Abstract
This study aims to evaluate the fermentative profile, fermentative losses, microbial populations, aerobic stability, chemical composition, and in situ degradability of total mixed ration silages based on forage cactus associated with xerophytic legumes. The treatments consisted of four total mixed ration silages based on forage cactus and concentrate (TMRC), associated with legumes such as Gliricidia sepium (TMRG), Leucaena (TMRL), and Senna obtusifolia (TMRS). There was a significant difference (p < 0.05) among the evaluated treatments for the pH and NH3-N (% of total N) variables. The pH values remained within the ideal range, from 4.2 to 4.4, and the NH3-N content ranged from 2.21 to 0.85%. The dry matter recovery for the evaluated treatments averaged 89%. The lactic acid bacteria (LAB) counts ranged from 5.0 to 6.3 log CFU/g among the evaluated silages, with TMRC presenting the lowest count at 5 log CFU/g in comparison with the total mixed ration silages associated with legume plants. All of the total mixed ration silages associated with legumes presented an average mold count of 3.3 log CFU/g. Yeast populations were observed only for TMRG at 5 log CFU/g. A higher aerobic stability was observed for TMRC, followed by TMRG and then TMRS. In conclusion, the total mixed ration silages associated with G. sepium and S. obtusifolia can be recommended based on their fermentation and nutritional value.
1. Introduction
In semi-arid regions, the scarcity of quality bulky feed is the main limiting factor for ruminant production. During drought periods, livestock rely on native cactus plants and foliage deposited in the soil as their primary sources of feed. This is because most of the shrubs and trees in the region, which represent important foraging resources, have a deciduous habit [1]. However, this senescent plant material has a low nutritional value, resulting in poor livestock performance. Therefore, the search for alternative feeds is imperative [2].
Among the species found in semi-arid regions are xerophytic legumes such as gliricidia (Gliricidia sepium), Leucaena (Leucaena leucocephala), and Senna obtusifolia, which produce high yields of nutritious green leaves during the rainy season [3]. Another notable species is forage cactus (Nopalea cochenilifera Salm-Dyck), which is widely spread in the Brazilian semi-arid region due to its adaptation to adverse climatic conditions and its high energy and water contents [4,5].
Considering that the production of these legume plants is concentrated during the rainy season, the excess forage can be stored as silage. However, legume plants generally have limitations for the silage process, mainly due to their high buffering capacity and low soluble carbohydrate content. To overcome these limitations, forage palm can be used as an additive source of soluble carbohydrates, offering a low-cost and readily available solution [6,7]. Furthermore, mixing forage palm silage with legume plants can help balance the soluble carbohydrate and crude protein contents of these forage species. When supplied separately, these contents may not meet the appropriate standards for silage and animal feed [2,8].
One approach to making legume plant silage associated with forage palm viable is by incorporating concentrated ingredients to increase the silage’s dry matter content. This mixture offers several advantages, including better nutrient utilization, increased digestibility of the concentrated ingredients, reduced nutritional losses in the silage, and the ability to acquire and store ingredients at lower costs during the rainy season [9,10]. Additionally, Santos et al. [10] reported that total ration silages improved rumen energy and protein efficiency and stabilized microbial activities in the silage.
Therefore, the study of G. sepium, L. leucocephala, and S. obtusifolia for total ration silage associated with forage palm aims to innovate the exploration of these xerophytic legume plants and employ new technologies for ruminant production systems in arid and semi-arid regions. Given the significance of these goals, the objective is to evaluate the effects of associating legume plants with forage palm silage as a complete ration on fermentative losses, fermentative profiles, microbial populations, aerobic stability, and nutritional value.
2. Materials and Methods
The experiment was carried at the Forage Sector belonging to the Universidade Federal do Maranhão, located in the Chapadinha-MA municipality, latitude 03°44′33″ S, longitude 43°21′21″ W. The climate is hot and humid tropical (Aw) according to Koppen’s classification. The experimental procedures were approved by the university’s Ethics Committee for Animal Experimentation under protocol number 23115.011059/2015-26.
The statistical design adopted for this experiment was completely randomized, with four treatments and five replicates. The obtained treatments were the control treatment (total mixed ration silage based on forage cactus and concentrate—TMRC); total mixed ration silage based on forage cactus and G. sepium—TMRG; total mixed ration silage based on forage cactus and L. leucocephala—TMRL; and total mixed ration silage based on forage cactus and S. obtusifolia—TMRS.
The forage cactus used was the miúda cultivar (Nopalea cochenillifera Salm Dyck), and the secondary cladodes with two years of regrowth were harvested from a forage cactus cultivation. The legume plants were collected from an agroforestry field. For the formulation of the experimental rations, the following concentrate ingredients were used: ground corn, soybean meal, and minerals. The chemical compositions of the ingredients that were used are described in Table 1.

Table 1.
Chemical compositions of the ingredients of the diets.
The experimental diets were formulated to meet the nutritional exigences of lambs with 20 kg body mass and to supply gains of 200 g/day, according to the NRC [11] recommendations. The proportions of the ingredients and the chemical compositions of the ensiled diets can be observed in Table 2.

Table 2.
