Genome-Wide Identification, Characterization, and Expression Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene Family Reveals Its Importance in Chloroplasts of Brassica oleracea L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification of BoGGPPS Gene Family Members in B. oleracea
2.3. Phylogenetic Analyses of GGPPS
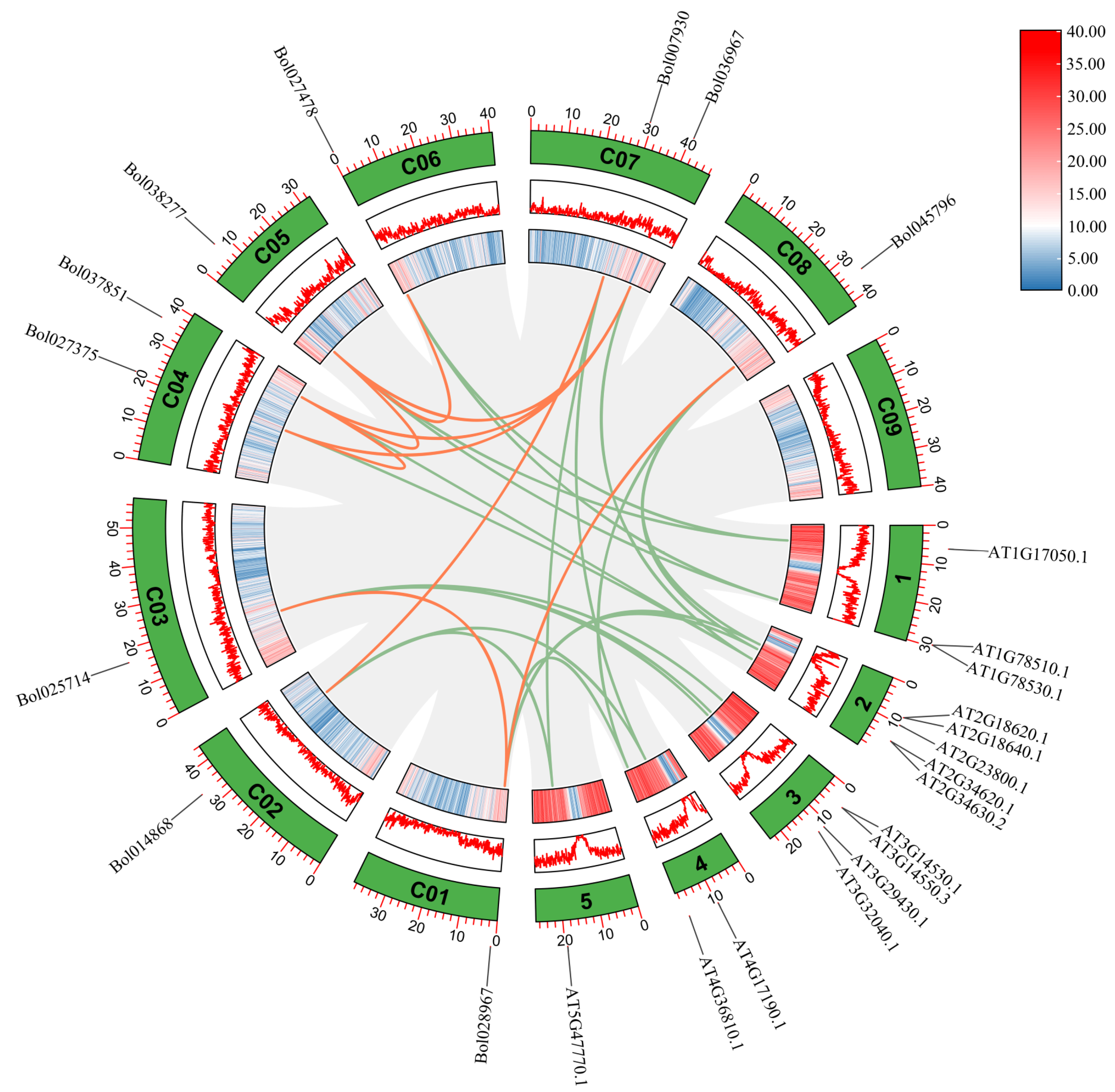
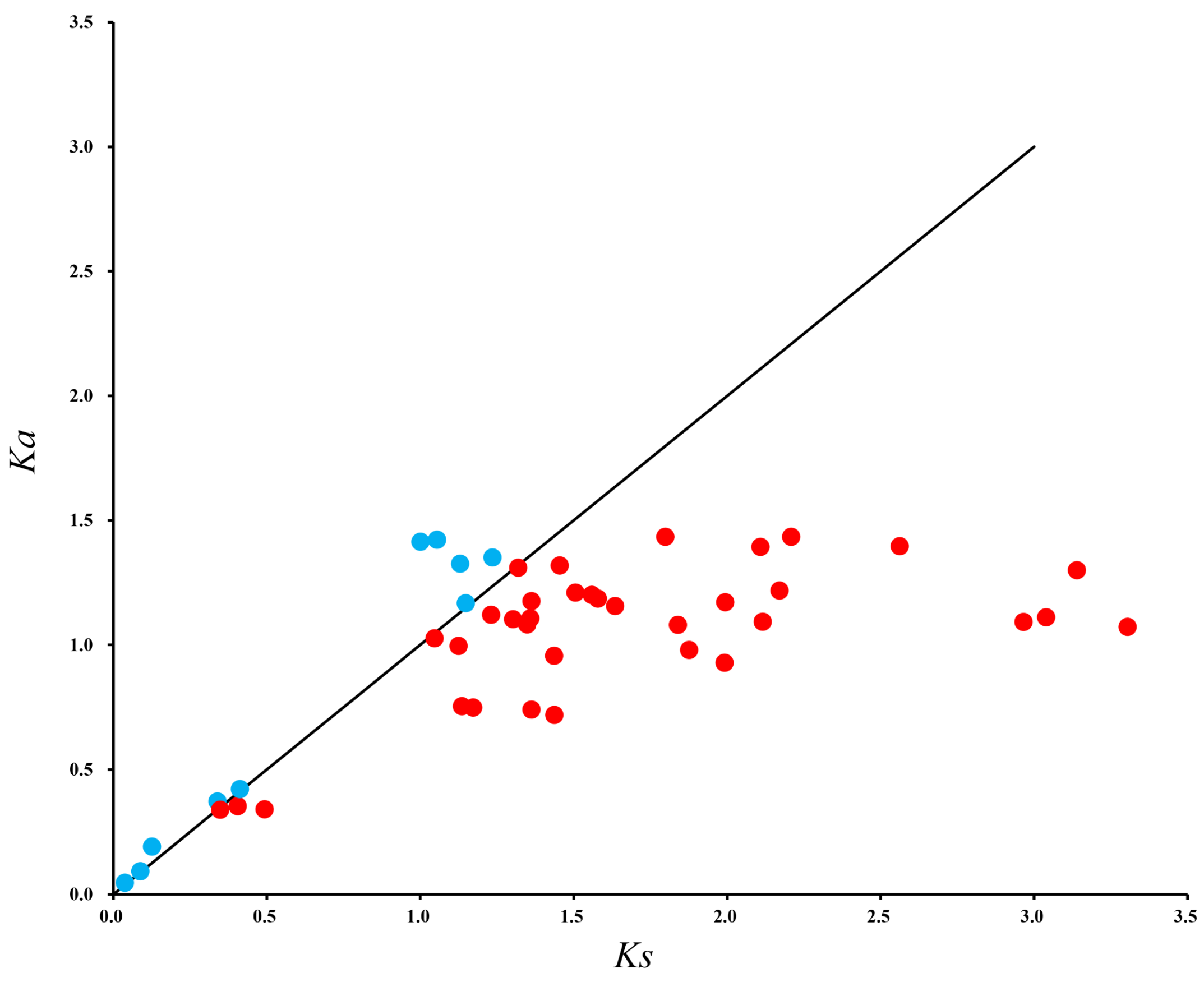
2.4. Collinearity, Distribution, and Ka/Ks Ratios of BoGGPPS Genes
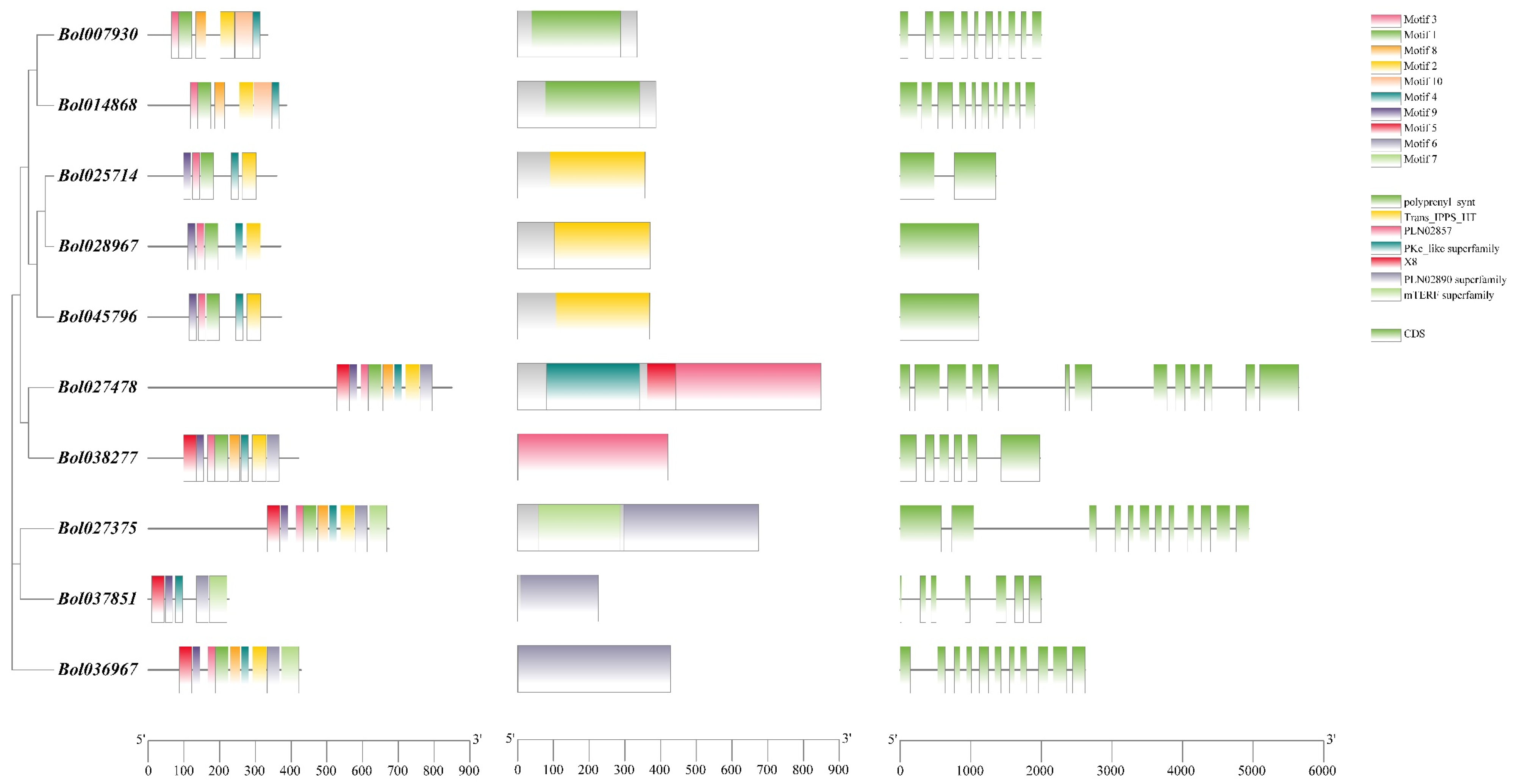
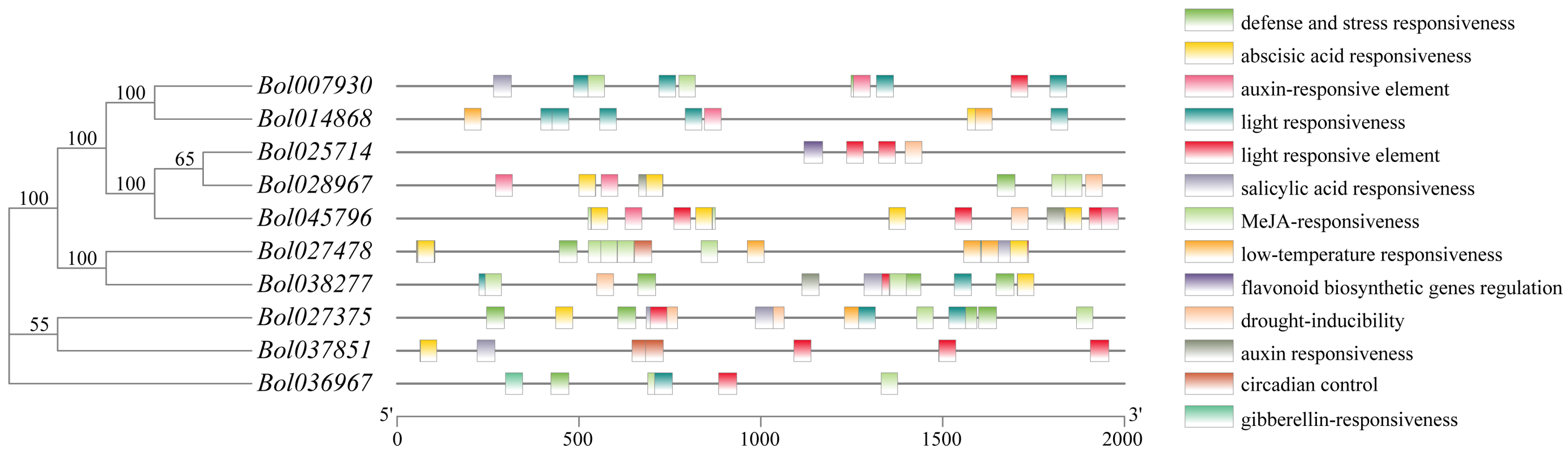
2.5. Gene Structure, Conserved Motif Distribution, and Cis-Elements Analysis
2.6. Three-Dimensional Prediction of the BoGGPPS Protein Structure
2.7. Expression Analysis of B. oleracea BoGGPPS Genes Using RNA-Seq Data
2.8. RNA Extraction, cDNA Synthesis, and qRT-PCR
2.9. Subcellular Localization and Confocal Laser Scanning Microscopy
3. Results
3.1. Identification and Phylogenetic Relationship of the BoGGPPS Gene Family in B. oleracea
3.2. Collinearity, Distribution, and Ka/Ks Analysis
3.3. Gene Structure, Domain and Conserved Motif Analysis
3.4. Analysis of Cis-Acting Elements in BoGGPPS Genes
3.5. Three-Dimensional Structure Prediction of GGPS Proteins
