Positioning Portugal in the Context of World Almond Production and Research
Abstract
:1. Introduction
2. Characterization of Edaphoclimatic Requirements
3. Almond Diseases and Pests
4. Almond Cultivars Grown in Portugal
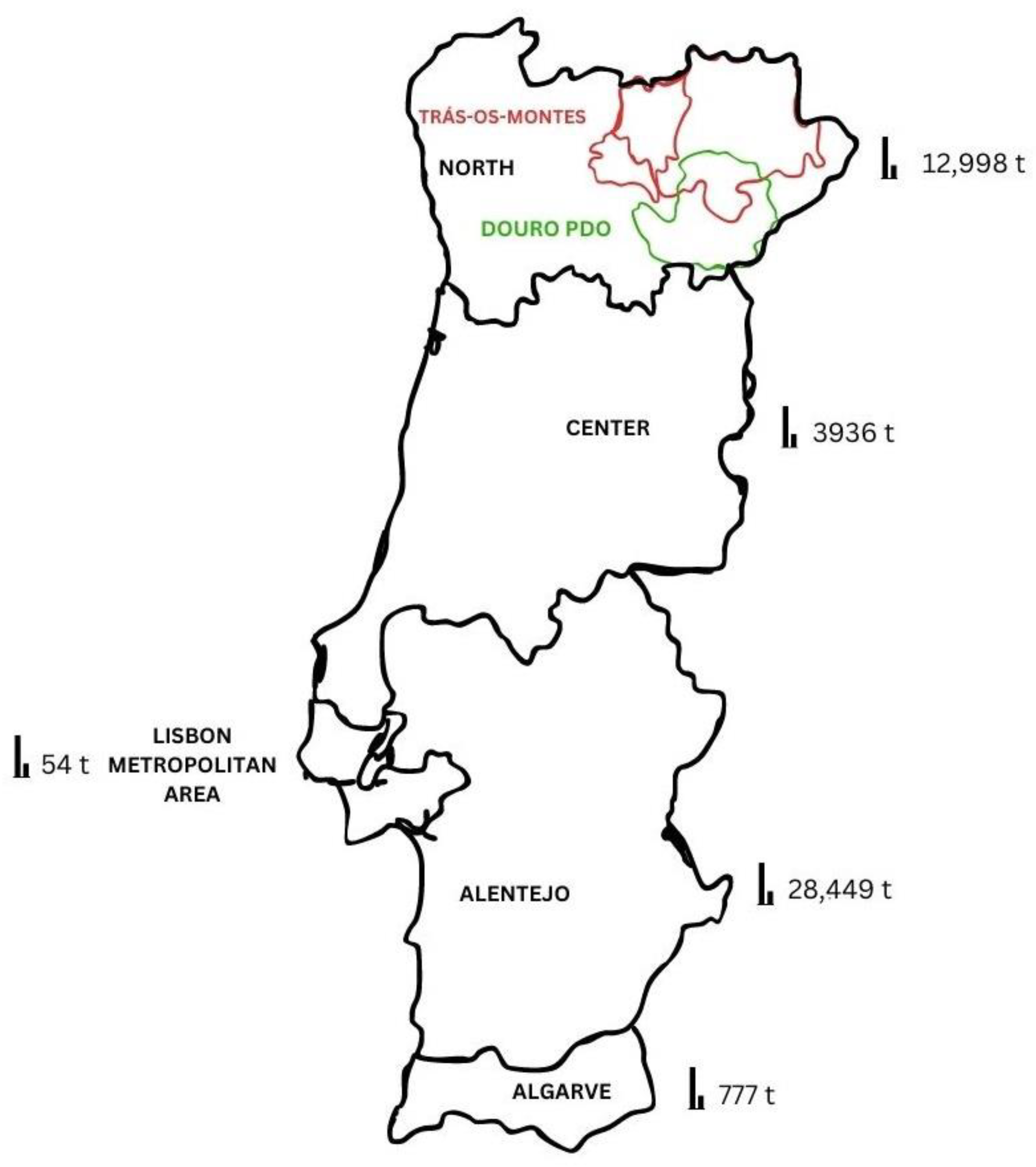
5. Almond Value Chain: Socio-Economic Importance
6. Almond Genetic Resources, Genetic Diversity and Breeding Approaches
6.1. Genetic Resources
6.2. Genetic Diversity
6.3. Breeding Approaches
6.4. Prospects for the Portuguese Almond Research and Production
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–207. [Google Scholar] [CrossRef]
- Martí, A.V.F.I.; Forcada, C.F.I.; Kamali, K.; Cabetas, M.J.R.; Wirthensohn, M. Molecular Analyses of Evolution and Population Structure in a Worldwide Almond [Prunus Dulcis (Mill.) D.A. Webb Syn. P. Amygdalus Batsch] Pool Assessed by Microsatellite Markers. Genet. Resour. Crop Evol. 2014, 62, 205–219. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Martínez-Gómez, P. Almond Breeding. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 207–258. [Google Scholar] [CrossRef]
- Rodrigues, Â.M. Manual Técnico Amendoeira: Estado Da Produção: Frutos Secos: Da Produção à Comercialização; Centro Nacional de Competências dos Frutos Secos: Bragança, Portugal, 2017; pp. 1–499. [Google Scholar]
- Homet-Gutiérrez, P.; Schupp, E.W.; Gómez, J.M. Naturalization of Almond Trees (Prunus Dulcis) in Semi-Arid Regions of the Western Mediterranean. J. Arid Environ. 2015, 113, 108–113. [Google Scholar] [CrossRef]
- Zeinalabedini, M.; Khayam-Nekoui, M.; Grigorian, V.; Gradziel, T.M.; Martínez-Gómez, P. The Origin and Dissemination of the Cultivated Almond as Determined by Nuclear and Chloroplast SSR Marker Analysis. Sci. Hortic. 2010, 125, 593–601. [Google Scholar] [CrossRef]
- Sana, S.; Akhter, N.; Amjum, F.; Khan, S.G.; Akram, M. Genetic Diversity in Almond (Prunus Dulcis). In Prunus—Recent Advances; Küden, A.B., Küden, A., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Company, R.S.I.; Gradziel, T.M. Almonds: Botany, Production and Uses; CABI: Oxfordshire, UK, 2017; pp. 1–494. [Google Scholar]