Percentage and chemical compositions of diets at the time of ensiling.
Experimental PVC silos with dimensions of 0.10 m in diameter and 0.35 m in length, with Bunsen valves for the exhaustion of gases from the fermentation process, were used. Placed at the bottom of each silo was 1 kg of sand for the capture of the effluents produced by the silages. The sand and the ensiled mass were separated with a Sombrite® screen to prevent the material from touching the sand.
The forage cactus was processed to a 2 cm size, approximately. The legume plants were also ground to a particle size of 2 cm using a forage harvester attached to the tractor. After mixing the concentrate ingredients and the forages, the manual packaging of the ensiled mass in the interior of the silo followed. The compaction density obtained was approximately 718 kg/m3; on average, 2.150 kg of fresh forage was put in each experimental silo, and the silos were kept at room temperature (26–29 degrees).
The opening of the silos occurred at 60 days after the ensilage of the rations. At the moment of the opening, the weighing of each sealed silo as well as the weighing of the silo without the lid and the weighing of the silo after removing the silage were performed. Every weight was registered and used for the calculation of the losses by gasses, effluent, and dry matter recovery, according to the formulae described by Jobim et al. [12]. Losses by gasses: PG = ((PSf − Psa)/(FMf × DMf)) × 100, in which PG: gas loss during storage (% of initial DM); PSf: weight of the silo at the ensilage; PSa: weight of the silo at the opening; FMs: forage mass at the ensilage; DMf: DM of the ensiled forage.
Losses by effluents: PE = (Pef × 1.000)/Mvi, in which PE: losses by effluents; Pef: effluent’s weight (weight of the empty set after opening–weight of the empty set prior to the filling); Mvi: amount of ensiled forage dry mass.
Dry matter content: DMR = ((FMab × DMab)/(FMfe × DMfe)) × 100, in which DMR: dry matter recovery index; FMab: forage mass at the opening; DMab: DM content at the opening; FMfe: forage mass during the closing; DMfe: DM content at the closing.
The concentration of soluble carbohydrates was obtained using the sulfuric acid phenol method [13]. For the obtention of the pH, 25 g of silage was collected and 100 mL of distilled water was added, and the material remained at rest for 1 h; later, the readings were performed using a potentiometer [14]. A total of 12.5 g of silage was collected for the determination of the ammoniac nitrogen concentration (N-NH3), in which 100 mL of H2SO4 0.2 N solution was added, and the samples remained for rest for 48 h; after the rest, a filtering was performed, and the extract that was used for the reading in the spectrophotometer at a 630 nm wavelength was obtained [15]. For the calculations, the total nitrogen of the sample was considered [16].
After the opening of the silos, 25 g of fresh silage sample was collected, in which 225 mL of distilled water was added. The targeted dilutions were 10−2 to 10−6, according to the methodology proposed by González and Rodríguez [17]. For the counting of lactic acid bacteria (LAB), the samples were plated to MRS plates (Difco) using Petri plates, and immediately afterwards, the plates were incubated at 37 °C for 48 h in a BOD greenhouse before the counting. The molds (M) and yeasts (L) were quantified using the pour-plating technique in potato dextrose agar (Difco) acidified by the addition of 1.5% (w/v) tartaric acid at 10%; after the plating, the Petri plates were incubated at 25 °C for 5 days. The colonies that were considered susceptible to counting were those that had from 30 to 300 CFUs (colony-forming unities).
The assay on the aerobic stability of the silages had a total elapsed time of 120 h. The experimental silos were placed in a room with a controlled room temperature at 25 °C. Room temperature was monitored by means of a thermometer hanging in the air. The silages’ temperatures were measured at each half hour with digital thermometers that were inserted in the center of the mass. The aerobic stability of the silage was calculated as the time spent, in hours, for the silage to increase to 2 °C above room temperature [18].
The samples of the ingredients of the rations, of the silages, and of the ensilages were placed in aluminum trays, labeled, weighed, and taken to forced circulation ovens at 55 °C for 72 h in the Laboratório de Análise de Alimentos e Nutrição Animal-LAANA belonging to the Universidade Federal da Paraíba-UFPB (Campus II, Areia). After the pre-drying, the samples were processed in a knife mill into 1 mm sieves. In the pre-dried samples, the dry matter (method 934.01), crude protein (Kjeldahl method 920.870), ethereal extract (EE, method 920.39), and ash (method 930.05) were determined and analyzed according to AOAC [19]. The neutral detergent fiber analysis (NDF) and the acid detergent fiber (ADF) were determined according to Van Soest et al. [20]. The hemicellulose (HEM) was obtained by calculating the difference between the NDF and ADF [16].
The total digestible nutrients (TDNs) were estimated according to the equation proposed by Cappelle et al. [21] for the category of each feed. The digestible energy (DE) was estimated using the formula DE (Mcal) = 0.04409 × TDN. For the estimation of the metabolizable energy (ME), the following formula was used: ME × (Mcal) = 1.01 × DE − 0.45 [22]. The FNCs were calculated according to the equation described by Weiss as follows [23]: FNC = 100 − (%CP + %EE + %MM + %NDF).