3.6. Expression Analysis of BoGGPPS Genes in Different Tissues
3.7. Subcellular Localization of the Bol028967 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudareva, N.; Negre, F. Practical applications of research into the regulation of plant volatile emission. Curr. Opin. Plant Biol. 2005, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Al-Taweel, A. Terpenes and Terpenoids; BoD–Books on Demand: London, UK, 2018. [Google Scholar]
- Banthorpe, D.; Wirz-Justice, A. Terpene biosynthesis. Part I. Preliminary tracer studies on terpenoids and chlorophyll of Tanacetum vulgare L. J. Chem. Soc. C Org. 1969, 4, 541–549. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zebec, Z.; Wilkes, J.; Jervis, A.J.; Scrutton, N.S.; Takano, E.; Breitling, R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr. Opin. Chem. Biol. 2016, 34, 37–43. [Google Scholar] [CrossRef]
- Haney, S.L.; Wills, V.S.; Wiemer, D.F.; Holstein, S.A. Recent advances in the development of mammalian geranylgeranyl diphosphate synthase inhibitors. Molecules 2017, 22, 886. [Google Scholar] [CrossRef]
- Muehlebach, M.E.; Holstein, S.A. Geranylgeranyl diphosphate synthase: Role in human health, disease and potential therapeutic target. Clin. Transl. Med. 2023, 13, e1167. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.; Barja, M.V.; Manzano, D.; Llorente, B.; Schipper, B.; Beekwilder, J.; Rodriguez-Concepcion, M. A Single Arabidopsis Gene Encodes Two Differentially Targeted Geranylgeranyl Diphosphate Synthase Isoforms. Plant Physiol. 2016, 172, 1393–1402. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.Y.; Gutensohn, M.; Jiang, L.; Zhang, P.; Zhang, D.; Dudareva, N.; Lu, S. A recruiting protein of geranylgeranyl diphosphate synthase controls metabolic flux toward chlorophyll biosynthesis in rice. Proc. Natl. Acad. Sci. USA 2017, 114, 6866–6871. [Google Scholar] [CrossRef]
- Feng, W.; Mehari, T.G.; Fang, H.; Ji, M.; Qu, Z.; Jia, M.; Wang, D.; Ditta, A.; Khan, M.K.R.; Cao, Y.; et al. Genome-wide identification of the geranylgeranyl pyrophosphate synthase (GGPS) gene family involved in chlorophyll synthesis in cotton. BMC Genom. 2023, 24, 176. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Qin, L.; Zhang, C.; Cao, L.; Li, H.; Ma, X. Overexpression of geranyl diphosphate synthase 1 (NnGGPPS1) from Nelumbo nucifera enhances carotenoid and chlorophyll content and biomass. Gene 2023, 881, 147645. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Methylome and transcriptome analyses of three different degrees of albinism in apple seedlings. BMC Genom. 2022, 23, 310. [Google Scholar] [CrossRef]