- Doll, D.A.; Freire De Andrade, J.; Serrano, P. Produção de Amêndoa Em Portugal: Tendências de Plantação e Desafios de Produção Num Setor Em Desenvolvimento; AGRO.GES: Cascais, Portugal, 2021. [Google Scholar]
- Blanca, G.; Díaz de la Guarda, C. Prunus L. In Flora Iberica; Garmendia, F.M., Navarro, C.C., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 1998; pp. 444–466. [Google Scholar]
- Chin, S.W.; Shaw, J.; Haberle, R.C.; Wen, J.; Potter, D. Diversification of Almonds, Peaches, Plums and Cherries—Molecular Systematics and Biogeographic History of Prunus (Rosaceae). Mol. Phylogenet. Evol. 2014, 76, 34–48. [Google Scholar] [CrossRef]
- Verma, M.K.; Awasthi, O.P.; Giri, R. Prospectus of Temperate Fruit Production in Subtropical Climate. In Proceedings of the National Seminar on “Precision Farming in Horticulture”, Jhalawar, India, 28–29 December 2010. [Google Scholar]
- Brittain, C.; Kremen, C.; Garber, A.K.; Klein, A.M. Pollination and Plant Resources Change the Nutritional Quality of Almonds for Human Health. PLoS ONE 2014, 9, e90082. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M. Almond (Prunus Dulcis) Breeding. In Breeding Plantation Tree Crops: Temperate Species; Priyadarshan, P.M., Mohan Jain, S., Eds.; Springer Science + Bussiness Media: New York, NY, USA, 2009; pp. 1–31. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Casas-Agustench, P.; Salas-Huetos, A. Cultural and Historical Aspects of Mediterranean Nuts with Emphasis on Their Attributed Healthy and Nutritional Properties. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S1–S6. [Google Scholar] [CrossRef]
- Méndez, J.; Vicedo, P.V.; Martín, R.G.; Sánchez, J.N. Comportamiento de Nuevas Variedades de Almendro En El Campo de Cartagena. 2021. Available online: https://www.carm.es/web/descarga?IDCONTENIDO=20865&ALIAS=PUBT&RASTRO=c2889$m58245,58256,58865&IDADIC=15713&ARCHIVO=Texto+Completo+1+Comportamiento+de+nuevas++variedades+de+almendro+en+el++campo+de+Cartagena.pdf (accessed on 27 July 2023).