For the in situ assay of the ruminal degradability, a 600 kg cross-bred cattle in 2 runs with a fistulated rumen was used. The animal was fed twice a day, in the morning and in the afternoon, with a concentrated mixture (corn meal and soybean meal) and roughage (based on corn and forage cactus silage), and a mineral core. The silage and ingredient samples that were pre-dried at 55 °C for 72 h were processed in a knife mill in 2 mm sieves. The experimental design was the completely randomized with four treatments and seven incubation times (0, 6, 12, 24, 48, 72 and 96 h) [24]; in each incubation period, four nonwoven fabric bags containing 4 g of the sample were incubated.
After the incubation period, each bag was washed in running water until the water turned crystal clear. Subsequently, the bags were put into a forced air circulation oven at 55 °C for 72 h. For the determination of the dry matter reduction at the period zero, the bags were washed in water bath for one hour at 39 °C to simulate rumen’s temperature. The bags from the period zero also went through the same procedures that the bags that were incubated in the rumen went through.
The dry matter reduction percentage at each period was calculated with the proportion of the feed that disappeared from the bag after incubation in the rumen.
For the evaluation of the dry matter degradation parameters (DMD), the following equation, proposed by Orskov and Mcdonald [25], was used:
Dt = A + B × (1 − ect) onde, where
Dt = degraded fraction in time t (%);
A = soluble fraction (%);
B = potentially degradable insoluble fraction in the rumen (%);
C = B fraction degradation rate (h − 1);
T = time (hours).
The effective degradability of the dry matter (ED) was estimated according to the model proposed by Orskov and Mcdonald [25], in which k corresponds to the flow rate of solid in the rumen (2%/h; 5%/h and 8%/h). The equation used was as follows:
ED = A + (B × c/c + k)
The data were submitted to variance analysis and the means were compared using the Tukey test at a 5% probability level. The SAS™ program’s PROC MIXED (Edition University, SAS Institute Inc., Cary, NC, USA) procedures were used with the following statistical model:
where: Yik is the dependent variable in the experiment, measured by the experimental unity “k” of the silage “i”; µ is the general constant; Si is the effect of the silages; and εik is the effect of the random error. The in situ degradability data were statistically analyzed using the following statistical model:
where: Yijk = observed value; μ = general constant; Ti = treatment effect; Aj = effect of incubation time in the rumen; PAjk = effect of the interaction between treatment and time; eijk = random error associated with each observation.
Yik = µ +Si + εik,
Yijk = μ + Ti + Aj + PAjk + eijk
3. Results
For the fermentation characteristics, a difference was observed regarding the pH (p < 0.001), ammoniacal nitrogen (NH3-N, p < 0.001), and effluent loss (EL, p < 0.05) values of the total mixed ration silages (Table 3). The levels of water-soluble carbohydrates (WSC, p = 0.1965), gas loss (GL, p = 0.5301) and dry matter recovery (DMR, p = 0.4035) did not show significant differences.

Table 3.
Fermentation characteristics of total mixed ration silages based on forage cactus and legume plants.
The highest pH value was observed for the total mixed ration silages based on forage cactus and G. sepium TMRG; this value was 4.4. The highest concentrations of NH3-N were obtained by the TMRC and TMRG with 2.20 and 2.21%, respectively. Regarding the TMRL and TMRS, lower concentrations of NH3-N were observed, and they were 0.85 and 1.01%, respectively. The TMRC registered the highest PE (40.13 kg/ton NM); however, it did not differ from the TMRG and TMRL, while the lowest EL was 23.36 kg/ton NM, which was observed for the TMRS, which did not differ from the TMRG or TMRL.
For microbial populations, the lactic acid bacteria (LAB) ranged from 5.0 to 6.3 log (CFU/g) for the evaluated total mixed ration silages (Figure 1). The TMRC presented the lowest count, 5 log (CFU/g), in comparison with the total mixed ration silages associated with legume plants.
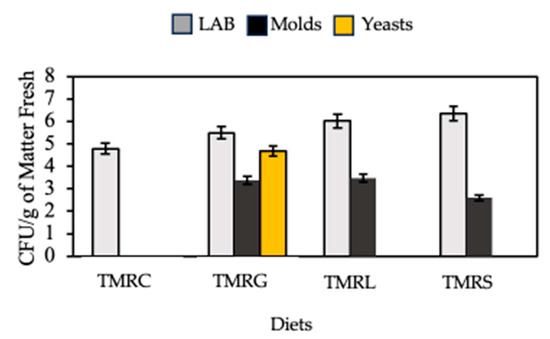
Figure 1.
Microbial populations in total mixed ration silages based on forage cactus and legume plants. TMRC = total mixed ration silage based on forage cactus and concentrate; TMRG = total mixed ration silage based on forage cactus and G. sepium; TMRL = total mixed ration silage based on forage cactus and L. leucocephala; TMRS = total mixed ration silage based on forage cactus and S. obtusifolia.
For the mold population, it was observed that all the total mixed ration silages associated with legume plants presented an average count of 3.3 log (CFU/g), except for the TMRC, which presented a null count for this microbial group (Figure 1).
For the yeast population, it was observed that the TMRG treatment presented a count of 5 log (CFU/g); on the other side, the TMRC, TMRL and TMRS presented null counts (Figure 1).