- Beck, G.; Coman, D.; Herren, E.; Ruiz-Sola, M.A.; Rodríguez-Concepción, M.; Gruissem, W.; Vranová, E. Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, H.; Zong, Y.; Tu, Z.; Li, H. Isolation, expression, and functional analysis of the geranylgeranyl pyrophosphate synthase (GGPPS) gene from Liriodendron tulipifera. Plant Physiol. Biochem. PPB 2021, 166, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, P.; Zhou, Y.J.; Wang, X.J.; Zhao, Y.J.; Liu, Y.J.; Tong, Y.R.; Hu, T.Y.; Huang, L.Q.; Gao, W. Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep. 2015, 34, 2179–2188. [Google Scholar] [CrossRef]
- Tata, S.K.; Jung, J.; Kim, Y.H.; Choi, J.Y.; Jung, J.Y.; Lee, I.J.; Shin, J.S.; Ryu, S.B. Heterologous expression of chloroplast-localized geranylgeranyl pyrophosphate synthase confers fast plant growth, early flowering and increased seed yield. Plant Biotechnol. J. 2016, 14, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Sci. 2010, 2, 1090. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.Y.; Zhu, Q.H.; Chen, X.; Luo, J.C. GSDS: A gene structure display server. Yi Chuan Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. Comput. Softw. J. Sci. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Orlova, I.; Nagegowda, D.A.; Kish, C.M.; Gutensohn, M.; Maeda, H.; Varbanova, M.; Fridman, E.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; et al. The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. Plant Cell 2009, 21, 4002–4017. [Google Scholar] [CrossRef]
- Rasouli, O.; Ahmadi, N.; Monfared, S.R.; Sefidkon, F. Physiological, phytochemicals and molecular analysis of color and scent of different landraces of Rosa damascena during flower development stages. Sci. Hortic. 2018, 231, 144–150. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, M.; Yuan, Q.; Zhang, F.; Tang, H. Sequence Analysis of the Geranylgeranyl Pyrophosphate Synthase Gene in Cabbage. IOP Conference Series: Materials Science and Engineering 2018, 452, 022074. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Wei, Z.; Yu, D.; Li, Y.h.; Gan, L.; Li, F.; Wang, Z. Genome-wide characterization and expression analysis of geranylgeranyl diphosphate synthase genes in cotton (Gossypium spp.) in plant development and abiotic stresses. BMC Genom. 2020, 21, 561. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A. The fate of duplicated genes: Loss or new function? BioEssays News Rev. Mol. Cell. Dev. Biol. 1998, 20, 785–788. [Google Scholar] [CrossRef]
- Friedman, R.; Hughes, A.L. Gene duplication and the structure of eukaryotic genomes. Genome Res. 2001, 11, 373–381. [Google Scholar] [CrossRef]
- De Bodt, S.; Maere, S.; Van de Peer, Y. Genome duplication and the origin of angiosperms. Trends Ecol. Evol. 2005, 20, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Guo, Y.; Qiu, L.J. Genome-wide identification and evolutionary analysis of leucine-rich repeat receptor-like protein kinase genes in soybean. BMC Plant Biol. 2016, 16, 58. [Google Scholar] [CrossRef]
- Davis, J.C.; Petrov, D.A. Preferential duplication of conserved proteins in eukaryotic genomes. PLoS Biol. 2004, 2, E55. [Google Scholar] [CrossRef]
- Min, D.D.; Tang, M.Q.; Li, G.; Qu, X.S.; Miao, J.H. Cloning and expression analysis of GGPPS gene from Panax notoginseng. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2015, 40, 2090–2095. [Google Scholar]
- Zhao, L. Cloning, bioinformatic analysis and expression analysis of RgGGPPS2 gene from Rehmannia glutinosa. Chin. Tradit. Herb. Drugs 2017, 48, 2269–2278. [Google Scholar]
- Lv, Y.; Chen, Y.; Wang, P. Cloning and Expression Analysis of GGPPS Gene from Mango (Mangifera indica). Mol. Plant Breed. 2017, 15, 65–70. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]