- Arquero, O. Manual Del Almendro; Junta de Andalucía, Consejería de Agricultura, Pesca y Desarrollo Rural: Sevilla, Spain, 2013; pp. 1–80. [Google Scholar]
- Malagón, J. Cultivo del Almendro; Conselleria d’Agricultura: Valencia, Spain, 2020; pp. 1–4. [Google Scholar]
- Hernández, D.; Moreno, P. El Cultivo Del Almendro, Cultivos Leñosos: Frutales de Zonas Áridas; Antonio Madrid Vicente: Madrid, Spain, 2019. [Google Scholar]
- Queirós, F. Manual de Boas Práticas de Fruticultura, 213th ed.; Frutas, Legumes e Flores, em parceria com INIAV, I.P. (Estação Nacional de Fruticultura Vieira Natividade) e COTR: Oeiras, Portugal, 2020. [Google Scholar]
- Grasselly, C.; Duval, H. L’Amandier; Maisonneuve et Larose: Paris, France, 1980; pp. 1–445. [Google Scholar]
- AJAP: Associação dos Jovens Agricultores de Portugal. Manual das boas Práticas para Culturas Emergentes: A Cultura da Amêndoa; AJAP: Lisboa, Portugal, 2017. [Google Scholar]
- Torguet, L.; Maldonado, M.; Miarnau, X. Importancia y Control de las Enfermedades en el Cultivo del Almendro. Available online: https://archivo.revistaagricultura.com/almendro/cultivos/importancia-y-control-de-las-enfermedades-en-el-cultivo-del-almendro_10697_38_13324_0_1_in.html (accessed on 27 July 2023).
- Gort, J.A.; Sánches, J. Controlo de plagas y enfermedades en el cultivo del Almendro. Vida Rural 2011, 332, 68–74. [Google Scholar]
- McKay, S.; Shtienberg, D.; Sedgley, M.; Scott, E.S. Anthracnose on Almond in Australia: Disease Progress and Inoculum Sources of Colletotrichum Acutatum. Eur. J. Plant Pathol. 2014, 139, 773–783. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X. Epidemiology and Management of Brown Rot on Stone Fruit Caused by Monilinia laxa. Eur. J. Plant Pathl. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Pons-Solé, G.; Miarnau, X.; Torguet, L.; Lázaro, E.; Civera, A.V.; Luque, J. Airborne Inoculum Dynamics of Polystigma Amygdalinum and Progression of Almond Red Leaf Blotch Disease in Catalonia, NE Spain. Ann. Appl. Biol. 2023, 183, 33–42. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Purcell, A.H. Biological Traits of Xylella Fastidiosa Strains from Grapes And Almonds. Appl. Environ. Microbiol. 2003, 69, 7447–7452. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.M.; Andrews, K. Effects of Spider Mites on Almond Tree Growth and Productivity. J. Econ. Entomol. 1978, 71, 555–558. [Google Scholar] [CrossRef]
- Mdellel, L.; Adouani, R.; Kamel, M.B.H. Aphid on Almond and Peach in Tunisia: Species, Bioecology, Natural Enemies and Control Methods. In Fruit Industry; Kahramanoğlu, İ., Wan, C., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Marcotegui, A.; Sánchez-Ramos, I.; Pascual, S.; Fernández, C.E.; Cobos, G.; Armendáriz, I.E.; Cobo, A.; González-Núñez, M. Kaolin and Potassium Soap with Thyme Essential Oil to Control Monosteira unicostata and Other Phytophagous Arthropods of Almond Trees in Organic Orchards. J. Pest Sci. 2015, 88, 753–765. [Google Scholar] [CrossRef]
- Hamby, K.A.; Nicola, N.; Niederholzer, F.; Zalom, F.G. Timing Spring Insecticide Applications to Target Both Amyelois Transitella (Lepidoptera: Pyralidae) and Anarsia Lineatella (Lepidoptera: Gelechiidae) in Almond Orchards. J. Econ. Entomol. 2015, 108, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Zhang, N.; Rijal, J.P.; Parker, L.E.; Ostoja, S.; Pathak, T.B. Climate Change Impacts on Insect Pests for High Value Specialty Crops in California. SSRN 2023. [Google Scholar] [CrossRef]
- Centro Nacional de Competências dos Frutos Secos (CNCFS). Available online: http://www.wp.cncfs.pt/ (accessed on 27 July 2023).
- Instituto Nacional de Estatística, I.P. (INE). Estatíscas da Produção Vegetal. Available online: https://www.ine.pt (accessed on 7 August 2023).