There was difference (p < 0.05) among the aerobic stability of the evaluated total mixed ration silages; the TMRC and the TMRG presented the highest aerobic stability values: 118.9 and 119.7 h, respectively. On the other hand, the TMRL and the TMRS presented the lowest aerobic stabilities, 87.3 and 92.13 h; up until that time, the silages kept their aerobic stability. The TMRL and the TMRS reached the highest temperatures, 31.12 °C and 30.82 °C, indicating the break of the aerobic stability of these treatments. Concerning the TMRG and the TMRC, the lower temperatures were registered to be 26.56 and 26.84 °C, respectively, and the break of the aerobic stability was not observed (Table 4.).

Table 4.
Aerobic stability of total mixed ration silages based on forage cactus associated with legume plants.
There was a difference (p < 0.05) regarding the dry matter (DM), ether extract (EE), neutral detergent fiber (NDF), acid detergent fiber (ADF), and the total digestible nutrient (TDN) contents of the total mixed ration silages (Table 5). No difference was observed for the content’s ash (p = 0.0008), crude protein (CP, p = 0.1526), hemicellulose (HEM, p = 0.0076), the total carbohydrates (TC, p = 0.7408), and non-fibrous carbohydrates (NFC, p = 0.1482).

Table 5.
Chemical composition of total mixed ration silages based on forage palm associated with legume plants.
Every total mixed ration silage presented DM contents above 30%, except for the TMRG, which presented the lowest DM content at 29.52%. The highest EE values were observed for the silages associated with legume plants, and those were 2.09 and 2.84%. The highest NDF contents were 24.99%, 22.74%, and 21.56% for the TMRL, TMRS, and TMRG, respectively, except for the TMRC, which presented the lowest NDF content at 19.77%. The highest ADF content was observed for the TMRS at 12.07%, and this treatment did not differ from the TMRL at 11.63. On the other hand, the ADF content of the TMRL was equal to the TMRG at 10.71%. The lowest ADF content was 6.42%, which was observed for the TMRC. The highest TDN was registered for the TMRC, which was 85.68%.
In Table 6, it is observed that the total mixed ration silages associated with legume plants presented the highest potentially degradable insoluble fraction in the rumen values (B), and those values ranged from 63.24% to 65.51%, except for the TMRC, which had the lowest potentially degradable insoluble fraction in the rumen at 55.81%.

Table 6.
In situ ruminal degradability parameters of the dry matter of the total mixed ration silage based on forage cactus associated with legume plants.
For the in situ ruminal degradability parameters, it was observed that the TMRC presented the highest soluble fraction at 39.67%. The lowest values of A were registered for the TMRL and the TMRS, being 32.15 and 30.17%, respectively (Table 6). For the degradation rate parameters (c) and the effective degradation (ED) of the DM, it was observed that the TMRC stood out, presenting the highest (c) at 3.5%/h, as well as the highest ED for the three evaluated flow rates (2%/h, 5%/h, and 8%/h).
4. Discussion
All the evaluated silages presented pH values within the ideal pH range established by McDonald et al. [26], although a slower reduction in the pH probably occurred when compared with the corn silages, by the buffering effect exerted by the forage palm’s mucilage as well as by the anions that are present in the legume plants. McDonald et al. [26] mentioned that well-fermented silages present pH values ranging from 3.8 to 4.2, which suggests that by the end of the fermentative process, there was the control of undesired microorganisms such as Clostridium genus bacteria and enterobacteria, which grow under high pH ranges, around 5.
The lowest soluble carbohydrate contents were observed for the total mixed ration silages associated with legume plants. These low values might be attributed to the intense consumption of this substrate by the homofermenting and heterofermenting LAB, since the legume plants and the forage palm promoted the buffering of the medium.
The low ammonia content yield is consonant with the crude protein contents of the total mixed ration silages, pointing towards the fact that there was no reduction in this nutrient during fermentation. The low N-NH3 concentrations observed in this work indicate that an intense proteolysis did not occur, since it is recommended that the N-NH3 contents in well-fermented silages do not exceed 10% of the total N [27]. This corroborates with the results reported by Santos et al. [10] when they evaluated the total mixed ration silages based on forage palm and G. sepium. Despite the buffering exerted by the legume plants, the ammonia production was reduced, suggesting that the preservation of the protein value of the rations was preserved, according to Table 5.
When compared with the corn silage, the total mixed ration silages based on forage palm with the addition of legume plants presented a good fermentative profile due to the adequate dry matter content and the soluble carbohydrate content, which favored the occurrence of lactic fermentation in the ensiled mass without a significant loss of ammonia. It should be noted that despite the legume-containing silages being more susceptible to the proteolysis occurrence, it was not observed in the present work.
The EL values obtained can be considered low and close to that cited by Rabelo et al. [28] when evaluating corn silage, which was 35.43 kg/ton NM. It can be considered that the losses by effluents were low, given that the dry matter contents of the total mixed ration silages were above 30%; however, the presence of legume plants in the total mixed ration silages promoted for a lesser lixiviation of humidity.