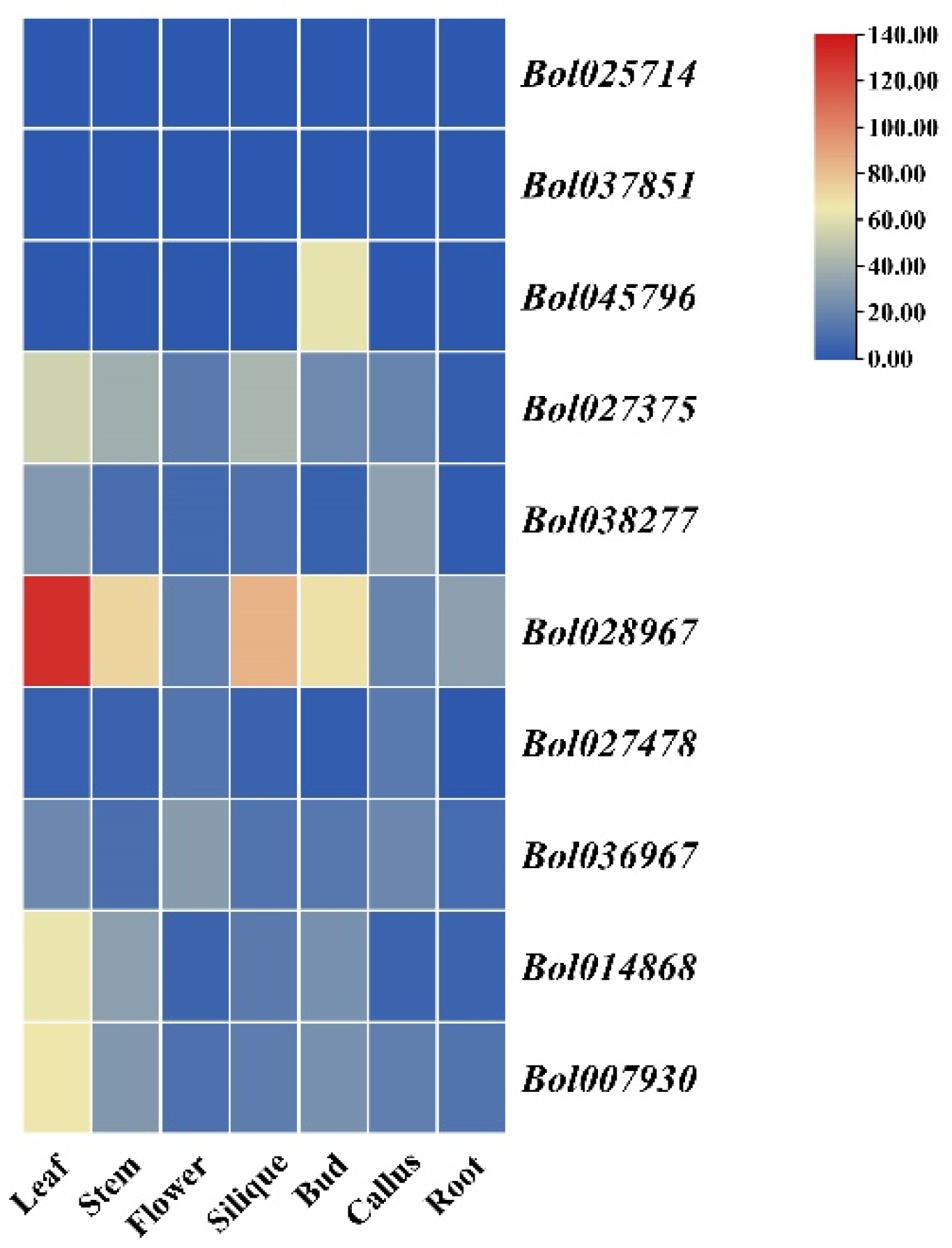

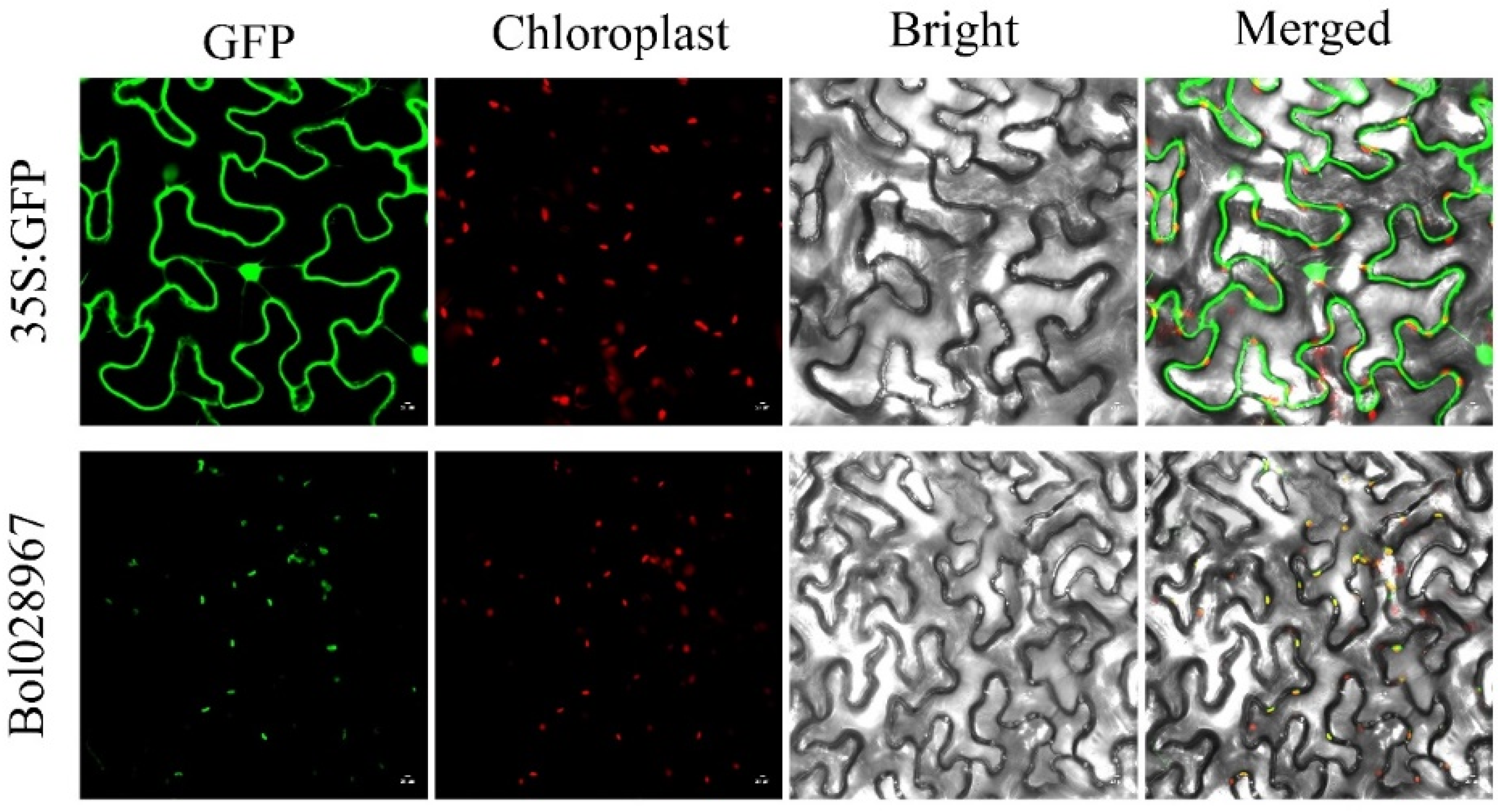
| Gene ID | Chr. | Genomic Location | CDS Length (bp) | Protein Length (aa) | Predicted Localization |
|---|---|---|---|---|---|
| Bol028967 | C01 | 1,144,276–1,145,388 | 1113 | 370 | Chloroplast/Plastid. |
| Bol014868 | C02 | 33,838,601–33,840,507 | 1161 | 386 | Mitochondrion |
| Bol025714 | C03 | 16,628,201–16,629,558 | 1077 | 358 | Chloroplast/Plastid |
| Bol027375 | C04 | 21,181,019–21,185,959 | 2022 | 673 | Chloroplast |
| Bol037851 | C04 | 35,798,177–35,800,174 | 678 | 225 | Chloroplast |
| Bol038277 | C05 | 6,816,936–6,818,915 | 1263 | 420 | Chloroplast |
| Bol027478 | C06 | 694,779–700,422 | 2550 | 849 | Chloroplast/Nucleus |
| Bol007930 | C07 | 29,493,460–29,495,460 | 1002 | 333 | Mitochondrion |
| Bol036967 | C07 | 40,793,183–40,795,804 | 1284 | 427 | Chloroplast |
| Bol045796 | C08 | 34,611,666–34,612,781 | 1116 | 371 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Fang, Z.; Zhang, N.; Yang, L.; Zhang, Y.; Zhuang, M.; Lv, H.; Ji, J.; Wang, Y. Genome-Wide Identification, Characterization, and Expression Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene Family Reveals Its Importance in Chloroplasts of Brassica oleracea L. Agriculture 2023, 13, 1615. https://doi.org/10.3390/agriculture13081615
Yan L, Fang Z, Zhang N, Yang L, Zhang Y, Zhuang M, Lv H, Ji J, Wang Y. Genome-Wide Identification, Characterization, and Expression Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene Family Reveals Its Importance in Chloroplasts of Brassica oleracea L. Agriculture. 2023; 13(8):1615. https://doi.org/10.3390/agriculture13081615
Chicago/Turabian StyleYan, Longxiang, Zhiyuan Fang, Na Zhang, Limei Yang, Yangyong Zhang, Mu Zhuang, Honghao Lv, Jialei Ji, and Yong Wang. 2023. "Genome-Wide Identification, Characterization, and Expression Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene Family Reveals Its Importance in Chloroplasts of Brassica oleracea L." Agriculture 13, no. 8: 1615. https://doi.org/10.3390/agriculture13081615
APA StyleYan, L., Fang, Z., Zhang, N., Yang, L., Zhang, Y., Zhuang, M., Lv, H., Ji, J., & Wang, Y. (2023). Genome-Wide Identification, Characterization, and Expression Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene Family Reveals Its Importance in Chloroplasts of Brassica oleracea L. Agriculture, 13(8), 1615. https://doi.org/10.3390/agriculture13081615