- Vargas, F.J.; Romero, M.A.; Clavé, J.; Vergés, J.; Santos, J.; Batlle, I. ‘Vayro’, ‘Marinada’, ‘Constantí’, and ‘Tarraco’ Almonds. Hortscience 2008, 43, 535–537. [Google Scholar] [CrossRef]
- Calvo, Á.; Gómara, E. Almendro. Resultado de 12 Años de Experimentación Con Variedades; Navarra agraria: Navarra, Spain, 2011; pp. 1–5. [Google Scholar]
- Arquero, O.; Casado, B.; Salguero, A.; Viñas, M. Sistemas de Formación y Poda. In Manual del Cultivo del Almendro; Arquero, O., Ed.; Junta de Andalucía, Consejería de Agricultura, Pesca y Desarrollo Rural: Sevilla, Spain, 2013; pp. 39–46. [Google Scholar]
- Monteiro, A.; Cordeiro, V.; Laranjo, J. A Amendoeira: Com Especial Referência a Trás-Os-Montes e Alto Douro; João Azevedo Editor, D.L.: Mirandela, Portugal, 2003; pp. 1–186. [Google Scholar]
- Miarnau, X.; Alegre, S.; Vargas, F. Productive potential of six almond cultivars under regulated deficit irrigation. In XIV GREMPA Meeting on Pistachios and Almonds; Zakynthinos, G., Ed.; CIHEAM/FAO/AUA/TEI Kalamatas/NAGREF Zaragoza: Athens, Greece, 2010; pp. 267–271. [Google Scholar]
- Hernández, D.M.S.; López-Cortés, I. La Producción Integrada Em El Almendro; UPV: Valência, Espanha, 2009; pp. 1–226. [Google Scholar]
- International Nut and Dried Fruit Council Foundation. Nuts Dried Fruits Statistical Yearbook; International Nut and Dried Fruit Council Foundation: Reus, Spain, 2019. [Google Scholar]
- Waycott, R.; Saa, S. Principales Características Del Sector de La Almendra En California: Los Inicios, La Consolidación, Las Perspectivas. Dialnet 2020, 36, 12–19. [Google Scholar]
- Iglesias, I. El Almendro en España: Situación, Innovación Tecnológica, Costes y Retos Para una Producción Sostenible. Available online: https://www.interempresas.net/Horticola/Articulos/316394-almendro-Espana-situacion-innovacion-tecnologica-costes-retos-produccion-sostenible.html (accessed on 8 August 2023).
- Almond Board of California. Almond Almanac. 2019. Available online: https://www.almonds.com/sites/default/files/2020-04/2019_Almanac.pdf (accessed on 24 May 2023).
- Almond Board of California. Almond Almanac. 2022. Available online: https://www.almonds.com/sites/default/files/2023-04/2022_Almanac.pdf (accessed on 29 May 2023).
- Pathak, T.B.; Maskey, M.L.; Rijal, J. Impact of Climate Change on Navel Orangeworm, a Major Pest of Tree Nuts in California. Sci. Total Environ. 2021, 755, 142657. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 8 August 2023).
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and Fatty Acid Profiles, A-tocopherol and Sucrose Contents, and Antioxidant Capacities of Understudied Portuguese Almond Cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef]
- Rodrigues, I.; Bento, A.; Reis, C.; Pereira, J.A. Monitorização das principais pragas da amendoeira em pomares da região de Trás-os-Montes; 4º Simpósio Nacional de Fruticultura: Faro, Portugal, 2020; pp. 450–455. [Google Scholar]
- Cabo, P.; Matos, A. Amendoeira: Estado da Comercialização: Frutos Secos: Da Produção à Comercialização; Centro Nacional de Competências dos Frutos Secos: Bragança, Portugal, 2017; pp. 1–107. Available online: http://www.wp.cncfs.pt/2017/04/02/amendoeira-estado-da-comercializacao (accessed on 16 May 2023).
- Borràs, E.; Amigo, J.M.; Van Den Berg, F.; Boqué, R.; Busto, O. Fast and Robust Discrimination of Almonds (Prunus amygdalus) with Respect to Their Bitterness by Using near Infrared and Partial Least Squares-Discriminant Analysis. Food Chem. 2014, 153, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Karatay, H.; Sahin, A.; Yilmaz, O.; Aslan, A. Major Fatty Acids Composition of 32 Almond (Prunus dulcis (Mill.) D. A. Webb.) Genotypes Distributed in East and Southeast of Anatolia. Turk. Biyokim. Derg. 2014, 39, 307–316. [Google Scholar] [CrossRef]
- Volpi, G. Demonstrating the Presence of Cyanide in Bitter Seeds While Helping Students Visualize Metal–Cyanide Reduction and Formation in a Copper Complex Reaction. J. Chem. Educ. 2016, 93, 891–897. [Google Scholar] [CrossRef]
- Javaid, T.; Mahmood, S.; Saeed, W.; Alam, M.Q. A Critical Review on Cultivars and Benefits of Almond (Prunus Dulcis). Act. Sci. Nutr. Health 2019, 3, 70–72. [Google Scholar] [CrossRef]
- Loghavi, M.; Souri, S.; Khorsandi, F. Physical and Mechanical Properties of Almond (Prunus Dulcis l. Cv. 7Shahrood). In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Louisville, Kentucky, 7–10 August 2011. [Google Scholar] [CrossRef]
- Toomey, M.B.S.V.; Nickum, B.S.E.A.; Flurer, C.L. Cyanide and Amygdalin as Indicators of the Presence of Bitter Almonds in Imported Raw Almonds. J. Forensic Sci. 2012, 57, 1313–1317. [Google Scholar] [CrossRef]