The EL values obtained in the present work were superior to the values observed by Brito et al. [7], and that can be attributed to the low dry matter content of the forage palm and the legume plants as well as the low quantity of mucilage in the mixture.
In the current study, dry matter recoveries (DMRs), with an average of 89%, were obtained for the total mixed ration silages. An amount of recovered dry matter similar to the result obtained in this study was reported by Carvalho et al. [29] when evaluating the silages of G. sepium with three different residue heights (70, 90, and 110 cm), which obtained a DMR index of 89.37% with the lowest residue height. In general, these fermentative losses can be attributed to the occurrence of heterolactic fermentations in the ensiled mass. The legume plants’ compositions might have been one of the preponderant factors for the observation of heterofermentations, because the legume plants present anions and orthophosphates that act in the buffering of the ensiled mass and in the reduction in the velocity of the pH drop.
All the silages had a satisfactory acid lactic bacteria propagation. This microbial group had growth conditions and dominium over the ensiled mass, which might have taken to a higher production of lactic and acetic acids, corroborating with the low final pH values observed. Santos et al. [10] reported that the silo’s environment is per se a limiting factor to the growth of certain microorganisms, which could be explained as a function of condition changes in the medium.
The populations of white mold that were observed in this work were, probably, originated from spores of filamentous fungi, since there was no oxygen during the fermentative process and every silage presented an adequate compaction density, because the mucilage in the forage palm helped in the aggregation of the concentrated ingredients and the particles of the legume plants, reducing the oxygen amount within the silo to a maximum [10]. The molds (filamentous fungi) grow better under aerobic conditions and act in a pH range from 5 to 6. The ideal growth temperature of this microorganism is situated between 25 and 35 °C [26].
Given the absence of oxygen within the silo, the adequate compaction that was promoted, and in the face of the findings from the literature [26], it can be deduced that the counts observed in the present work were from the filamentous fungi spores in the total mixed ration silages associated with legume plants. A similar fact was reported by Guim et al. [30] and by Ávila et al. [31], evaluating silages made of withered tropical grasses with and without microbial inoculation. The authors affirmed that the counts performed during the opening of the silos were from fungal spores. This shows that the total ration silages based on forage palm associated with legume plants present low counts of this microorganism, which does not affect the nutritional quality of the silages; when the mold count exceeds 6 log CFU/g FM, the dry matter losses might reach 40% [32].
The absence of yeasts observed in this work is possibly related to the high production of acetic acid as a consequence of the heterofermentative process, in which the acetic acid has an anti-fungi effect and might have controlled the yeast growth [33]. The presence of this microorganism in the TMRG might be related with the lower dry matter content observed in this treatment (Table 5), since yeasts are microorganisms that present good development under these conditions. In general, yeast is the starter of the silage’s aerobic degradation, and act by consuming soluble carbohydrates and organic acids, increasing the pH and the silage’s temperature [34].
The metabolic activity of the filamentous fungi possibly promoted the temperature rises for the TMRL and the TMRS, which presented the lowest aerobic stabilities observed in the present work.
Maximum temperatures that are superior to the temperatures observed in this work were cited by Silva Brito et al. [7], who evaluated mixed silage based on forage palm with different levels of G. sepium addition (0, 25, 50, 75, and 100%). The authors reported maximum temperatures ranging from 32.77 to 27.07 °C, in which the highest temperature was observed for the treatment without the legume plant. In a certain way, the maximum temperatures described by Silva Brito et al. [7] were higher than the temperatures observed in the present work, but it was verified that in both works, the silages that contained the G. sepium legume did not present a temperature increase, which is a fact that might be intimately related to the acetic acid concentration that controlled the growth of silage-degrading microorganisms.
In the present work, the TMRL and the TMRS presented the highest elapsed times to reach the maximum temperatures, 115.10 and 110.70 h, and those were the treatments in which a higher mold count was also observed. Therefore, it can be affirmed that the slower temperature rise is due to the presence of filamentous fungi, which are microorganisms with a slow development and dominium compared to yeast [33]. For the TMRC and the TMRG, the lowest elapsed times to reach the maximum temperature were registered, which were 26.84 °C and 26.58 °C, respectively. It is worth pointing out that the observed temperatures are below the temperature range that indicates the break of the aerobic stability of the silages, which occurs from 27 °C onward.
The highest aerobic stabilities seen in this work could be attributed to the absence of yeast in the TMRC treatment, as well as the control of the yeast population in the TMRG during the exposure to oxygen. Since the TMRL and the TMRS were sporulated with filamentous fungi, it can be presumed that this microorganism’s spores were activated when it came in touch with the oxygen. From then on, there was a growth and beginning of metabolic activities, resulting in a rise in the silage mass’s temperature and, because of that, those total ration silages presented the lowest observed aerobic stabilities.
The forage cactus, when included in total ration diets, fulfilled its function of triggering lactic and acetic fermentation, which reflects a better fermentative pattern in the same way that the acetic acid, which was also produced during the fermentative process, fulfilled an important role by guaranteeing aerobic stability in the silages [10]. The dry matter contents observed in the present work are within the ideal range for the occurrence of an adequate fermentation, from 30 to 35%, as reported by McDonald et al. [26], except for the TMRG, which presented the lowest dry matter content, which is a fact that could be attributed to a possible heterolactic fermentation that resulted in dry matter loss.
