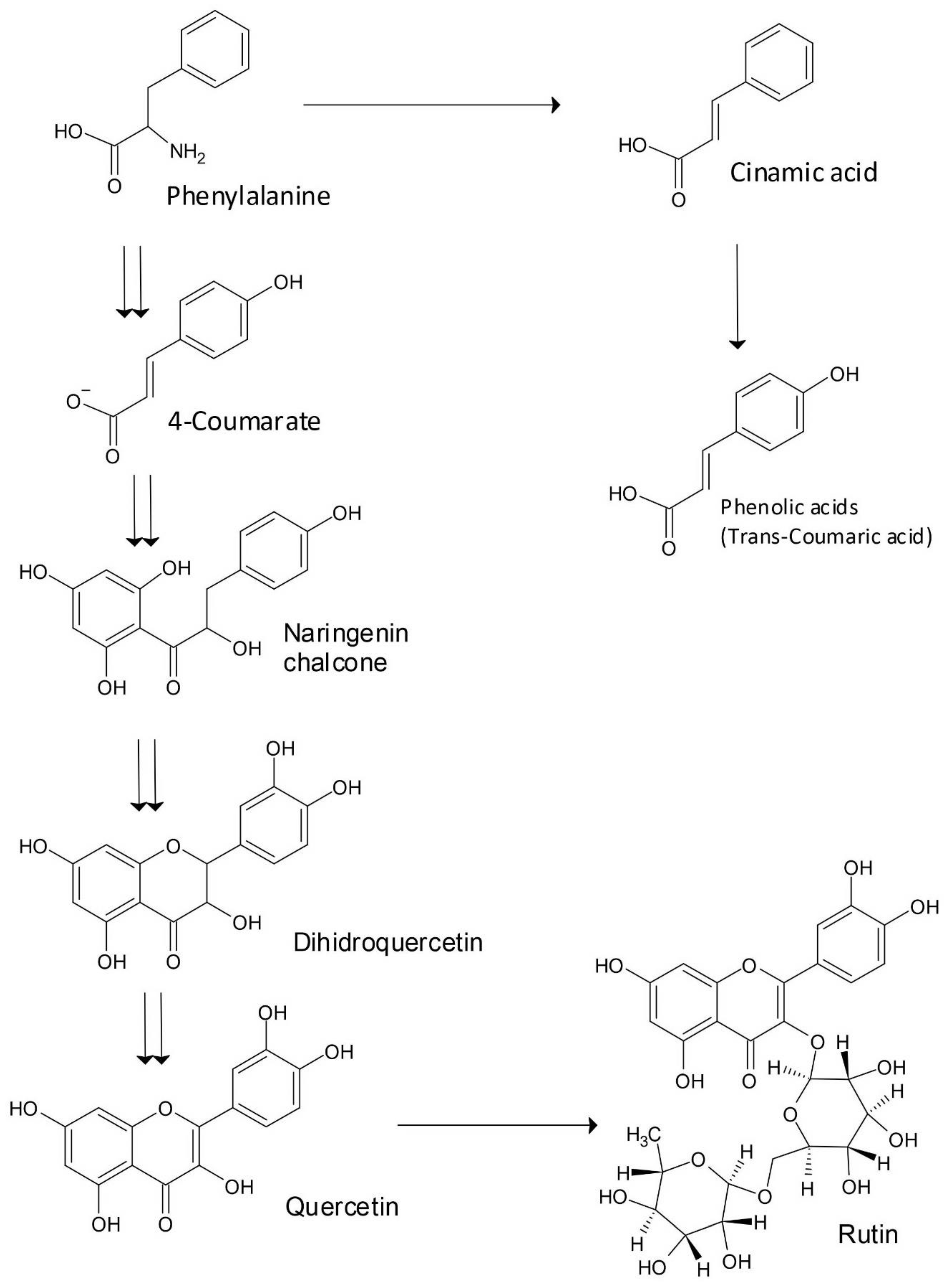
1. Introduction
2. Bioactivity of Flavonoids Rutin and Quercetin
3. Precautions for Possible Adverse Effects of Tartary Buckwheat Metabolites
4. Main Goals and Methods for Breeding Tartary Buckwheat
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreft, I. (Ed.) Bitter Seed Tartary Buckwheat; Slovenian Academy of Sciences and Arts; Maribor: Fagopyrum—Slovenian Association for Buckwheat Promotion: Ljubljana, Slovenia, 2022. [Google Scholar]
- Kreft, I.; Vollmannová, A.; Lidiková, J.; Musilová, J.; Germ, M.; Golob, A.; Vombergar, B.; Kocjan Ačko, D.; Luthar, Z. Molecular Shield for Protection of Buckwheat Plants from UV-B Radiation. Molecules 2022, 27, 5577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.; et al. FtSAD2 and FtJAZ1 Regulate Activity of the FtMYB11 Transcription Repressor of the Phenylpropanoid Pathway in Fagopyrum Tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef]
- Chitarrini, G.; Nobili, C.; Pinzari, F.; Antonini, A.; De Rossi, P.; Del Fiore, A.; Procacci, S.; Tolaini, V.; Scala, V.; Scarpari, M.; et al. Buckwheat Achenes Antioxidant Profile Modulates Aspergillus Flavus Growth and Aflatoxin Production. Int. J. Food Microbiol. 2014, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal Biotransformation of Chlorogenic and Caffeic Acids by Fusarium Graminearum: New Insights in the Contribution of Phenolic Acids to Resistance to Deoxynivalenol Accumulation in Cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Huang, Y.; Jiang, Y.; Liu, Y.; Wen, W.; Li, H.; Shao, J.; Wang, C.; Zhu, X. Antioxidant Capacity, Metal Contents, and Their Health Risk Assessment of Tartary Buckwheat Teas. ACS Omega 2020, 5, 9724–9732. [Google Scholar] [CrossRef] [PubMed]
- Nobili, C.; De Acutis, A.; Reverberi, M.; Bello, C.; Leone, G.P.; Palumbo, D.; Natella, F.; Procacci, S.; Zjalic, S.; Brunori, A. Buckwheat Hull Extracts Inhibit Aspergillus Flavus Growth and AFB1 Biosynthesis. Front. Microbiol. 2019, 10, 1997. [Google Scholar] [CrossRef]
- Vombergar, B.; Luthar, Z. Starting Points for the Study of the Effects of Flavonoids, Tannins and Crude Proteins in Grain Fractions of Common Buckwheat (Fagopyrum esculentum Moench) and Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Folia Biol. Geol. 2018, 59, 101. [Google Scholar] [CrossRef]
- Vombergar, B. Rutin and Quercetin in Common Buckwheat and Tartary Buckwheat Flour. Folia Biol. Geol. 2020, 61, 257–280. [Google Scholar] [CrossRef]
- Vombergar, B. Rutin and Quercetin in Common and Tartary Buckwheat Flour and Dough. Fagopyrum 2021, 38, 43–53. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K. Acute and Subacute Toxicity Studies on Rutin-Rich Tartary Buckwheat Dough in Experimental Animals. J. Nutr. Sci. Vitaminol. 2015, 61, 175–181. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Characterization of Rutin-Rich Bread Made with ‘Manten-Kirari’, a Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 2015, 21, 733–738. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K.; Otsuka, S. Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Plants 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and Technological Properties of the Flour and Bran from Common and Tartary Buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat Phenolic Metabolites in Health and Disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Zivcak, M.; Phan Tran, L.-S. The Contribution of Buckwheat Genetic Resources to Health and Dietary Diversity. Curr. Genom. 2016, 17, 193–206. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Kosǐr, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Sytar, O.; Kosyan, A.; Taran, N.; Smetanska, I. Anthocyanin’s as Marker for Selection of Buckwheat Plants with High Rutin Content. Gesunde Pflanz. 2014, 66, 165–169. [Google Scholar] [CrossRef]
- Kuwabara, T.; Han, K.H.; Hashimoto, N.; Yamauchi, H.; Shimada, K.I.; Sekikawa, M.; Fukushima, M. Tartary Buckwheat Sprout Powder Lowers Plasma Cholesterol Level in Rats. J. Nutr. Sci. Vitaminol. 2007, 53, 501–507. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of Rutin-Rich Tartary Buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in Body Weight Reduction Related to Its Antioxidant Properties: A Randomised, Double-Blind, Placebo-Controlled Study. J. Funct. Foods 2016, 26, 460–469. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Mukasa, Y.; Takigawa, S.; Yokota, S.; Ishiguro, K.; Noda, T. Breeding of ‘Manten-Kirari’, a Non-Bitter and Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Breed. Sci. 2014, 64, 344–350. [Google Scholar] [CrossRef]
- Li, L.; Lietz, G.; Seal, C. Buckwheat and CVD Risk Markers: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Shimaoka, I.; Kayashita, J.; Yokoyama, F.; Nakajoh, M.; Kato, N. A Buckwheat Protein Product Suppresses Gallstone Formation and Plasma Cholesterol More Strongly than Soy Protein Isolate in Hamsters. J. Nutr. 2000, 130, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High Protein Buckwheat Flour Suppresses Hypercholesterolemia in Rats and Gallstone Formation in Mice by Hypercholesterolemic Diet and Body Fat in Rats Because of Its Low Protein Digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef]
- Tomotake, H.; Yamamoto, N.; Kitabayashi, H.; Kawakami, A.; Kayashita, J.; Ohinata, H.; Karasawa, H.; Kato, N. Preparation of Tartary Buckwheat Protein Product and Its Improving Effect on Cholesterol Metabolism in Rats and Mice Fed Cholesterol-Enriched Diet. J. Food Sci. 2007, 72, S528–S533. [Google Scholar] [CrossRef]
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary Buckwheat in Human Nutrition. Plants 2021, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kwon, S.J.; Choi, J.Y.; Ju, Y.H.; Roy, S.K.; Lee, D.-G.; Park, C.H.; Woo, S.-H. Variation of Rutin and Quercetin Contents in Tartary Buckwheat Germplasm. Fagopyrum 2019, 36, 51–65. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative Metabolomics Study of Tartary (Fagopyrum tataricum (L.) Gaertn) and Common (Fagopyrum esculentum Moench) Buckwheat Seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, K.; Guo, W.; Zhang, C.; Chen, H.; Xu, T.; Lu, Y.; Wu, Q.; Li, Y.; Chen, Y. Aspergillus Niger Fermented Tartary Buckwheat Ameliorates Obesity and Gut Microbiota Dysbiosis through the NLRP3/Caspase-1 Signaling Pathway in High-Fat Diet Mice. J. Funct. Foods 2022, 95, 105171. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.; Zhu, H.; He, Z.; Liu, J.; Hao, W.; Jiao, R.; et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The Temperature Threshold for the Transformation of Rutin to Quercetin in Tartary Buckwheat Dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Yoshihashi, T. Heat-Treatment of Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Provides Dehulled and Gelatinized Product with Denatured Rutinosidase. Food Sci. Technol. Res. 2019, 25, 613–618. [Google Scholar] [CrossRef]
- Asami, Y.; Ikeda, S.; Ikeda, K. Leaving Buckwheat Noodles after Their Making and Subsequent Cooking Leads to Remarkable Changes in Mechanical Characteristics. Fagopyrum 2022, 39, 5–11. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.B.; Choi, Y.S.; Heo, K.; Kim, S.L.; Lee, K.C.; Chang, K.J.; Lee, H.B. Rutin Content in Food Products Processed from Groats, Leaves, and Flowers of Buckwheat. Fagopyrum 2000, 17, 63–66. [Google Scholar]
- Park, M.O.; Kim, H.J.; Choi, I.Y.; Park, C.H. Development and Utilization of Buckwheat Sprouts in Korea. Fagopyrum 2022, 39, 19–26. [Google Scholar] [CrossRef]
- Ikeda, K.; Ishida, Y.; Ikeda, S.; Asami, Y.; Lin, R. Tartary, but Not Common, Buckwheat Inhibits α-Glucosidase Activity: Its Nutritional Implications. Fagopyrum 2017, 34, 13–18. [Google Scholar] [CrossRef]
- Luo, K.; Zhou, X.; Zhang, G. The Impact of Tartary Buckwheat Extract on the Nutritional Property of Starch in a Whole Grain Context. J. Cereal Sci. 2019, 89, 102798. [Google Scholar] [CrossRef]
- Li, Y.; Gao, S.; Ji, X.; Liu, H.; Liu, N.; Yang, J.; Lu, M.; Han, L.; Wang, M. Evaluation Studies on Effects of Quercetin with Different Concentrations on the Physicochemical Properties and in Vitro Digestibility of Tartary Buckwheat Starch. Int. J. Biol. Macromol. 2020, 163, 1729–1737. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of Tartary Buckwheat, Rutin, and Quercetin on Lipid Metabolism in Rats during High Dietary Fat Intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Skrabanja, V.; Liljeberg Elmståhl, H.G.M.; Kreft, I.; Björck, I.M.E. Nutritional Properties of Starch in Buckwheat Products: Studies in Vitro and In Vivo. J. Agric. Food Chem. 2001, 49, 490–496. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Lineva, A.; Benković, E.T.; Kreft, S.; Kienzle, E. Remarkable Frequency of a History of Liver Disease in Dogs Fed Homemade Diets with Buckwheat. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2019, 47, 242–246. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef]
- Valido, E.; Stoyanov, J.; Gorreja, F.; Stojic, S.; Niehot, C.; Kiefte-de Jong, J.; Llanaj, E.; Muka, T.; Glisic, M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients 2023, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jiao, W.; Tang, Z.; Wang, C.; Li, Q.; Wei, M.; Song, S.; Du, L.; Jin, Y. Quercetin Inclusion Complex Gels Ameliorate Radiation-Induced Brain Injury by Regulating Gut Microbiota. Biomed. Pharmacother. 2023, 158, 114142. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Okahata, C.; Onozato, M.; Minami, T.; Maeshima, M.; Ogihara, K.; Yamazaki, S.; Takahashi, Y.; Nakamura, S. Buckwheat Flour and Its Starch Prevent Age-Related Cognitive Decline by Increasing Hippocampal BDNF Production in Senescence-Accelerated Mouse Prone 8 Mice. Nutrients 2022, 14, 2708. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood–Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wolff, A.; Antfolk, M.; Brodin, B.; Tenje, M. In Vitro Blood–Brain Barrier Models—An Overview of Established Models and New Microfluidic Approaches. J. Pharm. Sci. 2015, 104, 2727–2746. [Google Scholar] [CrossRef]
- Gupta, S.; Dhanda, S.; Sandhir, R. Anatomy and Physiology of Blood-Brain Barrier. In Brain Targeted Drug Delivery System; Academic Press: Cambridge, MA, USA, 2019; pp. 7–31. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and Related Polyphenols: New Insights and Implications for Their Bioactivity and Bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Woon, C.K.; Hui, W.K.; Abas, R.; Haron, M.H.; Das, S.; Lin, T.S. Natural Product-Based Nanomedicine: Recent Advances and Issues for the Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2022, 20, 1498–1518. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, H.; Li, P.; Zhao, H.; Li, R.; Yang, F. Quercetin Administration Following Hypoxia-Induced Neonatal Brain Damage Attenuates Later-Life Seizure Susceptibility and Anxiety-Related Behavior: Modulating Inflammatory Response. Front. Pediatr. 2022, 10, 73. [Google Scholar] [CrossRef]
- Cen, J.; Zhang, R.; Zhao, T.; Zhang, X.; Zhang, C.; Cui, J.; Zhao, K.; Duan, S.; Guo, Y. A Water-Soluble Quercetin Conjugate with Triple Targeting Exerts Neuron-Protective Effect on Cerebral Ischemia by Mitophagy Activation. Adv. Healthc. Mater. 2022, 11, 2200817. [Google Scholar] [CrossRef] [PubMed]
- Taïlé, J.; Bringart, M.; Planesse, C.; Patché, J.; Rondeau, P.; Veeren, B.; Clerc, P.; Gauvin-Bialecki, A.; Bourane, S.; Meilhac, O.; et al. Antioxidant Polyphenols of Antirhea Borbonica Medicinal Plant and Caffeic Acid Reduce Cerebrovascular, Inflammatory and Metabolic Disorders Aggravated by High-Fat Diet-Induced Obesity in a Mouse Model of Stroke. Antioxidants 2022, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Dogra, N.; Jakhmola-Mani, R.; Potshangbam, A.M.; Buch, S.; Pande Katare, D. CXCR4 as Possible Druggable Target Linking Inflammatory Bowel Disease and Parkinson’s Disease. Metab. Brain Dis. 2023, 1, 1079–1096. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, A.R.; Gonçalves, I.; Quintela, T.; Preissner, R.; Santos, C.R.A. The Druggability of Bitter Taste Receptors for the Treatment of Neurodegenerative Disorders. Biochem. Pharmacol. 2022, 197, 114915. [Google Scholar] [CrossRef] [PubMed]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The Effects of Buckwheat Leaf and Flower Extracts on Antioxidant Status in Mouse Organs. Oxid. Med. Cell. Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.M.; Lee, D.G.; Cho, S.; Yoon, Y.H.; Cho, E.J.; Lee, S. The N-Butanol Fraction and Rutin from Tartary Buckwheat Improve Cognition and Memory in an In Vivo Model of Amyloid-β-Induced Alzheimer’s Disease. J. Med. Food 2015, 18, 631–641. [Google Scholar] [CrossRef]