- Amirshaghaghi, Z.; Rezaei, K.; Rezaei, M.H. Characterization and Functional Properties of Protein Isolates from Wild Almond. J. Food Meas. Charact. 2017, 11, 1725–1733. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Lapsley, K.; Ellis, P.R. A Review of the Impact of Processing on Nutrient Bioaccessibility and Digestion of Almonds. Int. J.Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef]
- Martinez, M.L.; Penci, M.C.; Marin, M.A.; Ribotta, P.D.; Maestri, D.M. Screw Press Extraction of Almond (Prunus Dulcis (Miller) D.A. Webb): Oil Recovery and Oxidative Stability. J. Food Eng. 2013, 119, 40–45. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Rodriguez-Garcia, J.; Chiralt, A.; González-Martínez, C. Effect of High Pressure Homogenisation and Heat Treatment on Physical Properties and Stability of Almond and Hazelnut Milks. LWT- Food Sci. Technol. 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Chaouali, N.; Gana, I.; Amira, D.; Khelifi, F.; Nouioui, A.; Masri, W.; Belwaer, I.; Ghorbel, H.; Hedhili, A. Potential Toxic Levels of Cyanide in Almonds (Prunus Amygdalus), Apricot Kernels (Prunus Armeniaca), and Almond Syrup. Int. Sch. Res. Notices. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Giusti, M.M.; Balasubramaniam, V.M. Effect of High Pressure Processing on Dispersive and Aggregative Properties of Almond Milk. J. Sci. Food Agric. 2016, 96, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health Issues and Technological Aspects of Plant-Based Alternative Milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z. The Uses and Properties of Almond Oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Čolić, S.; Zec, G.; Natić, M.; Fotiric-Aksic, M. Almond (Prunus Dulcis) Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 149–180. [Google Scholar] [CrossRef]
- Harlan, J.R.; De Wet, J.M.J. Toward A Rational Classification of Cultivated Plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Warschefsky, E.; Klein, L.L.; Miller, A.J. Using Living Germplasm Collections to Characterize, Improve, and Conserve Woody Perennials. Crop Sci. 2019, 59, 2365–2380. [Google Scholar] [CrossRef]
- Miller, A.J.; Matasci, N.; Schwaninger, H.; Aradhya, M.K.; Prins, B.; Zhong, G.Y.; Simon, C.; Buckler, E.S.; Myles, S. Vitis Phylogen omics: Hybridization Intensities from a SNP Array Outperform Genotype Calls. PLoS ONE 2013, 8, e78680. [Google Scholar] [CrossRef]
- Klein, A.M.; Brittain, C.; Hendrix, S.D.; Thorp, R.; Williams, N.; Kremen, C. Wild Pollination Services to California Almond Rely on Semi-Natural Habitat. J. Appl. Ecol. 2012, 49, 723–732. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañan, G.; Juan, T.; Alonso, J.M.; Espiau, M.T.; Company, R.S.I. Oil Content, Fatty Acid Composition and Tocopherol Concentration in the Spanish Almond Genebank Collection. Sci. Hortic. 2014, 177, 99–107. [Google Scholar] [CrossRef]
- Currò, S.; La Malfa, S.; Distefano, G.; Long, G.; Sottile, F.; Gentile, A. Analysis of S-Allele Genetic Diversity in Sicilian Almond Germplasm Comparing Different Molecular Methods. Plant Breed. 2015, 134, 713–718. [Google Scholar] [CrossRef]
- Tamura, M.; Ushijima, K.; Sassa, H.; Hirano, H.; Tao, R.; Gradziel, T.M.; Dandekar, A.M. Identification of Self-Incompatibility Genotypes of Almond by Allele-Specific PCR Analysis. Theor. Appl. Genet. 2000, 101, 344–349. [Google Scholar] [CrossRef]
- Channuntapipat, C.; Wirthensohn, M.; Ramesh, S.A.; Batlle, I.; Arús, P.; Sedgley, M.; Collins, G. Identification of Incompatibility Genotypes in Almond (Prunus Dulcis Mill.) Using Specific Primers Based on the Introns of the S-Alleles. Plant Breed. 2003, 122, 164–168. [Google Scholar] [CrossRef]
- Ministério da Agricultura e do Mar. Plano Nacional para os Recursos Genéticos Vegetais; Instituto Nacional de Investigação Agrária e Veternaria, I.P. (INIAV): Oeiras, Portugal, 2015. [Google Scholar]
- Martí, A.F.I.; Alonso, J.M.; Espiau, M.T.; Cabetas, M.J.R.; Company, R.S.I. Genetic Diversity in Spanish and Foreign Almond Germplasm Assessed by Molecular Characterization with Simple Sequence Repeats. J. Am. Soc. Hortic. Sci. 2009, 134, 535–542. [Google Scholar] [CrossRef]
- Padilla, G.; Company, R.S.I.; Ordás, A. Molecular Characterization of Almond Accessions from the Island of La Palma (Canary Islands, Spain) Using SSR Markers. Plant Genet. Resour. 2014, 12, 323–329. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Romero, A.; Batlle, I.; Dicenta, F. Molecular characterization of Spanish autochthonous almond breeding collections using SSRs. In XVI GREMPA Meeting on Almonds and Pistachios; Kodad, O., López-Francos, A., Rovira, M., Rafael Socias i Company, Eds.; CIHEAM Zaragoza: Meknes, Morrocco, 2016; pp. 111–114. Available online: http://om.ciheam.org/article.php?IDPDF=00007374 (accessed on 9 August 2023).