The EE values of the total mixed ration silages are considered to be low; however, this did not prevent the lactic acid bacteria from acting inside the silo, fermenting, and acidifying the medium. This result indicates that fermentations, predominantly lactic fermentation, occurred, which allowed for the control of proteolytic microorganisms during fermentation. This result might be related with the low N-NH3 content of the silages. Santos et al. [10] worked with total mixed ration silage based on forage palm and G. sepium and observed that the lactic fermentation was predominant, with no proteolysis. This response pattern was similar to the pattern obtained in the present work, in which the total ration silages were also associated with legume plants.
In general, the total ration silages based on forage palm, isolate forage palm, and mixed silages of forage palm and G. sepium present positive responses regarding the control of undesired fermentations, with low ammoniac nitrogen concentrations, which allows for the maintenance of the crude protein content of the silages [7]. The crude protein contents obtained for the total mixed ration silages associated with legume plants were around 16%; therefore, the ration silages meet the nutritional demands of sheep, which includes approximately 11% of crude protein in order to promote diary gains of 200 g [11].
Since the total ration silages were made of forage palm and different legume plants in different proportions, it is convenient to conclude that the forages that were used had differentiated chemical compositions, so a dilution of the fiber possibly occurred (NDF, ADF, and HEM); therefore, a reduction in the levels of fibrous components in silages is expected. Mokoboki et al. [35], who worked with silage based on forage palm containing different molasses levels (0, 8, 16, and 24% on a dry matter basis), observed that the ADF contents of the silages reduced as the molasses level increased.
The NFC contents of the ration silages were superior to the contents obtained during the ensilage, which demonstrates that a higher solubilization of fibrous components occurred during fermentation, and with it, a higher liberation of sugars, starch, and pectin also occurred. The non-fibrous carbohydrates presented degradation rates that ranged from high to medium. These fractions are readily fermented in the rumen [36]. In the present work, the total mixed ration silages presented high NFC contents. These values were superior to the values recommended by the NRC [22], in which the NFC contents in rations for ruminants must be at a maximum of 44%.
The total mixed ration silages presented adequate TDN contents, so it meets the nutritional demands of sheep with a 20 kg live weight, in which the energetic demands are 66% TDN for 200 g diary gain [11]. It is worth mentioning that the total ration silages obtained meet the protein and energy demands of sheep according to the NRC [11]. In the face of the observed reports from the literature, it can be concluded that the ration silages based on forage palm and legume plants can be used as sheep feed, since the lowest NDF contents obtained would not be a limiting factor to the silage’s usage. Therefore, it be-comes relevant to propose that performance assays with animals should be conducted in order to test the total ration silages obtained.
The soluble fraction (A) of the TMRC was high and is in accordance with the composition of that treatment, which presented a higher proportion of concentrate ingredients in comparison to the other total ration silages (Table 1), which, in a certain way, resulted in a higher observed NFC content. In turn, this fraction was rapidly fermented in the rumen and was readily used.
The A fraction obtained was 38.28% for the total ration silage based on forage palm with 0% Buffel grass addition. It is worth mentioning that, despite the total ration silages being made of forage palm, both presented distinct concentrated ingredients (wheat bran) that were added to the ensilages in different proportions. The higher proportion of concentrated ingredients and the high NFC content possibly contributed to the higher soluble fraction observed in the present work. Therefore, this result is a reflex of the composition of the total ration silages that were associated with the legume plants, which presented the highest NDF contents and reduced NFC contents when compared to the TMRC (Table 5).
The total ration silages associated with legume plants presented a higher usage potential of the fibrous fraction by the ruminal microbiota, since for these silages, the highest fibrous carbohydrate contents were observed. The literature reports that fiber degradation could be affected by the protein level in the diet; however, it was noticed that all of the total ration silages presented good crude protein levels, which shows that the protein levels probably did not limit the activity of fibrous-component-degrading microorganisms.
The total ration silages associated with legume plants showed the lowest degradation rates (c), which ranged from 1.15%/h to 1.45%/h, and also showed the lowest effective degradation (ED) of dry matter. It can be concluded that the TMRC presented a higher concentration of NFC in its composition, and the NDF and ADF contents were relatively low when compared with the silages that were associated with legume plants. The lowest degradation rates and ED were observed for the total ration silages associated with legume plants. This could be attributed to the higher fiber content of those silages, since the microorganisms that use the cell wall components, such as hemicellulose and cellulose, ferment more slowly than the ruminal microorganisms that use NFC.
In the face of the results obtained in this work, it can be concluded that the total ration silages based on forage palm associated with legume plants exhibited a predominantly heterolactic fermentative pattern, in which low N-NH3 productions were registered. The final pH remained within the adequate range (4.2 to 4.4). The total ration silages presented high TDN contents. The TMRG and TMRC followed by the TMRS presented the highest aerobic stabilities at 119.70, 118.90, and 92.13 h, respectively.