- Eguchi, K.; Anase, T.; Osuga, H. Plant Production Science Development of a High-Performance Liquid Chromatography Method to Determine the Fagopyrin Content of Tartary Buckwheat (Fagopyrum tartaricum Gaertn.) and Common Buckwheat (F. esculentum Moench). Plant Prod. Sci. 2009, 12, 475–480. [Google Scholar] [CrossRef]
- Benković, E.T.; Žigon, D.; Friedrich, M.; Plavec, J.; Kreft, S. Isolation, Analysis and Structures of Phototoxic Fagopyrins from Buckwheat. Food Chem. 2014, 143, 432–439. [Google Scholar] [CrossRef]
- Kočevar Glavač, N.; Stojilkovski, K.; Kreft, S.; Park, C.H.; Kreft, I. Determination of Fagopyrins, Rutin, and Quercetin in Tartary Buckwheat Products. LWT—Food Sci. Technol. 2017, 79, 423–427. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, K.T. Fagopyrins in Different Parts of Common Buckwheat (Fagopyrum esculentum) and Tartary Buckwheat (F. tataricum) during Growth. J. Food Compos. Anal. 2020, 86, 103354. [Google Scholar] [CrossRef]
- Szymański, S.; Majerz, I. Theoretical Studies on the Structure and Intramolecular Interactions of Fagopyrins—Natural Photosensitizers of Fagopyrum. Molecules 2022, 27, 3689. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Wieslander, G. A Review on Epidemiological and Clinical Studies on Buckwheat Allergy. Plants 2021, 10, 607. [Google Scholar] [CrossRef]
- Vogrinčič, M.; Kreft, I.; Filipič, M.; Žegura, B. Antigenotoxic Effect of Tartary (Fagopyrum tataricum) and Common (Fagopyrum esculentum) Buckwheat Flour. J. Med. Food 2013, 16, 944–952. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Evaluation of the Mutagenicity Potential of Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Using the Ames Test. J. Agric. Chem. Environ. 2016, 5, 100–105. [Google Scholar] [CrossRef][Green Version]
- Okamoto, T. Safety of Quercetin for Clinical Application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; Patton, E.; VanderVeen, B.N.; Unger, C.; Aladhami, A.; Enos, R.T.; Madero, S.; Chatzistamou, I.; Fan, D.; Murphy, E.A.; et al. Sub-Chronic Oral Toxicity Screening of Quercetin in Mice. BMC Complement. Med. Ther. 2022, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I. Breeding of Determinate Buckwheat. Fagopyrum 1989, 9, 57–59. [Google Scholar]
- Fesenko, N.V. A Genetic Factor Responsible for the Determinate Type of Plants in Buckwheat. Genetica 1968, 4, 165–166. [Google Scholar]
- Fesenko, N.V.; Martinenko, G.E. Contemporary Buckwheat Breeding Work in Russia. In Current Advances in Buckwheat Research, Proceedings of the 6th International Symposium Buckwheat, Ina, Japan, 24–29 August 1995; Shinshu University Press: Nagano, Japan, 1995; pp. 269–275. [Google Scholar]
- Ohsawa, R. Invited Review Current Status and Prospects of Common Buckwheat Breeding in Japan. Breed. Sci. 2020, 70, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Funatsuki, H.; Suvorova, G.; Sekimura, K. Determinate Type Variants in Japanese Buckwheat Lines. Breed. Sci. 1996, 46, 275–277. [Google Scholar] [CrossRef]