- Savoia, M.A.; Del Faro, L.; Venerito, P.; Gaeta, L.; Palasciano, M.; Montemurro, C.; Sabetta, W. The Relevance of Discovering and Recovering the Biodiversity of Apulian Almond Germplasm by Means of Molecular and Phenotypic Markers. Plants 2022, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Rigoldi, M.P.; Rapposelli, E.; De Giorgio, D.; Resta, P.; Porceddu, A. Genetic Diversity in Two Italian Almond Collections. Electron. J. Biotechnol. 2015, 18, 40–45. [Google Scholar] [CrossRef]
- Pavan, S.; Delvento, C.; Mazzeo, R.; Ricciardi, F.; Losciale, P.; Gaeta, L.; D’Agostino, N.; Taranto, F.; Sánchez-Pérez, R.; Ricciardi, L.M.; et al. Almond Diversity and Homozygosity Define Structure, Kinship, Inbreeding, and Linkage Disequilibrium in Cultivated Germplasm, and Reveal Genomic Associations with Nut and Seed Weight. Hortic. Res. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Rahimi-Dvin, S.; Gharaghani, A.; Pourkhaloee, A. Genetic Diversity, Population Structure, and Relationships among Wild and Domesticated Almond (Prunus Spp.) Germplasms Revealed by ISSR Markers. Adv. Hort. Sci. 2020, 34, 287–300. [Google Scholar] [CrossRef]
- Goonetilleke, S.N.; March, T.J.; Wirthensohn, M.G.; Arús, P.; Walker, A.R.; Mather, D.E. Genotyping by Sequencing in Almond: SNP Discovery, Linkage Mapping, and Marker Design. G3: Genes Genon. Genet. 2018, 8, 161–172. [Google Scholar] [CrossRef]
- Tavassolian, I.; Rabiei, G.; Gregory, D.; Mnejja, M.; Wirthensohn, M.G.; Hunt, P.W.; Gibson, J.P.; Ford, C.M.; Sedgley, M.; Wu, S.B. Construction of an Almond Linkage Map in an Australian Population Nonpareil × Lauranne. BMC Genom. 2010, 11, 1–10. [Google Scholar] [CrossRef]
- Martins, M.; Tenreiro, R.; Oliveira, M.M. Genetic Relatedness of Portuguese Almond Cultivars Assessed by RAPD and ISSR Markers. Plant Cell Rep. 2003, 22, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, L.; Apostolova, E.; Neves, A.; Marreiros, A.; Leitão, J. Genetic Diversity Assessment of the Almond (Prunus Dulcis (Mill.) D.A. Webb) Traditional Germplasm of Algarve, Portugal, Using Molecular Markers. Plant Genent. Resour. 2014, 12 (Suppl. S1), S164–S167. [Google Scholar] [CrossRef]
- Dicenta, F.; Egea, J.; Ortega, E.; Martínez-Gómez, P.; Sánchez-Pérez, R.; Rubio, M.; Martínez-García, P.J.; Gómez, E.M.; del Cueto, J.; Sánchez-Prudencio, A.; et al. Almond Breeding: Important issues and challenges for research. In XVI GREMPA Meeting on Almonds and Pistachios; Kodad, O., López-Francos, A., Rovira, M., Socias i Company, R., Eds.; CIHEAM Zaragoza: Morocco, 2016; pp. 23–28. Available online: http://om.ciheam.org/om/pdf/a119/00007357.pdf (accessed on 18 August 2023).