5. Conclusions
The legume plants (G. sepium and S. obtusifolia), when ensiled along with the forage palm in the form of total ration, gave origin to aerobically stable silages, with an adequate profile that is fermentative, microbiological, and nutritional. The ration silages that were obtained could be used as animal feed because they meet the nutritional demands of sheep. It becomes convenient to propose that animal performance assays should be conducted to test the total mixed ration silages associated with legume plants, with the aim of evaluating the forms of usage and its contribution to the improvement in sheep performance, within the conditions and the reality of the farms.
Author Contributions
Conceptualization, R.R. and R.L.; methodology, R.R.; software, A.P.; validation, R.R., F.N.S., E.M.S., A.P. and D.F.; formal analysis, R.L., R.S., E.S., M.R. and M.T.; investigation, R.R., R.L. and B.E.M.; resources, R.R., E.M.S. and A.Z.; data curation, A.M.S. and K.V.; writing—original draft preparation, R.R. and R.L.; writing—review and editing, F.N.S.; A.P. and D.O.-V. visualization, R.R., F.N.S. and A.Z.; supervision, R.R.; project administration, R.R.; funding acquisition, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES-Brazil), finance code 001; the National Council for Scientific and Technological Development (CNPq); and the Foundation for Research Support and Scientific and Technological Development of Maranhão (FAPEMA-Brazil).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the financial support received from the FAPEMA (Brazil) and the contributions made by Clésio dos Santos Costa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinheiro, F.M.; Nair, P.K.R. Silvopasture in the Caatinga biome of Brazil: A review of its ecology, management, and development opportunities. For. Syst. 2018, 27, eR01S. [Google Scholar] [CrossRef]
- Gusha, J.; Halimani, T.E.; Katsande, S.; Zvinorova, P.I. The effect of Opuntia ficus indica and forage legumes based diets on goat productivity in smallholder sector in Zimbabwe. Small Rum. Res. 2015, 125, 21–25. [Google Scholar] [CrossRef]
- Adelson, J.; Neto, S.; Da, V.; Oliveira, S.; De Lima Valença, R. Leguminosas adaptadas como alternativa alimentar para ovinos no semiárido—Revisão. Rev. Ciên. Agrovet. 2015, 14, 191–200. Available online: https://www.revistas.udesc.br/index.php/agroveterinaria/article/view/5786 (accessed on 22 June 2023).
- Pessoa, D.V.; de Andrade, A.P.; Magalhães, A.L.R.; Teodoro, A.L.; dos Santos, D.C.; de Araújo, G.G.L.; de Medeiros, A.N.; Nascimento, D.B.D.; Valença, R.d.L.; Cardoso, D.B. Forage nutritional differences within the genus Opuntia. J. Arid. Environ. 2020, 181, 104243. [Google Scholar] [CrossRef]
- Filho, R.R.R.; Santos, D.C.; Véras, A.S.C.; Siqueira, M.C.B.; Novaes, L.P.; Mora-Luna, R.; Monteiro, C.C.F.; Ferreira, M.A. Can spineless forage cactus be the queen of forage crops in dryland areas? J. Arid. Environ. 2021, 186, 104426. [Google Scholar] [CrossRef]
- Ali, M.F.; Tahir, M. An overview on the factors affecting water-soluble carbohydrates concentration during ensiling of silage. J. Plant Environ. 2021, 3, 63–80. [Google Scholar] [CrossRef]
- Brito, G.S.M.S.; Santos, E.M.; de Araújo, G.G.L.; de Oliveira, J.S.; Zanine, A.d.M.; Perazzo, A.F.; Campos, F.S.; Lima, A.G.V.d.O.; Cavalcanti, H.S. Mixed silages of cactus pear and gliricidia: Chemical composition, fermentation characteristics, microbial population and aerobic stability. Sci. Rep. 2020, 10, 6834. [Google Scholar] [CrossRef]
- Silva, T.S.; de Araujo, G.G.L.; Santos, E.M.; de Oliveira, J.S.; Campos, F.S.; Godoi, P.F.A.; Gois, G.C.; Perazzo, A.F.; Ribeiro, O.L.; Turco, S.H.N. Water intake and ingestive behavior of sheep fed diets based on silages of cactus pear and tropical forages. Trop. Anim. Health Prod. 2021, 53, 244. [Google Scholar] [CrossRef]
- Nogueira, M.D.S.; Araújo, G.G.L.; Santos, E.M.; Neto, S.G.; de Oliveira, J.S.; Perazzo, A.F.; de Moura Zanine, A.; Pinho, R.M.A.; Corrêa, Y.R.; Pereira, D.M. Feed Alternatives with Cactus Forage Silage for Animal Nutrition. Int. J. Agric. Biol. 2019, 22, 1393–1398. [Google Scholar]
- Santos, F.N.S.; Santos, E.M.; Oliveira, J.S.; Medeiros, G.R.; Zanine, A.M.; Araújo, G.G.L.; Perazzo, A.F.; Lemos, M.L.P.; Pereira, D.M.; Cruz, G.F.L.; et al. Fermentation profile, microbial populations, taxonomic diversity and aerobic stability of total mixed ration silages based on Cactus and Gliricidia. J. Agric. Sci. 2020, 158, 396–405. [Google Scholar] [CrossRef]