- Wang, Y.; Campbell, C.G. Tartary Buckwheat Breeding (Fagopyrum tataricum L. Gaertn.) through Hybridization with Its Rice-Tartary Type. Euphytica 2007, 156, 399–405. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Z.; Liang, C.; Liao, K.; Xiang, D.; Huang, J.; Wei, C.; Shi, T.; Chen, Q. Agronomic and Metabolomics Analysis of Rice-Tartary Buckwheat (Fagopyrum tataricum Gaertn) Bred by Hybridization. Sci. Rep. 2022, 12, 11986. [Google Scholar] [CrossRef] [PubMed]
- Mukasa, Y.; Suzuki, T.; Honda, Y. Suitability of Rice-Tartary Buckwheat for Crossbreeding and for Utilization of Rutin. Japan Agric. Res. Q. JARQ 2009, 43, 199–206. [Google Scholar] [CrossRef][Green Version]
- Duan, Y.; Yin, G.; He, R.; Yang, X.; Cai, S.; Wang, Y.; Lu, W.; Sun, D.; Wang, L.; Wang, Y.; et al. Identification of Candidate Genes for Easily-Shelled Traits in Tartary Buckwheat Based on BSA-Seq and RNA-Seq Methods. Euphytica 2022, 218, 91. [Google Scholar] [CrossRef]
- Wang, D.; Yang, T.; Li, Y.; Deng, F.; Dong, S.; Li, W.; He, Y.; Zhang, J.; Zou, L. Light Intensity—A Key Factor Affecting Flavonoid Content and Expression of Key Enzyme Genes of Flavonoid Synthesis in Tartary Buckwheat. Plants 2022, 11, 2165. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Zhang, K.; Li, S.; Chen, Y.; He, M.; He, F.; Gao, B.; Yang, D.; Fan, Y.; et al. Rewiring of the Seed Metabolome during Tartary Buckwheat Domestication. Plant Biotechnol. J. 2023, 21, 150–164. [Google Scholar] [CrossRef]
- He, Y.; Zhang, K.; Li, S.; Lu, X.; Zhao, H.; Guan, C.; Huang, X.; Shi, Y.; Kang, Z.; Fan, Y.; et al. Multiomics Analysis Reveals the Molecular Mechanisms Underlying Virulence in Rhizoctonia and Jasmonic Acid–Mediated Resistance in Tartary Buckwheat (Fagopyrum tataricum). Plant Cell 2023, 35, 2773–2798. [Google Scholar] [CrossRef]
- Meng, H.L.; Sun, P.Y.; Wang, J.R.; Sun, X.Q.; Zheng, C.Z.; Fan, T.; Chen, Q.F.; Li, H.Y. Comparative Physiological, Transcriptomic, and WGCNA Analyses Reveal the Key Genes and Regulatory Pathways Associated with Drought Tolerance in Tartary Buckwheat. Front. Plant Sci. 2022, 13, 985088. [Google Scholar] [CrossRef]
- Luthar, Z.; Fabjan, P.; Mlinarič, K. Biotechnological Methods for Buckwheat Breeding. Plants 2021, 10, 1547. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreft, I.; Golob, A.; Germ, M. A Crop of High Nutritional Quality and Health Maintenance Value: The Importance of Tartary Buckwheat Breeding. Agriculture 2023, 13, 1783. https://doi.org/10.3390/agriculture13091783
Kreft I, Golob A, Germ M. A Crop of High Nutritional Quality and Health Maintenance Value: The Importance of Tartary Buckwheat Breeding. Agriculture. 2023; 13(9):1783. https://doi.org/10.3390/agriculture13091783
Chicago/Turabian StyleKreft, Ivan, Aleksandra Golob, and Mateja Germ. 2023. "A Crop of High Nutritional Quality and Health Maintenance Value: The Importance of Tartary Buckwheat Breeding" Agriculture 13, no. 9: 1783. https://doi.org/10.3390/agriculture13091783
APA StyleKreft, I., Golob, A., & Germ, M. (2023). A Crop of High Nutritional Quality and Health Maintenance Value: The Importance of Tartary Buckwheat Breeding. Agriculture, 13(9), 1783. https://doi.org/10.3390/agriculture13091783