- Miarnau, X.; Zazurca, L.; Torguet, L.; Zúñiga, E.; Batlle, I.; Alegre, S.; Luque, J. Cultivar Susceptibility and Environmental Parameters Affecting Symptom Expression of Red Leaf Blotch of Almond in Spain. Plant Dis. 2021, 105, 940–947. [Google Scholar] [CrossRef]
- Dicenta, F.; Martínez-Gómez, P.; Martínez-Pato, E.; Gradziel, T.M. Screening for Aspergillus flavus Resistance in Almond. Hortscience 2003, 38, 266–268. [Google Scholar] [CrossRef]
- Gainza, F.; Opazo, I.; Guajardo, V.; Meza, P.; Ortiz, M.; Pinochet, J.; Muñoz, C. Rootstock Breeding in Prunus Species: Ongoing Efforts and New Challenges. Chil. J. Agric. Res. 2015, 75, 6–16. [Google Scholar] [CrossRef]
| Diseases or Pests | Affected Organs | Symptoms | Means of Combat | Reference |
|---|---|---|---|---|
| Anthracnose (Colletotrichum acutatum) | All | Necroses; Leaves and fruit lesions | Elimination of the attacked organs; Aeration of the crown; Rational fertilization; Preventive treatments | [25] |
| Brown Rot Blossom Blight (Monilinia laxa) | Flowers | Necrosis of flower buds; Fruits mummify | Destruction of affected branches; Promote the aeration of the canopy; Nitrogen supplementation; Avoid prolonged irrigation | [26] |
| Ochre staining (Polystigma amygdalinum) | Leaves | Yellow spots of leaves; Reduced photosynthetic capacity | Destroy fallen leaves; Use fungicides | [27] |
| Xylella fastidiosa | All | Wilting and plant death; Leaf scorching; Decrease in productivity of orchards | There are no effective means of control | [28] |
| Tetranchid mites (Tetranychus urticae Koch and Panonychus ulmi) | Leaves | Pale yellow, whitish and silver spots | Reduce water stress; Nitrogen fertilization | [29] |
| Aphids (Myzus persicae Sulzer, Brachycaudus amygdalinus and Brachycaudus helichrysi Kalt) | Branches and leaves | Curling and deformation of leaves and young twigs, shorter internodes; Reduction in production | Chemical control through the application of insecticides | [30] |
| Monosteira unicostata | Leaves and fruits | Leaves with yellow spots | No biological control methods known | [31] |
| Anarsia lineatella Zeller | Branches and leaves | Damages in young shoots and fruits | Treatment with Bacillus thuringiensis and kaolin; Sexual confusion method | [32] |
| Grapholita molesta | Shoots and fruits | Destruction of young shoots (malformations) | Application of insecticides; Technique of sexual confusion; Application of Bacillus thuringiensis | [33] |
| Cultivar | Region | Pollination | Flowering Season | Productivity |
|---|---|---|---|---|
| ‘Bonita’ | Terra Quente | Cross | Early-mid | Medium |
| ‘Casanova’ | Alto Douro | Cross | Early-mid | Medium |
| ‘Dona Virtude’ | Alto Douro | Cross | Very early | Low |
| ‘Gama’ | Alto Douro | Cross | Early-mid | Low |
| ‘Marcelina Grada’ | Terra Quente | Cross | Early-mid | Medium-low |
| ‘Mourisca’ | Alto Douro | Cross | Early-mid | Low |
| ‘Parada’ | Alto Douro | Cross | Early-mid | Medium |
| ‘Romeira’ | Alto Douro | Cross | Early-mid | Low |
| ‘Verdeal’ | Alto Douro | Cross | Early-mid | Very High |
| ‘Boa Casta’ | Algarve | Cross | Early-mid | Low |
| ‘Bonita de São Brás’ | Algarve | Cross | Early-mid | Medium |
| ‘Duro Amarelo’ | Algarve | Cross | Early-mid | Low |
| ‘José Dias’ | Algarve | Cross | Early | Low |
| Country | Cultivar | Plant Material | Pollination | Flowering Season | Productivity |
|---|---|---|---|---|---|
| Spain | ‘Belona’ | ‘Blanquerna’ × ‘Belle d’Aurons’ | Self | Late | High |
| ‘Constantí’ | ‘FGFD2’ × ‘open pollination’ | Self | Late | High | |
| ‘Desmayo Largueta’ | Tarragona | Cross | Early | High | |
| ‘Guara’ | Clonal Selection (Zaragosa) | Self | Late | High | |
| ‘Marcona’ | Alicante | Cross | Early-Mid | Very High | |
| ‘Marinada’ | ‘Lauranne’ × ‘Glorieta’ | Self | Late | Very High | |
| ‘Marta’ | ‘Ferragnes’ × ‘Tuono’ | Self | Late | High | |