- National Research Council—NRC. Nutrient Requirements of Small Ruminants; National Academy Press: Washington, DC, USA, 2007; 381p. [Google Scholar]
- Jobim, C.C.; Nussio, L.G.; Reis, R.A.; Schmidt, P. Avanços metodológicos na avaliação da qualidade da forragem conservada. Rev. Bras. Zootec. 2007, 36, 101–119. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smitch, F. Colorimetric method for determination of sugars and related su stances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bolsen, K.K.; Lin, C.; Brent, C.R. Effects of silage additives on the microbial succession and fermentation process of alfafa and corn silages. J. Dairy Sci. 1992, 75, 3066–3083. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Detmann, E.; Souza, M.A.; Filho, S.C.V.; Queiroz, A.C.; Berchielli, T.T.; Saliba, E.O.S.; Cabral, L.S.; Pina, D.S.; Ladeira, M.M.E.; Azevedo, J.A.G. Métodos para Análise de Alimentos—INCT—Ciência Animal, 1st ed.; Suprema: Visconde do Rio Branco, Brasil, 2012; 214p. [Google Scholar]
- González, G.; Rodríguez, A.A. Effect of Storage Method on Fermentation Characteristics, Aerobic Stability, and Forage Intake of Tropical Grasses Ensiled in Round Bales. J. Dairy Sci. 2003, 86, 926–933. [Google Scholar] [CrossRef]
- Taylor, C.C.; Kung, L. The Effect of Lactobacillus buchneri 40,788 on the Fermentation and Aerobic Stability of High Moisture Corn in Laboratory Silos. J. Dairy Sci. 2002, 85, 1526–1532. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feed. II. A rapid method for the determination of fiber and lignin. J. AOAC Int. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Cappelle, E.R.; Filho, S.D.C.V.; Da Silva, J.F.C.; Cecon, P.R. Estimativas do Valor Energético a partir de Características Químicas e Bromatológicas dos Alimentos. Rev. Bras. Zootec. 2001, 30, 1837–1856. [Google Scholar] [CrossRef]
- National Research Council—NRC. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Weiss, W.P. Energy prediction equations for ruminant feeds. Proceedings 1999, 1, 176–185. [Google Scholar] [CrossRef]
- Sampaio, I.B.M.; Pike, D.J.; Owen, E. Optimal design for studying dry matter degradation in the rumen. Arq. Bras. Med. Vet. Zootec. 1995, 47, 373–383. [Google Scholar]
- Orskov, E.R.; Mcdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Oklahoma, OK, USA, 1991; 340p. [Google Scholar]
- Costa, R.F.; Pires, D.A.d.A.; Moura, M.M.A.; de Sales, E.C.J.; Rodrigues, J.A.S.; Rigueira, J.P.S. Agronomic characteristics of sorghum genotypes and nutritional values of silage. Acta Sci. Anim. Sci. 2016, 38, 127–133. [Google Scholar] [CrossRef][Green Version]
- Rabelo, C.H.S.; de Rezende, A.V.; Rabelo, F.H.S.; Nogueira, D.A.; Senedese, S.S.; Vieira, P.d.F.; Bernardes, C.L.; Carvalho, A. Silagens de milho inoculadas microbiologicamente em diferentes estádios de maturidade: Perdas fermentativas, composição bromatológica e digestibilidade in vitro. Ciên. Rural. 2014, 44, 368–373. [Google Scholar] [CrossRef]
- de Carvalho, G.; Rebouças, R.; Campos, F.; Santos, E.; Araújo, G.; Gois, G.; de Oliveira, J.; Oliveira, R.; Rufino, L.d.A.; Azevedo, J.; et al. Intake, digestibility, performance, and feeding behavior of lambs fed diets containing silages of different tropical forage species. Anim. Feed. Sci. Technol. 2017, 228, 140–148. [Google Scholar] [CrossRef]
- Guim, A.; De Andrade, P.; Iturrino-Schocken, R.P.; Franco, G.L.; Ruggieri, A.C.; Malheiros, E.B. Estabilidade aeróbica de silagens de capim-elefante (Pennisetum purpureum, Schum) emurchecido e tratado com inoculante microbiano. Rev. Bras. Zootec. 2002, 31, 2176–2185. [Google Scholar] [CrossRef]
- Ávila, C.L.d.S.; Pinto, J.C.; Figueiredo, H.C.P.; de Morais, A.R.; Pereira, O.G.; Schwan, R.F. Estabilidade aeróbia de silagens de capim-mombaça tratadas com Lactobacillus buchneri. Rev. Bras. Zootec. 2009, 38, 779–787. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. Silage Sci. Technol. 2015, 42, 31–93. [Google Scholar] [CrossRef]
- Mokoboki, K.; Sebola, N.; Matlabe, G. Effects of molasses levels and growing conditions on nutritive value and fermentation quality of Opuntia cladodes silage. J. Anim. Plant Sci. 2016, 28, 4488–4495. Available online: http://www.m.elewa.org/JAPS (accessed on 22 June 2023).
- Hall, M.B. Challenges with nonfiber carbohydrate methods. J. Anim. Sci. 2003, 81, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).