| ‘Masbovera’ | ‘Primorskyi’ × ‘Cristomorto’ | Cross | Late | High | |
| ‘Penta’ | ‘CEBAS S5133’ × ‘Lauranne’ | Self | Very Late | High | |
| ‘Soleta’ | ‘Blanquerna’ × ‘Belle d’ Aurons’ | Self | Late | High | |
| ‘Tardona’ | ‘CEBAS S5133’ × ‘Grasselly R1000’ | Self | Very Late | High | |
| ‘Tarraco’ | ‘Ferralise’ × ‘Tuono’ × ‘Anxaneta’ | Cross | Very Late | Very High | |
| ‘Vayro’ | ‘Primorskij’ × ‘Cristomorto’ × ‘Lauranne’ | Self | Late | Very High | |
| France | ‘Ferraduel’ | ‘Cristomorto’ × ‘Ai’ | Cross | Late | Very High |
| ‘Ferragnes’ | ‘Cristomorto’ × ‘Ardéchoise’ | Cross | Half late | Very High | |
| ‘Ferrastar’ | ‘Cristomorto’ × ‘Ardéchoise’ | Cross | Late | Very High | |
| ‘Lauranne’ | ‘Farragnes’ × ‘Tuono’ | Self | Late | Very High | |
| USA | ‘Nonpareil’ | California | Cross | Half late | Very High |
| ‘Texas’ | California | Cross | Half Late | High |
| Rootstock | Origin | Advantages | Disadvantages | |
|---|---|---|---|---|
| Almond ‘Francos de amendoeira’ | Sexual propagation through seeds of different almond cultivars (e.g., ‘Verdeal’ and ‘José Dias’) | Highly rustic rootstock; Resistant to water scarcity; Well adapted to arid, stony and calcareous soils | Difficulty in transplanting due to its pivotal root; Sensitive to soil diseases; Sensitive to hypoxia; Restricted compatibility with grafted cultivar; Development and behavior heterogeneity in the orchard; Low productivity and late entry into production | |
| Peach ‘Francos de Pessegueiro’ | Sexual propagation through seeds of different peach cultivars | Highly rustic rootstock; Resistant to water scarcity; Fasciculate root system; High compatibility with several cultivars of almonds; Good adaptation to transplanting; Quick entry into production; Higher homogeneity of production | Low resistance to lime, which leads the cultivars to manifest iron chlorosis | |
| Clonal Plums | Plum clones | Resistant to root asphyxia, iron chlorosis and soil diseases | Drawbacks when used on almond trees, with localized and displaced incompatibilities at the physiological level | |
| Interspecific Hybrids | Peach × Almond | e.g., GF 677, GF 557, Hansen, Adafuel, GxN-15 | Deep rooting; Adaptation to the rainfeed and to calcareous soils | Sensitive to root waterlogging and soil diseases; Some of these (e.g., GF 677) are only propagated in vitro, dificulting its mass propagation |
| Plum × Almond | e.g., Cadaman, Rootpac 20 (Densipac), Rootpac 40 (Nanopac), Rootpac 70 (Purplepac), Rootpac 90 (Greenpac), Rootpac R (Replantpac) | Depending on the rootstock has tolerance to root asphyxia and soil diseases; Good compatibility with most of the cultivars | Depending on the rootstock is sensitive to root asphyxia and soil diseases | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, C.R.; Sousa, B.; Silva, J.; Braga, M.; Araújo, S.d.S.; Sales, H.; Pontes, R.; Nunes, J. Positioning Portugal in the Context of World Almond Production and Research. Agriculture 2023, 13, 1716. https://doi.org/10.3390/agriculture13091716
Campos CR, Sousa B, Silva J, Braga M, Araújo SdS, Sales H, Pontes R, Nunes J. Positioning Portugal in the Context of World Almond Production and Research. Agriculture. 2023; 13(9):1716. https://doi.org/10.3390/agriculture13091716
Chicago/Turabian StyleCampos, Carolina Ribeiro, Beatriz Sousa, Joana Silva, Megan Braga, Susana de Sousa Araújo, Hélia Sales, Rita Pontes, and João Nunes. 2023. "Positioning Portugal in the Context of World Almond Production and Research" Agriculture 13, no. 9: 1716. https://doi.org/10.3390/agriculture13091716
APA StyleCampos, C. R., Sousa, B., Silva, J., Braga, M., Araújo, S. d. S., Sales, H., Pontes, R., & Nunes, J. (2023). Positioning Portugal in the Context of World Almond Production and Research. Agriculture, 13(9), 1716. https://doi.org/10.3390/agriculture13091716








